Abstract
OBJECTIVES
The objective of this multicenter survey is to characterize the use of direct thrombin inhibitors (DTIs) in the pediatric population. The results of this survey may be used to design a prospective multicenter study with the ultimate goal of developing a dosing/titration recommendation for the use of DTIs in the pediatric population.
METHODS
This is a multicenter, descriptive study to survey hospitals around the country regarding the use of DTIs (argatroban, bivalirudin, and lepirudin) in the pediatic population. Institutional review board approval was obtained. The survey consisted of 42 questions and was designed utilizing Survey Monkey. The survey was emailed to members of the Pediatric Pharmacy Advocacy Group. Listserv members who responded to the survey within 4 weeks of when the survey was emailed were included in the study. Descriptive statistics were performed utilizing Microsoft Excel 2007.
RESULTS
Responses were obtained from 56 institutions from 29 states in the United States. Multiple agents are available on formulary with argatroban being the most common (~80%). The large majority of institutions (41.1%) utilize DTIs 2 to 4 times a year with an additional 33.9% utilizing them less than twice a year. There is no consistent approach to dosing and titration amongst pediatric institutions.
CONCLUSIONS
There are a wide variety of methods used by pediatric institutions with regard to dosing and titration of DTIs. Recently published prospective studies and package insert updates should help guide practitioners toward a more consistent approach to dosing of these high-risk medications.
INDEX TERMS: argatroban, bivalirudin, direct thrombin inhibitors, lepirudin, pediatrics
INTRODUCTION
Pediatric venous thromboembolic events (VTEs) are traditionally thought to be rare with a reported rate of 0.07 cases per 10,000 children.1 There is a wide incidence range published in the literature from 4.9 to 8 per 10,000 admissions.2–4 A recent study evaluating the incidence of VTE in the United States found an increase of 70% in VTE admissions from 2001 to 2007. Neonates, infants, and adolescents experienced the highest rates of VTE. During this time frame, the incidence of VTE increased significantly in neonates (44 to 75 per 10,000 admissions) and in infants (25 to 50 per 10,000 admissions).5
Due to the high incidence of VTE, neonates and infants are thought to be at high risk of developing VTE. Causes of VTE in this populaton are multifactorial. Among other risk factors, the presence of a central venous catheter is the single most common risk factor in children.5 A developing coagulation system may also play a role in the higher risk of VTE in neonates and infants. Other risk factors for VTE may be present more often in the neonatal/infant population as more premature infants are surviving long-term. Contributing factors may also include advancements in treatment and supportive care for severely ill pediatric patients, underlying chronic illness, and idiopathic VTE.5,6
Standard of therapy for VTE in the pediatric population is heparin, with increasing use of enoxaparin due to its mutiple benefits regarding ease of use, decreased risk for heparin-induced thrombocytopenia (HIT), and less monitoring.7 Heparin use in neonates and infants can be challenging due to varying degrees of intrinsic heparin resistance, partly from decreased amounts of antithrombin. Direct thrombin inhibitors (DTIs) such as argatroban, bivalirudin, or lepirudin have been suggested for use in the pediatric population due to their ability to provide anticoagulation without the necessity for adequate antithrombin concentrations. In the adult population, DTIs are utilized primarily for patients with HIT.8
However, given the perceived benefit of use in the pediatic population, there is limited data regarding the use of DTIs in this population for VTE, let alone in the infant population. The updated 2012 anticoagulation guidelines outlining treatment of VTE in neonates and children do not mention DTIs as a therapy option.9 Two prospective studies have been published looking at bivalirudin and argatroban in the pediatric population, along with an abstract regarding bivalirudin in pediatric patients > 6 months of age.10–12 The remainder of available literature is either retrospective in nature or limited to case reports. There are a total of 33 case reports in the literature of DTI use, with the most being with argatroban. A dosing and titration standard for DTI use is not currently known for the neonatal, infant, and pediatric population.
The aim of this survey is to characterize DTI use in pediatric patients across the United States. The results of this survey will potentially be employed to design a prospective multicenter study with the ultimate goal of developing a dosing and titration recommendation for the use of DTIs in the pediatric population.
MATERIALS AND METHODS
This is a multicenter, descriptive study to survey hospitals around the country regarding the use of DTIs in the pediatic population. Institutional review board approval was obtained. The objective of this multicenter survey is to characterize the current use of DTIs in the pediatric population.
The survey consisted of 42 questions, which included collection of institutional demographic data along with detailed questions regarding their use of argatroban, bivalirudin, and lepirudin. The survey was designed utilizing Survey Monkey (Palo Alto, CA) and was emailed to pharmacy practitioners identified as being members of the Pediatric Pharmacy Advocacy Group (PPAG) in the last quarter of 2011.
Institutions on the PPAG listserv that responded to the survey were included in the study. Institutions were excluded if they did not respond within the 4-week period of the survey being emailed. It was expected that 100 hospitals would receive the survey with an anticipated completion rate of 50%. Descriptive statistics were performed utilizing Microsoft Excel 2007.
RESULTS
Responses were obtained from 56 institutions from 29 states within the United States. Of those responding, 33.9% were free-standing pediatric hospitals, 62.5% were integrated pediatric and adult hospitals, and 3.6% were reported as other. Of the 56 institutions, 85.7% were teaching institutions. There was a wide variety in pediatric bed size (Figure 1), Pediatric Intensive Care Unit bed space (Figure 2), and Neonatal Intensive Care Unit bed space (Figure 3). Of these institutions, 35.7% have a physician-led anticoagulation service and 41.1% have a hematology/anticoagulation pharmacist on site. Pharmacy consult services for anticoagulation are available in 58.9% of respondents. With regard to utilization of a standard protocol for the use of any DTI, 33.9% of respondents have a protocol.
Figure 1.
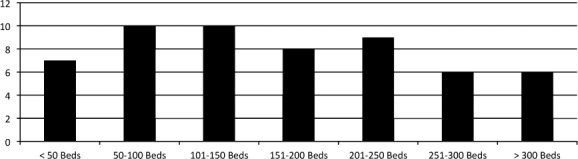
Number of Pediatric Beds.
Figure 2.
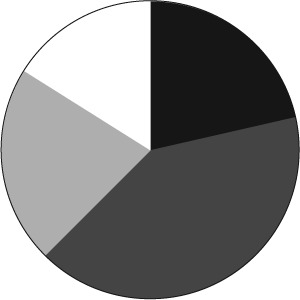
Number of Pediatric Intensive Care Unit Beds. ▪ <10 Beds;  10–20 Beds;
10–20 Beds;  20–30 Beds; □ >30 Beds
20–30 Beds; □ >30 Beds
Figure 3.
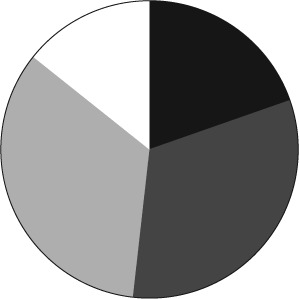
Number of Neonatal Intensive Care Unit Beds. ▪ <20 Beds;  20–40 Beds;
20–40 Beds;  40–60 Beds; □ >60 Beds
40–60 Beds; □ >60 Beds
The large majority of institutions (41.1%) utilize DTIs 2 to 4 times a year with an additional 33.9% utilizing them less than twice a year. Approximately 16% of institutions use a DTI more than 4 times a year while 9% never use a DTI. DTIs are used for treatment of thrombus in the presence of HIT in approximately 50% of respondents with approximately 40% using DTIs sometimes in the presence of HIT. When patients experience thrombus extension despite a therapeutic activated prothrombin time (aPTT) with heparin therapy, 5.4% of respondents switch to a DTI, 33.9% do not change therapy, and 60.7% sometimes switch to a DTI.
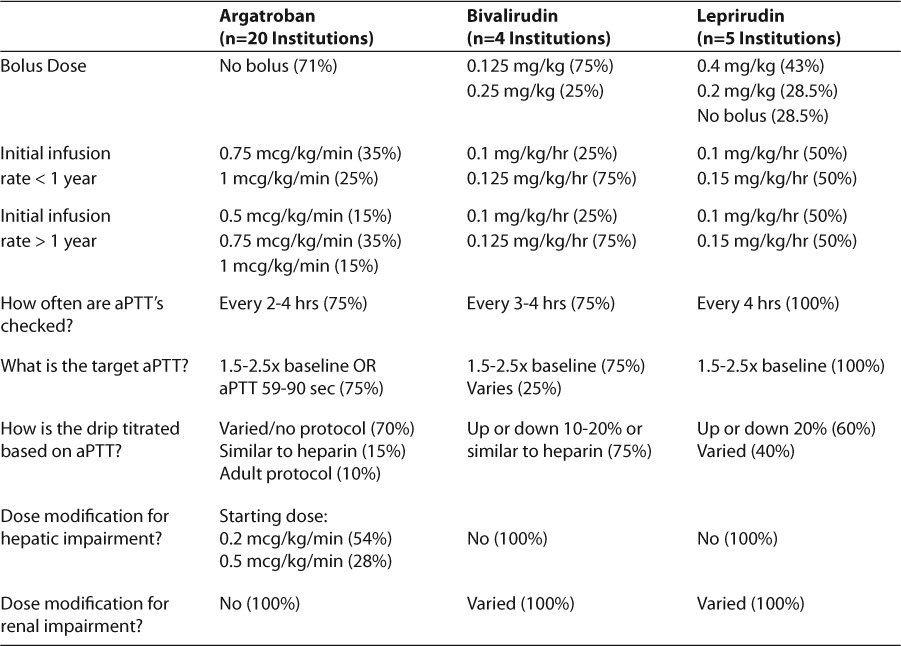
Several institutions had multiple DTIs on their formulary, with argatroban being the most common (~80%), followed by bivalirudin (41%) and lepirudin (4%). Several institutions (10%) did not have any DTI on formulary. Approximately 82% (45/55) of respondents use argatroban regardless of formulary status. A total of 20 institutions responded regarding details of argatroban dosing. Bolus doses were reported as not used in 71% of institutions. Of the remaining 29% of institutions, half occasionally utilized bolus doses of argatroban, while the other half reported varied use and dose. Initial infusion rates with argatroban were similar between patients < 1 year of age and > 1 year of age (Table 1). There was a large degree of variability in titration of argatroban based on aPTT, where 40% of institutions either did not have a protocol or titrate based on attending physician discretion. Of the remaining institutions, 35% adjust by 0.1 to 0.25 mcg/kg/min based on a variety of aPTT ranges, 15% utilize heparin aPTT ranges and adjustment recommendations,2,13 and 10% utilize adult argatroban recommendations.14 A majority of respondents check aPTT every 2 to 4 hours (75%) then daily after aPTTs are stable.
Table 1.
Initial Infusion Rate of Argatroban
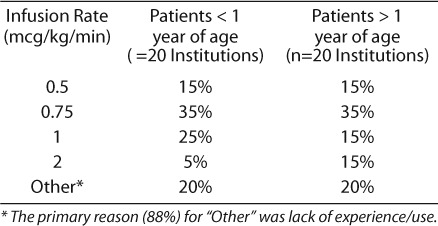
Four institutions reported use of bivalirudin with 75% utilizing a bolus dose of 0.125 mg/kg and 25% using 0.25 mg/kg. An initial infusion rate of 0.125 mg/kg/hr, regardless of patient age, was reported in 75% of respondents with 25% using a rate of 0.1 mg/kg/hr. Targets for aPTT were similar to other DTIs, with a target aPTT of 1.5 to 2.5× baseline (75%).
Five institutions reported use of lepirudin with 43% using bolus doses of 0.4 mg/kg regardless of patient age, 28.5% reporting bolus doses of 0.2 mg/kg, and 28.5% did not use bolus doses. Regardless of patient age, one-half of institutions use 0.1 mg/kg/hr, and the other half reported 0.15 mg/kg/hr. The initial infusion rate is titrated to an aPTT ratio of 1.5 to 2.5 with aPTTs being checked every 4 hours until stable levels achieved by 100% of respondents. Fifty percent of respondents titrate by 20% to reach goal, if the goal is exceeded, the drip is reinitiated at 50% of the infusion rate. Dosing used for all 3 DTIs are summarized in Table 2.
Table 2.
Summary Table of Dosing For All DTI Agents
DISCUSSION
One of the main barriers to utilizing DTIs in the neonatal/infant population is the lack of data regarding dosing and titration. That combined with its rarity of use result in many practitioners utilizing these potent anticoagulants without much guidance. As evidenced from the survey results, while many practitioners are utilizing adult protocols on pediatric patients, there still exists a wide variety in dosing and titration.
Young and colleagues11 published a multicenter, open-label study in 2011 looking at argatroban dosing in pediatric patients from 2003 to 2007. Approximately 44% of the subjects were < 6 months of age, but neonates with a corrected gestation of ≤ 44 weeks' gestation were excluded. Use of argatroban in this study was typically short term (mean of 3 days) and typically for use during invasive procedures. Dosing used in the study reflect the same methods employed by a majority of respondents to the survey, in that they were developed based on experience of physicians and adult protocols. There was a variety in dosing due to the variety of indications, underlying disease states, and patient age. They concluded that in critically ill pediatric patients, a starting dose of 0.75 mcg/kg/min (0.2 mcg/kg/min in patients with hepatic impairment) is reasonable and should be adjusted to achieve an aPTT 1.5 to 3 times baseline (maximum of 100 seconds).11
The investigators above performed pharmacokinetic (PK) and pharmacodynamic (PD) analysis on aPTT levels and argatroban serum concentrations drawn during their study. That data was reported to the Food and Drug Administration for prescribing information changes to the argatroban package insert (PI).15 Simulations were performed to derive optimal dosing, taking into consideration the parameters used to determine current adult protocols balancing expected benefit (percentage of patients falling within the target aPTT window) and risk (percentage exceeding target and at risk for bleeding). In adults, a starting dose of argatroban 2 mcg/kg/min is predicted that 67% of patients will attain a target aPTT with 2% exceeding the range. Based on serum aPTT levels drawn from 15 of 18 patients in the above study, a starting dose of 0.75 mcg/kg/min would achieve a target aPTT in 59% of pediatric patients with 1.2% exceeding the target range. These simulations also predicted that titrating by 0.25 mcg/kg/min would result in almost all patients reaching the target aPTT window.15
The study described above gives pediatric practitioners better information and guidance with regards to utilizing argatroban for a variety of indications. These PK and PD analyses were conducted based on a total of 15 patients, with a variety of ages. While their simulations found that hepatic function was more of an indicator of decreased clearance rather than age, repeating these simulations with a larger pediatric population may be beneficial. Additionally, the original study excluded all neonates ≤ 44 weeks' gestational age.
The PI for argatroban was revised in late 2011 to include dose recommendations in pediatric patients.14 The PI was updated to recommend in pediatric patients with normal hepatic function, an initial infusion rate of 0.75 mcg/kg/min and to titrate in increments of 0.1 to 0.25 mcg/kg/min. Those patients with critical illness with impaired hepatic function should be initiated at 0.2 mcg/kg/min and titrated in increments of 0.1 to 0.25 mcg/kg/min.12 From the results of the survey, only 35% of institutions are initiating at the dose recommended in the PI. With regard to patients with hepatic impairment, 50% of institutions are following PI recommendations. Prospective data also exist with bivalirudin indicating that a loading dose of 0.125 mg/kg followed by 0.125 mg/kg/hr should be utilized in pediatric patients.11,12 A larger majority of institutions are following these recommendations (75%). However, the sample size was extremely low (n=4).
Even though more data are becoming available regarding dosing and titration of DTIs in the pediatric population, implementation of these recommendations and consistency across institutions is needed. DTIs are considered a high-risk medication with a majority of institutions utilizing argatroban. While it is alarming that the large majority of institutions appear to be dosing them outside of the PI recommendations, as well as titrating in an inconsistent manner, this survey was sent out to practitioners about the same time as changes were made to the argatroban PI. Therefore, even though these studies were published, many may not have had access or were aware that these studies were available. By updating the PI, other tertiary literature sources commonly used by prescribers and pharmacists will be updated as well; hopefully making these dose recommendations more up front when looking for dosing information.
Another important point is the lack of consistency regarding titration of DTIs. There are well-accepted titration regimens in the adult population regarding how to titrate DTIs based on aPTT. As evidenced from results of the survey, 70% of institutions have no protocol on titration once a DTI is initiated. Studies utilized to update the PI did not include how to titrate based on aPTT other than indicating a range of 0.1 to 0.25 mcg/kg/min to target an aPTT 1.5 to 3 times baseline. Development of a more specific titration scheme based on resulted aPTTs is necessary to further insure safe and effective use of DTIs.
Overall, a large majority of institutions across the country are utilizing these high-risk medications in an inconsistent manner. Many of the doses and approaches reported concerning use of DTIs do not appear to be utilizing current available evidence. Education of both pharmacists and practitioners is necessary to ensure safe and appropriate dosing of these agents. Of note, adult recommendations for which DTI to use is different depending on the patient's renal or hepatic function, as well as indication (HIT, need for cardiac surgery, etc.).8 However, as the majority of data is available regarding argatroban, institutions utilizing other DTI agents in the pediatric population should consider utilizing argatroban. Institutions that chose to utilize bivalirudin should adhere to dosing recommendations from published prospective studies. More data are still needed via large prospective evaluation regarding dosing and titration of DTI agents as the data available are from limited sample size and duration of therapy. In the meantime, as healthcare providers it is our responsibility to utilize available literature to maximize the safe and effective use of these high-risk medications.
ACKNOWLEDGMENT
Portions of the manuscript have been previously published in the PPAG in the November/December 2012 newsletter. The author would like to acknowledge the PPAG research committee for all their help and support with the study.
ABBREVIATIONS
- aPTT
activated prothrombin time
- DTI
direct thrombin inhibitor
- HIT
heparin-induced thrombocytopenia
- PD
pharmacodynamic
- PI
package insert
- PK
pharmacokinetic
- PPAG
Pediatric Pharmacy Advocacy Group
- VTE
venous thromboembolic event
Footnotes
DISCLOSURE The author declares no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Parasuraman S, Goldhaber SZ. Venous thromboembolism in children. Circulation. 2006;113(2):e12–e16. doi: 10.1161/CIRCULATIONAHA.105.583773. [DOI] [PubMed] [Google Scholar]
- 2.Monagle P, Chalmers E, Chan A. Antithrombic therapy in children: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2008;133(suppl 6):e887s–e968s. doi: 10.1378/chest.126.3_suppl.645S. et al. [DOI] [PubMed] [Google Scholar]
- 3.Sandoval JA, Sheehan MP, Stonerock CE. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population risk. J Vasc Surg. 2008;47(4):837–843. doi: 10.1016/j.jvs.2007.11.054. et al. [DOI] [PubMed] [Google Scholar]
- 4.Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in neonates and children: data from the national hospital discharge survey. J Pediatr. 2004;145(4):563–565. doi: 10.1016/j.jpeds.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Raffini L, Huang YS, Witmer C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008. doi: 10.1542/peds.2009-0768. et al. [DOI] [PubMed] [Google Scholar]
- 6.Setty BA, O'Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer. 2012;59(2):258–264. doi: 10.1002/pbc.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AK, Deveber G, Monagle P. Venous thrombosis in children. Thromb Haemostasis. 2003;1(7):1443–1455. doi: 10.1046/j.1538-7836.2003.00308.x. et al. [DOI] [PubMed] [Google Scholar]
- 8.Linkins LA, Dans AL, Moores LK. Treatment and prevention of heparin-induced thrombocytopenia. Chest. 2012;141(suppl 2):e495s–e530s. doi: 10.1378/chest.11-2303. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monagle P, Chan A, Goldenberg NA. Antithrombic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis. Chest. 2012;141(suppl 2):e737s–801s. doi: 10.1378/chest.11-2308. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young G, Tarantino MD, Wohrley J. Pilot dose-finding and safety study of bivalirudin in infants less than 6 months of age with thrombosis. J Thromb Haemost. 2007;5(8):1654–1659. doi: 10.1111/j.1538-7836.2007.02623.x. et al. [DOI] [PubMed] [Google Scholar]
- 11.Young G, Boshkov LK, Sullivan JE. Argatroban therapy in pediatric patients requiring nonheparin anticoagulation: an open label, safety, efficacy, and pharmacokinetic study. Pediatr Blood Cancer. 2011;56(7):1103–1109. doi: 10.1002/pbc.22852. et al. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien SH, Yee D, Lira J. Atlanta, GA: December 2012. Prospective, open-label clinical trial of bivalirudin in children with venous thromboembolism. et al. Poster presented at: Annual Meeting of the American Society of Hematology. [Google Scholar]
- 13.Taketomo CK, Hodding JH, Kraus DM, editors. Pediatric Dosage Handbook. 16th ed. Hudson, OH: Lexi-comp; 2009. eds. [Google Scholar]
- 14.Argatroban [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2012. [Google Scholar]
- 15.Madabushi R, Cox DS, Hossain M. Pharmacokinetic and pharmacodynamic basis for effective argatroban dosing in pediatrics. J Clin Pharmacol. 2011;51(1):19–28. doi: 10.1177/0091270010365550. et al. [DOI] [PubMed] [Google Scholar]


