Abstract
OBJECTIVES
Earlier studies have indicated that the pharmacokinetics of mycophenolic acid (MPA) is influenced by polymorphisms of ABCC2, which encodes for the membrane transporter MRP2. The ABCC2 rs717620 A allele has been associated with enterohepatic recirculation of MPA, and our previous work had correlated the discontinuance of MPA with this allele in pediatric heart transplant patients. Therefore, we hypothesized that the ABCC2 rs717620 A allele would be associated with poorer outcomes including rejection with hemodynamic compromise (RHC), graft failure, and death in the pediatric heart transplant (PHTx) population receiving MPA.
METHODS
PHTx recipients from 6 institutions in the Pediatric Heart Transplantation Study (PHTS) from the period of 1993–2009, receiving MPA therapy, were genotyped for ABCC2 rs717620. Genotyping was accomplished by direct sequencing. Demographic and outcome data were limited to the data routinely collected as part of the PHTS and included RHC and mortality.
RESULTS
Two hundred ninety patients were identified who received MPA at some point post transplantation, of which 200 carried the GG genotype, 81 carried the AG genotype, and 9 carried the AA genotype. Follow-up time after transplantation was 6 years. RHC occurred in 76 patients and 18 patients died. In the 281 patients followed up more than 1 year, late RHC (>1 year post transplantation) occurred in 42 patients. While both RHC and late RHC were associated with the ABCC2 rs717620 GG genotype (hazard ratios: 1.80 and 4.57, respectively, p<0.05) in all patients, this association was not significant in PHTx patients receiving only MPA as the antiproliferative agent from the time of transplant (n=142).
CONCLUSIONS
ABCC2 rs717620 polymorphisms varied within racial groups. As a candidate gene assessment, the ABCC2 rs717620 AG and AA genotypes may be associated with improved, rather than poorer, RHC in PHTx patients receiving MPA therapy. ABCC2 rs717620 polymorphisms should be included in any expanded pharmacogenomic analysis of outcomes after pediatric heart transplantation.
INDEX TERMS: ABCC2 polymorphisms, heart transplantation, mycophenolic acid, pediatrics, pharmacogenetics, rejection
INTRODUCTION
Organ transplantation and its management is a complex process that requires maintenance of organ function, and management of the immune response, infections, and drug adverse effects and interactions. The influence of donor and recipient genetics on this process is still being worked out. One approach that has been taken is a candidate gene approach where single nucleotide polymorphisms (SNPs) are first tested individually, and then those SNPs with promise are tested as a group for associations with a disease state.1 The Pediatric Heart Transplant Study (PHTS) has taken this candidate gene approach, and the results of several multiple SNP analyses have been published.2–4 These previous studies had not examined SNPs for an important membrane transporter called the multidrug resistance protein 2 (MRP2).
The ABC transporter family plays a role in the disposition of multiple drugs, and MRP2 is encoded by ATP-binding cassette subfamily C member 2 (ABCC2). A genetic influence on the function of MRP2 is well known due to the inborn genetic error in ABCC2 in Dubin-Johnson syndrome of conjugated hyperbilirubinemia. MRP2 plays a role in the liver with enterohepatic recirculation, in gastrointestinal absorption, and in drug elimination in the renal proximal tubule. While multiple SNPs can be identified inABCC2,4 ABCC2 rs717620 (formerly referred to as C-24T) has been most extensively studied in transplant patients in association with mycophenolic acid (MPA) therapy.5–8
MPA and its metabolites are transported in the liver and kidney primarily by MRP2. This transport process in liver was initially recognized as having a role in the drug interaction between cyclosporine and MPA.9 In our previous study in pediatric heart transplant (PHTx) patients, 36% of the study population had MPA temporarily stopped or discontinued owing to gastrointestinal (GI) intolerance.10 While the exact mechanism of the intestinal adverse effects is not understood, this discontinuation of MPA or a reduction in MPA dosage due to GI intolerance has been associated with an increased risk of rejection and graft failure in renal allograft patients.11 Dose reduction for MPA due to GI intolerance has also been reported as a risk factor for increased rejection rates in adult heart transplant patients.12
Given the preliminary evidence for the association of ABCC2 polymorphisms with MPA discontinuation and the association of MPA dose reduction or discontinuation with organ transplant rejection, we hypothesized that ABCC2 polymorphisms may be a risk factor for rejection episodes, rejection with hemodynamic compromise (RHC), or death in PHTx patients. With an increasingly diverse set of transplant immuno-suppressive agents available, a pharmacogenetic effect on clinical outcomes could have important implications for drug selection algorithms for PHTx patients in the future. In accordance with the candidate gene approach of the PHTS, the objective of this study was to conduct an initial examination of the association between the long-term outcomes in PHTx patients receiving MPA and the ABCC2 rs717620 polymorphism.
MATERIALS AND METHODS
Participants
This study included PHTx recipients undergoing a transplant between January 1993 and December 2009 and followed up at 1 of the 6 centers participating in the National Heart, Lung and Blood Institute–sponsored Specialized Centers for Clinically Oriented Research (SCCOR) program “Optimizing Outcomes After Pediatric Heart Transplantation” and the PHTS. This SCCOR program included University of Pittsburgh, Stanford University, Loma Linda University, Washington University, Columbia University, and University of Alabama at Birmingham. Patients were enrolled in the study after obtaining approval of the institutional review boards of the participating centers, informed consent from parents or guardians, and assent from children of age to provide this. All participants were separately enrolled in the PHTS, a prospective, event-driven database study.4 All patients were maintained on immunosuppressive regimens and underwent rejection surveillance as per individual institutional protocols. For the purpose of this evaluation, patients were included if they received any product formulation of MPA.
Data Collection and Clinical Outcomes
All demographic, clinical, and rejection data were extracted from the PHTS database. Race was determined by parent-described response recorded at study entry. The PHTS defines rejection as “an event (biopsy or other criteria) leading to acute augmentation of immunosuppressive therapy,” and rejection episodes are reported on designated event forms.13–16 For each rejection event, hemodynamic status is reported and hemodynamic compromise, if present, is classified as mild, with worsening of cardiac function, or severe, requiring inotropic support. In this study, we investigated episodes of RHC, both mild and severe.
Only patients who received any formulation of MPA as part of their immunosuppressive regimen were eligible for inclusion in this analysis. Since the hypothesis related outcome to MPA therapy, a separate analysis was performed for patients who received MPA from the time of transplantation (initial immunosuppressive therapy) to reduce confounding factors. Mycophenolate plasma concentrations and drug doses were not collected as part of this study. No attempt to analyze the results by clinical center was made because of the relatively small number of patients.
Detection of Genetic Polymorphisms
A sample of 3 to 6 mL anticoagulated peripheral venous blood was obtained from each study participant. Genomic deoxyribonucleic acid (DNA) was extracted from each blood sample by using QIAamp DNA Blood Midi Kit and amplified by using REPLI-g Mini/Midi Kit (Qiagen Inc, CA). Genotypes were obtained by direct nucleotide sequencing at the ABCC2 locus (G > A transition; rs717620) by using nested polymerase chain reaction (PCR). The primary PCR primers were F1 5′-AACAACAATTCTCCTTCCTCAC-3′ and R1 5′-TAGGCTCACACTGGATAAGC-3′. The secondary PCR primers were F2 5′-TCTTGTTGGTGACCACCCTAAG-3′ and R2 5′-AGCTCTGTTGACATCTTTCAGTG-3′. For primary PCR, a 20 uL reaction was prepared and a 50 uL reaction was used for secondary PCR. Each reaction consisted of the following: 1× PCR buffer, 2 mmol/L of each deoxynucleotide triphosphate, 0.2 mmol/L magnesium chloride, 20 mmol/L forward and reverse primers, 1 unit of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA), and 5 uL DNA. The conditions for the primary PCR amplification were 94°C for 5 minutes, followed by 20 cycles of 94°C for 30 seconds, 68°C for 30 seconds, and 72°C for 30 seconds, with a final extension step at 72°C for 7 minutes. The secondary PCR conditions were 94°C for 5 minutes, followed by 40 cycles of 94°C for 30 seconds, 64°C for 30 seconds, and 72°C for 30 seconds, with a final extension step at 72°C for 7 minutes. Shrimp Alkaline Phosphatase (SAP)17 and Exonuclease I (Exo I) (USB, Cleveland, OH) were used to remove excess amplification primers and deoxyribonucleotide (dNTPs) before genotyping. The purification reaction was prepared with 1 unit SAP, 3 units Exo I, 1 unit SAP Dilute Buffer (10×), and 15 uL PCR product. The reaction conditions were 90 minutes at 37°C followed by 20 minutes at 70°C. Direct nucleotide sequencing PCR was performed by using the Big Dye Terminator Cycle Sequencing Ready Reaction kit V3.1 on an ABI Prism 3130xl Genetic Analyzer (Applied BioSystems, Foster City, CA). The following primers were used for sequencing: F3 5′-ACTAACTACCACTTGTTCTGAG-3′ and R3 5′-AACTGGTGAGTCTCCCTGTC-3′.
Statistical Analyses
Participating PHTx patients were grouped according to their ABCC2 rs717620 genotype. Clinical and demographic characteristics were compared between ABCC2 rs717620 groups by using chi-square statistics. Sixty-day rejection rates and severity of RHC were compared with Mantel-Haenszel chi-square statistics for ordered data. Time-to-event analyses were performed for rejection outcomes, for graft failure and death among all study patients, and for time to late rejection and late RHC events among patients with at least 1 year of follow-up. Unadjusted RHC rates at 5 years post transplantation were estimated by Kaplan-Meier methods and compared among subgroups defined by recipient race, age at transplantation, and genotype, using log-rank statistics. Individual Cox proportional hazards regression models were created to estimate the hazard ratio (HR) of the ABCC2 rs717620 genotype on the specified clinical outcomes, adjusting for recipient race and age at transplantation. A dominant model was used to compare patients with ABCC2 rs717620 GG to those with AG or AA. The main effect and interaction terms between the SNP and black race were included in the model to test whether the effect of the SNP on RHC varied significantly by race. In addition, the age-adjusted HR for the genotype was examined separately within each racial group. Adjusted HRs and 95% confidence intervals (CIs) are reported. All analyses were performed with SAS version 9.1 (Cary, NC). p values less than 0.05 were considered statistically significant. A subanalysis was conducted for patients receiving MPA as initial therapy from the time of transplantation.
RESULTS
From the PHTS population, 290 patients were identified as having received MPA at some time post transplantation. Fifty-four percent of the patients received only MPA as an antiproliferative agent, and 46% of patients had received both azathioprine and MPA during their posttransplant course. One hundred forty-two patients had received MPA as part of initial immunosuppressive therapy from the time of transplantation. The calcineurin antagonist immunosuppressant used for most of the patients was cyclosporine (71%), and tacrolimus was used in the remaining group (29%). Induction therapy was administered in 62% of the patient population. When drug therapy was compared across ABCC2 rs717620 genotypes, no significant difference was observed for any given genotype. The average patient follow-up time was 6 years post transplantation.
The demographic characteristics of the patients are given in Table 1. The patients were evenly divided between males and females, and 74% were of white race with 23% of all patients having Hispanic ethnicity. In this group, 26% were infants defined as younger than 1 year, and 24% were aged 13 years or older. Fifty percent had cardiomyopathy and 44% had congenital heart disease. While the patient characteristics for age, sex, Hispanic ethnicity, and etiology did not differ significantly, race varied by genotype group. The prevalence of the ABCC2 rs717620 genotypes by combined race/ethnicity categories is presented in Figure 1. The ABCC2 genotypes differed significantly by racial/ethnic groups, such that the black non-Hispanic group was more likely to have a GG genotype and was significantly less likely to be heterozygous (p=0.045; chi-square test). Only 1 homozygous ABCC2 rs717620 AA patient was found among the 46 black non-Hispanic patients. All genotypes within racial groups were in Hardy-Weinberg equilibrium.
Table 1.
Demographics of Patient Population (n=290)
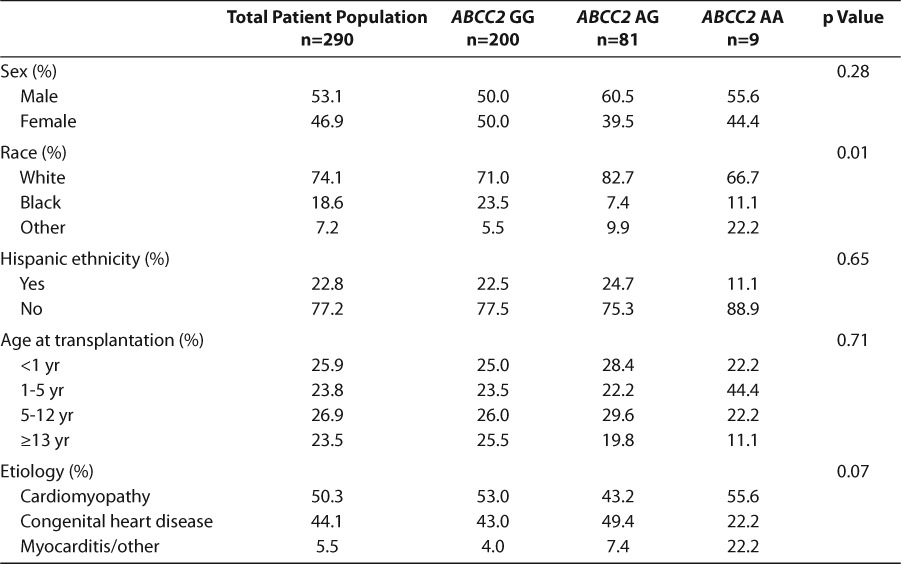
Figure 1.
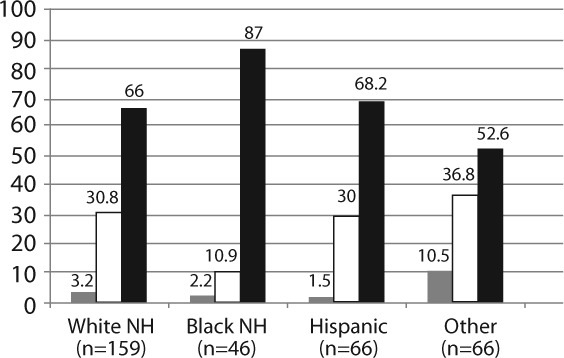
ABCC2 genotype frequency by race/ethnicity (p=0.042). NH, non-Hispanic.  AA;
AA;  ; AG;
; AG;  GG
GG
Of the 290 patients receiving MPA as immunosuppressive therapy, 28 suffered graft failure and 18 died (see Table 2). ABCC2 rs717620 polymorphisms were not associated with graft failure or death. One hundred and twenty-five patients had more than 1 episode of rejection, and ABCC2 rs717620 genotype was not associated with the occurrence of a second rejection episode. Seventy-six patients had RHC, and patients with ABCC2 rs717620 GG were more likely to have RHC after transplantation than those with AA or AG (5 year freedom from RHC: 69.4% GG vs 82.5% AG or AA, p=0.0016, Figure 2). Accounting for age and black race,18 patients with ABCC2 rs717620 GG had significantly greater risk of RHC than those with AA or AG (HR=1.80, 95% CI=1.01 to 3.20, p=0.045).
Table 2.
Association of Clinical Outcomes and ABCC2 rs717620 GG vs ABCC2 rs717620 AA or AG Polymorphisms Adjusted for Age and Race

Figure 2.
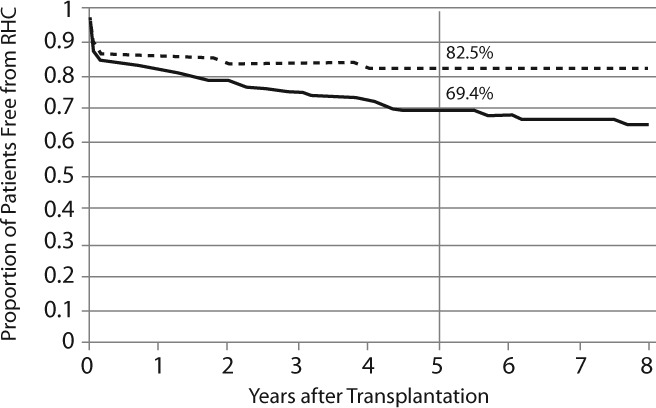
Rejection with hemodynamic compromise for patients with ABCC2 genotypes GG (solid line) and AG or AA (dashed line). Log rank statistic p=0.016.
Most of the patients (n=281) had been followed up for greater than 1 year post transplantation. Of this group, 116 patients had an episode of rejection after 1 year post transplantation. Forty-two patients had RHC greater than 1 year post transplantation, and the adjusted risk of late RHC was associated with ABCC2 rs717620 GG vs AA or AG (HR=4.57, 95% CI=1.60 to 13.0, p=0.0045). When the sample was limited to those patients receiving MPA as their initial immunosuppressive therapy, the relationships between ABCC2 rs717620 and RHC and late RHC were not statistically significant, and the estimated effects, as measured by the HR, were attenuated in this patient subset (see Figure 3).
Figure 3.
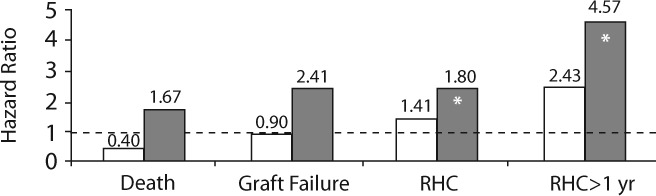
ABCC2 rs717620 genotypes and posttransplantation outcomes. The age and race-adjusted HRs for GG vs AA or AG are shown, and an asterisk (*) signifies a significant association (p<0.05). The dashed line represents HR=1 HR, hazard ratio.  Pts. Who Received MPA as Initial Therapy (n=142);
Pts. Who Received MPA as Initial Therapy (n=142);  All Pts. Who Received MPA (n=290)
All Pts. Who Received MPA (n=290)
DISCUSSION
Rejection with hemodynamic compromise in PHTx patients predicts a very poor outcome, with a 50% mortality within 5 years of the event.19 Most deaths occur within 6 months of the event, and these statistics have not changed in the last 15 years.16 Therefore, any factor contributing to RHC has to be seriously considered in PHTx patients. Risk factors previously associated with RHC have included older age of recipients, nonwhite race,16 and genetic polymorphisms of IL-10, FAS, and ACE II.2
Drug therapy is the primary defense against RHC, and MPA is currently the most commonly used antiproliferative drug therapy. The disposition and effect of MPA may be controlled by a number of gene polymorphisms and drug interactions. The target of MPA is inosine 5′-monophosphate dehydrogenase (IMPDH), and IMPDH is the product of 2 polymorphic genes, IMPDH1 and IMPDH2. Polymorphisms in the human genes IMPDH1 and IMPDH2 have been associated with IMPDH-MPA activity20–22 and acute rejection in organ transplant patients.23 Glucuronidation of MPA to MPA glucuronide is an important route of metabolism, and polymorphisms in UGT1A9 can affect the MPA gluronide (MPAG), and to a lesser extent, MPA exposure.24 Studies with MRP2 were driven by the documented interaction of MPA with cyclosporine,25,26 and previous reports5 have associated ABCC2 polymorphisms with a change in MPA exposure or trough plasma concentration.
The long-term implications of ABCC2 polymorphisms on transplant outcome have not been reported previously. This is the first report in a large group of PHTx patients with long-term follow up to document the association between serious episodes of rejection affecting heart function and ABCC2 rs717620 genotypes. Previous reports of ABCC2 genotypes in organ transplant patients have focused on effects on MPA kinetics and MPA-associated GI intolerance.
Cyclosporine-mediated inhibition of MRP2 does affect MPA plasma concentrations and MPA dosage requirement.25 However, the effect of ABCC2 polymorphisms on the pharmacokinetics of MPA is unclear. A report in 2006 suggested that the ABCC2 rs717620 A allele is associated with enhanced MPA enterohepatic recirculation and diarrhea in the first year after renal transplantation.5 Later findings in 200724 and in 20098 in renal allograft recipients could not confirm that there was any pharmacokinetic effect of the ABCC2 polymorphisms on plasma concentrations of MPA or the metabolite MPAG.
The GI adverse effects and diarrhea produced by the administration of MPA in either of its commercially available forms are a major problem in maintaining therapy. In a study of 72 PHTx patients at 1 center, Ohmann et al10 found that GI adverse effects were found in 36% of patients, leading to holding the drug for more than 2 days or drug discontinuation. While the incidence of any adverse GI effects did not differ between ABCC2 rs717620 genotypes, severe side effects leading to drug discontinuation were significantly associated with the A variant allele. In that study, only 2.4% of carriers of the GG genotype discontinued MPA for GI intolerance, compared with 44.4% of patients with an A allele at this locus (p< 0.001), with an adjusted odds ratio of 0.025 and a 95% CI of 0.002 to 0.27 (p=0.004). Transplant patients receiving MPA that have GI intolerance have been reported to be at greater risk of graft rejection or graft loss. In a study using the US Renal Data System database, 3675 renal transplant patients were identified who were receiving MPA at the diagnosis of a GI disorder.11 In these patients, an MPA dose reduction of 50% or more or MPA discontinuation was strongly associated with graft loss. In another study of adult heart transplant patients, sustained significant rejection was observed in 35% of patients not requiring an MPA dose reduction vs 67% of patients requiring an MPA dose reduction for adverse GI effects (p=0.002).12 In the current study, it was not possible to tell how many patients ultimately discontinued MPA therapy. However, our initial hypothesis that patients carrying an A allele at rs717620 would do poorly, based on their GI intolerance of MPA, was shown to be incorrect.
This analysis of a larger multicenter cohort suggests that carriers of the ABCC2 rs717620 A allele may in fact do better long term. When coupled with the results of the previous study by Ohmann et al,10 the ABCC2 rs717620 A allele may be associated with the positive attributes of increased enterohepatic recirculation of MPA and less RHC, while carrying an increased likelihood of GI adverse effects. This observation could also imply that the A allele patients are more responsive to the drug for reasons that we do not currently understand.
RHC continues to carry a very poor prognosis in PHTx patients, especially when associated with need for inotropic agents.16 Death most commonly occurs within 6 months of the RHC episode, and rejection is frequently the cause of death. Continued efforts at identifying those patients at risk for RHC are necessary so that alternative means of preventing RHC can be explored. Since cytokines such as IL-1, TNF, and IL-6 suppress the expression of MRP2 in animal models,27 patients with less enterohepatic recirculation of MPA, on the basis of ABCC2 polymorphisms, may be at further risk for diminished MPA efficacy with recurrent episodes of rejection. Drug concentration monitoring in PHTx patients for MPA has been recommended,28 but more complex blood sampling strategies may be necessary for a drug with enterohepatic recirculation.29
The current analysis has several important limitations. The PHTS database did not list specific MPA doses over time, plasma MPA concentrations, or reasons for switching antiproliferative therapy to or from MPA. Multiple centers were involved in caring for the PHTx patients, which could potentially affect management and outcome. Since this was a candidate gene study, interactions with other gene polymorphisms could not be assessed concurrently. Also, restriction of the patient population to only those initiating MPA therapy from the time of transplant reduces the number of patients, and the statistical significance of this observation is lost. Alternatively, patients whose therapy is switched to MPA after being initially treated with other adjunctive agents (notably azathioprine), or given adjunctive therapy for the first time, may be at greater risk for RHC than patients given MPA from the time of transplant
The ABCC2 rs717620 polymorphism, as a candidate gene SNP, may play a role in the complex interaction between the recipient, the donor organ, and regulation of the immune response in a transplant patient receiving MPA. This may be particularly true for vulnerable populations, such as black transplant recipients. As newer immunosuppressive regimens emerge, the ABCC2 rs717620 genotype could be 1 component of a drug selection algorithm in PHTx patients.
ABBREVIATIONS
- ABCC2
ATP–binding cassette subfamily C member 2
- CI
confidence interval
- DNA
deoxyribonucleic acid
- Exo I
Exonuclease I
- GI
gastrointestinal
- HR
hazard ratio
- IMPDH
inosine 5′-monophosphate dehydrogenase
- MPA
mycophenolic acid
- MPAG
mycophenolic acid gluronide
- MRP2
multidrug resistance protein 2
- PCR
polymerase chain reaction
- PHTS
Pediatric Heart Transplantation Study
- PHTx
pediatric heart transplant
- RHC
rejection with hemodynamic compromise
- SAP
Shrimp Alkaline Phosphatase
- SCCOR
Specialized Centers for Clinically Oriented Research
- SNPs
single nucleotide polymorphisms
Footnotes
DISCLOSURE This work was supported by 5P50 HL 074 732-03 from the National Heart Lung and Blood Institute, National Institutes of Health. The authors declare no conflicts of interest or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
DISCLAIMER The views expressed in this work are those of the authors and do not represent the position of the US Food and Drug Administration.
REFERENCES
- 1.Daly AK. Candidate gene case-control studies. Pharmacogenomics. 2003;4(2):127–139. doi: 10.1517/phgs.4.2.127.22629. [DOI] [PubMed] [Google Scholar]
- 2.Girnita DM, Webber SA, Ferrell R. Disparate distribution of 16 candidate single nucleotide polymorphisms among racial and ethnic groups of pediatric heart transplant patients. Transplantation. 2006;82(12):1774–1780. doi: 10.1097/01.tp.0000250656.33731.08. et al. [DOI] [PubMed] [Google Scholar]
- 3.Girnita DM, Ohmann EL, Brooks MM. Gene polymorphisms impact the risk of rejection with hemodynamic compromise: a multicenter study. Transplantation. 2011;91(12):1326–1332. doi: 10.1097/TP.0b013e31821c1e10. et al. [DOI] [PubMed] [Google Scholar]
- 4.Hsu DT, Naftel DC, Webber SA. Lessons learned from the pediatric heart transplant study. Congenit Heart Dis. 2006;1(3):54–62. doi: 10.1111/j.1747-0803.2006.00011.x. et al. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DR, Verbeke K. Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation. 2006;82(8):1074–1084. doi: 10.1097/01.tp.0000235533.29300.e7. et al. [DOI] [PubMed] [Google Scholar]
- 6.Miura M, Satoh S, Inoue K. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007;63(12):1161–1169. doi: 10.1007/s00228-007-0380-7. et al. [DOI] [PubMed] [Google Scholar]
- 7.Yang JW, Lee PH, Hutchinson IV. Genetic polymorphisms of MRP2 and UGT2B7 and gastrointestinal symptoms in renal transplant recipients taking mycophenolic acid. Ther Drug Monit. 2009;31(5):542–548. doi: 10.1097/FTD.0b013e3181b1dd5e. et al. [DOI] [PubMed] [Google Scholar]
- 8.Van Schaik RHN, Van Agteren M, De Fijter JW. UGT1A9 −275T>A/−2152C>T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86(3):319–327. doi: 10.1038/clpt.2009.83. et al. [DOI] [PubMed] [Google Scholar]
- 9.Westley IS, Brogan LR, Morris RG. Role of Mrp2 in the hepatic disposition of mycophenolic acid and its glucuronide metabolites: effect of cyclosporine. Drug Metab Dispos. 2006;34(2):261–266. doi: 10.1124/dmd.105.006122. et al. [DOI] [PubMed] [Google Scholar]
- 10.Ohmann EL, Burckart GJ, Brooks MM. Genetic polymorphisms influence mycophenolate mofetil-related adverse events in pediatric heart transplant patients. J Heart Lung Transplant. 2010;29(5):509–516. doi: 10.1016/j.healun.2009.11.602. et al. [DOI] [PubMed] [Google Scholar]
- 11.Bunnapradist S, Lentine KL, Burroughs TE. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006;82(1):102–107. doi: 10.1097/01.tp.0000225760.09969.1f. et al. [DOI] [PubMed] [Google Scholar]
- 12.Galiwango PJ, Delgado DH, Yan R. Mycophenolate mofetil dose reduction for gastrointestinal intolerance is associated with increased rates of rejection in heart transplant patients. J Heart Lung Transplant. 2008;27(1):72–77. doi: 10.1016/j.healun.2007.10.012. et al. [DOI] [PubMed] [Google Scholar]
- 13.Canter C, Naftel D, Caldwell R. Survival and risk factors for death after cardiac transplantation in infants: a multi-institutional study: The Pediatric Heart Transplant Study. Circulation. 1997;96(1):227–231. doi: 10.1161/01.cir.96.1.227. et al. [DOI] [PubMed] [Google Scholar]
- 14.Chin C, Naftel DC, Singh TP. Risk factors for recurrent rejection in pediatric heart transplantation: a multicenter experience. J Heart Lung Transplant. 2004;23(2):178–185. doi: 10.1016/S1053-2498(03)00059-7. et al. [DOI] [PubMed] [Google Scholar]
- 15.Gossett JG, Canter CE, Zheng J. Decline in rejection in the first year after pediatric cardiac transplantation: a multi-institutional study. J Heart Lung Transplant. 2010;629(6):625–632. doi: 10.1016/j.healun.2009.12.009. et al. [DOI] [PubMed] [Google Scholar]
- 16.Everitt MD, Pahl E, Schechtman KB. Rejection with hemodynamic compromise in the current era of pediatric heart transplantation: a multi-institutional study. J Heart Lung Transplant. 2011;30(3):282–288. doi: 10.1016/j.healun.2010.08.031. et al. [DOI] [PubMed] [Google Scholar]
- 17.Kidwai M, Venkataramanan R, Mohan R. Cancer chemotherapy and heterocyclic compounds. Curr Med Chem. 2002;9(12):1209–1228. doi: 10.2174/0929867023370059. et al. [DOI] [PubMed] [Google Scholar]
- 18.Girnita DM, Brooks MM, Webber SA. Genetic polymorphisms impact the risk of acute rejection in pediatric heart transplantation: a multi-institutional study. Transplantation. 2008;85(11):1632–1639. doi: 10.1097/TP.0b013e3181722edc. et al. [DOI] [PubMed] [Google Scholar]
- 19.Pahl E, Naftel DC, Canter CE. Death after rejection with severe hemodynamic compromise in pediatric heart transplant recipients: a multi-institutional study. J Heart Lung Transplant. 2001;20(3):279–287. doi: 10.1016/s1053-2498(00)00228-x. et al. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Zeevi A, Webber S. A novel variant L263F in human inosine 5′-monophosphate dehydrogenase 2 is associated with diminished enzyme activity. Pharmacogenet Genomics. 2007;17(4):283–290. doi: 10.1097/FPC.0b013e328012b8cf. et al. [DOI] [PubMed] [Google Scholar]
- 21.Devyatko E, Dunkler D, Bohdjalian A. Lymphocyte activation and correlation with IMPDH activity under therapy with mycophenolate mofetil. Clin Chim Acta. 2008;394(1–2):67–71. doi: 10.1016/j.cca.2008.04.006. et al. [DOI] [PubMed] [Google Scholar]
- 22.Sombogaard F, Van Schaik RH, Mathot RA. Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet Genomics. 2009;19(8):626–634. doi: 10.1097/FPC.0b013e32832f5f1b. et al. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Yang JW, Zeevi A. IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther. 2008;83(5):711–717. doi: 10.1038/sj.clpt.6100347. et al. [DOI] [PubMed] [Google Scholar]
- 24.Baldelli S, Merlini S, Perico N. C-440T/T-331C polymorphisms in the UG-T1A9 gene affect the pharmacokinetics of mycophenolic acid in kidney transplantation. Pharmacogenomics. 2007;8(9):1127–1141. doi: 10.2217/14622416.8.9.1127. et al. [DOI] [PubMed] [Google Scholar]
- 25.Hesselink DA, Van Hest RM, Mathot RaA. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant. 2005;5(5):987–994. doi: 10.1046/j.1600-6143.2005.00779.x. et al. [DOI] [PubMed] [Google Scholar]
- 26.Shipkova M, Armstrong VW, Kuypers D. Effect of cyclosporine withdrawal on mycophenolic acid pharmacokinetics in kidney transplant recipients with deteriorating renal function: preliminary report. Ther Drug Monit. 2001;23(6):717–721. doi: 10.1097/00007691-200112000-00020. et al. [DOI] [PubMed] [Google Scholar]
- 27.Fardel O, Jigorel E, Le Vee M. Physiological, pharmacological and clinical features of the multidrug resistance protein 2. Biomed Pharmacother. 2005;59(3):104–114. doi: 10.1016/j.biopha.2005.01.005. et al. [DOI] [PubMed] [Google Scholar]
- 28.Gajarski RJ, Crowley DC, Zamberlan MC. Lack of correlation between MMF dose and MPA level in pediatric and young adult cardiac transplant patients: does the MPA level matter? Am J Transplant. 2004;4(9):1495–1500. doi: 10.1111/j.1600-6143.2004.00534.x. et al. [DOI] [PubMed] [Google Scholar]
- 29.Baraldo M, Isola M, Feruglio MT. Therapeutic mycophenolic acid monitoring by means of limited sampling strategy in orthotopic heart transplant patients. Transplant Proc. 2005;37(5):2240–2243. doi: 10.1016/j.transproceed.2005.03.090. et al. [DOI] [PubMed] [Google Scholar]


