Summary
Objective
Developmental differences in structure and function have been reported along the hippocampal subregions. The aims of this study were to determine if there were volumetric differences in hippocampal head (HH), body (HB), tail (HT), and total hippocampus (TotH)) in children with nonlesional localization-related epilepsy relative to controls, and the relation between hippocampal subregions with episodic memory and clinical parameters.
Methods
Forty-eight children with nonlesional localization-related epilepsy, consisting of 29 left-sided and 19 right-sided epilepsy, and 27 healthy controls were recruited. All patients and controls underwent volumetric T1-weighted imaging, and verbal and nonverbal memory testing. The volume of hippocampal subregions was compared between patients and controls. The associations between left hippocampal subregions with verbal memory; right hippocampal subregions with nonverbal memory; and hippocampal subregions with age, age at seizure onset, and seizure frequency were assessed.
Results
Patients with left-sided epilepsy had smaller left HH (p = 0.003) and HB (p = 0.012), right HB (p = 0.021) and HT (p = 0.015), and right TotH (p = 0.020) volumes. Those with right-sided epilepsy had smaller right HT (p = 0.018) volume. There were no statistically significant differences between verbal and nonverbal memory in left-sided and right-sided epilepsy relative to controls (all p > 0.025). In left-sided epilepsy, there was a significant association between left HH volume with verbal memory (β = 0.492, p = 0.001). There was no significant association between left and right hippocampal subregions with verbal and nonverbal memory, respectively, in right-sided epilepsy and controls (all p > 0.002). In left-sided and right-sided epilepsy, there was no significant association between hippocampal subregions with age, age at seizure onset, and seizure frequency (all p > 0.002).
Significance
We have found hippocampal volume reduction, but did not identify a gradient in the severity of volume reduction along the hippocampal axis in children with localization-related epilepsy. Further study is needed to clarify if there are volumetric changes within the cornu ammonis subfields and dentate gyrus.
Keywords: Hippocampus, Localization-related epilepsy, Pediatric
Developmental differences in structure and function have been reported along the longitudinal axis of the hippocampus.1–3 A previous study indicated that the total volume of the hippocampus is unchanged from age 4–25 years; however, the posterior hippocampal volume decreases and the anterior hippocampal volume increases over time.1 A functional magnetic resonance imaging (fMRI) study showed that activity in the anterior hippocampus was associated with episodic retrieval in adults; in contrast, activity in the posterior hippocampus rather than the anterior hippocampus was associated with episodic retrieval in children.3
Volume reduction in the hippocampus has been demonstrated in pediatric localization-related epilepsy, including mesial temporal lobe epilepsy (TLE) and extratemporal lobe epilepsy.4–11 However, most of these studies have evaluated the whole hippocampus rather than subregions of the hippocampus. For the purpose of this study, the term “hippocampal subregions” was used to refer to hippocampal division to hippocampal head (HH), body (HB), and tail (HT), and not to subfields of the cornu ammonis (CA1, CA2, CA3, and CA4). Because developmental differences occur along the longitudinal axis of the hippocampus, vulnerability to seizure-related changes may also differ along the axis of the hippocampus in childhood epilepsy. Several studies of adult patients with mesial TLE have reported greater atrophy in subregions of the hippocampus.12–14 The aims of this study were to determine if there were volumetric differences in hippocampal subregions (HH, HB, HT, and total hippocampus [TotH]) in children with nonlesional localization-related epilepsy (overall epilepsy group) relative to controls, and to examine for potential relations between the volume of subregions and performance on episodic memory tasks as well as clinical parameters. The secondary aims of this study were to determine if there were volumetric differences in hippocampal subregions in subgroups with frontal lobe epilepsy (FLE) and TLE relative to controls, and the relations between volume of hippocampal subregions and episodic memory tasks as well as clinical parameters in these subgroups.
Methods
Subjects
The study has the approval of research ethics board at the Hospital for Sick Children and written informed consent was obtained from parents and assents from children. Children with localization-related epilepsy were recruited from the epilepsy clinic. Location of the epileptogenic zone was defined using seizure semiology, ictal and interictal video–electroencephalography (EEG), magnetoencephalography (MEG), and fluorodeoxyglucose–positron emission tomography (FDG-PET) scan. Forty-eight patients (27 female and 21 male) with nonlesional localization-related epilepsy were recruited with a mean age of 13.5 years (standard deviation [SD] 2.9; range 7.0–17.5 years). All patients had normal MRI on 3T scanner. Twenty-nine patients had left-sided epilepsy and 19 had right-sided epilepsy (Table 1). Twenty-four patients had FLE (13 left-sided and 11 right-sided), 18 had TLE (14 left-sided and 4 right-sided), 5 had multilobar epilepsy (2 left-sided and 3 right-sided), and one had right parietal lobe epilepsy. The mean age at seizure onset was 8.0 years (SD 3.7), the mean duration of epilepsy was 5.2 years (SD 3.1), mean seizure frequency was 9.1/week (SD 19.1), and mean number of antiepileptic medications was 2.1 (SD 0.7, range 1–3 medications). Twenty-seven healthy controls (12 female and 15 male) with no neurologic or psychiatric disorders were recruited. The mean age of controls was 13.9 years (SD 3.0; range 7.0–18.7 years). There was no significant difference between the age of patients and controls (p = 0.523).
Table 1.
Characteristics of patients and controls
| Patients (n = 48)
|
Controls (n = 27) | ||
|---|---|---|---|
| Left-sided epilepsy (n = 29) | Right-sided epilepsy (n = 19) | ||
| Age | 13.5 (2.9) | 13.5 (3.0) | 13.9 (3.0) |
| Female:male | 14:15 | 13:6 | 12:15 |
| Intelligence quotient | 91.2 (15.2) | 94.7 (16.0) | 113.2 (9.5) |
| Type of epilepsy | |||
| Frontal lobe | 13 | 11 | |
| Temporal lobe | 14 | 4 | |
| Other | 2 | 4 | |
| Age at seizure onset (years) | 7.7 (3.3) | 8.6 (4.2) | |
| Duration of epilepsy (years) | 5.4 (3.4) | 4.8 (2.9) | |
| Seizure frequency (per week) | 6.7 (11.0) | 12.7 (27.0) | |
| Number of antiepileptic medications | 2.1 (0.7) | 2.1 (0.7) | |
SD, standard deviation.
MRI scanning
MRI was performed on Philips 3T scanner (Achieva, Philips Medical System, Best, The Netherlands) using an eight-channel phased array head coil in all patients and controls. The imaging in patients and controls included axial volumetric three-dimensional (3D) T1 (TR/TE 4.9/2.3 msec, slice thickness 1 mm, field of view [FOV] 22 cm, matrix 220 × 220), angled to the anterior commissure-posterior commissure plane. Patients had additional sequences done on the same scanner including axial and coronal fluid-attenuated inversion recovery (FLAIR), T2-weighted, and proton density.
Hippocampal segmentation
Automated segmentation of the whole hippocampus was carried out using FreeSurfer, v5.1.0 (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer uses probabilistic information estimated from a large training set of expert measurements to automatically assign a neuroanatomic label to each voxel in the brain. The technical details of the FreeSurfer whole brain segmentation procedure have been described in detail in previous publications.15,16 All FreeSurfer segmentation of the whole hippocampus was visually inspected for accuracy prior to segmentation into the three principal subregions. Subsequently the hippocampus was manually segmented into three principal subregions using ITK-SNAP, v2.4.0.17 The subregions were defined based on Duvernoy18 and Malykhina et al.19 guidelines, and consisted of HH, HB, and HT. Both of the head-body and body-tail boundaries were identified in the coronal planes, perpendicular to the anterior commissure-posterior commissure plane. The most posterior slice of the HH was the first slice where the uncal apex was clearly present.18 The first slice where the fornix was clearly seen in full profile was marked as the boundary between HB and HT.19 The left and right hippocampal labels were extracted from the FreeSurfer whole brain segmentation and converted into whole hippocampal masks. The structural T1 image and the hippocampus mask were then loaded into ITK-SNAP while maintaining the FreeSurfer boundaries; the hippocampus was subdivided into HH, HB, and HT (Fig. 1).
Figure 1.
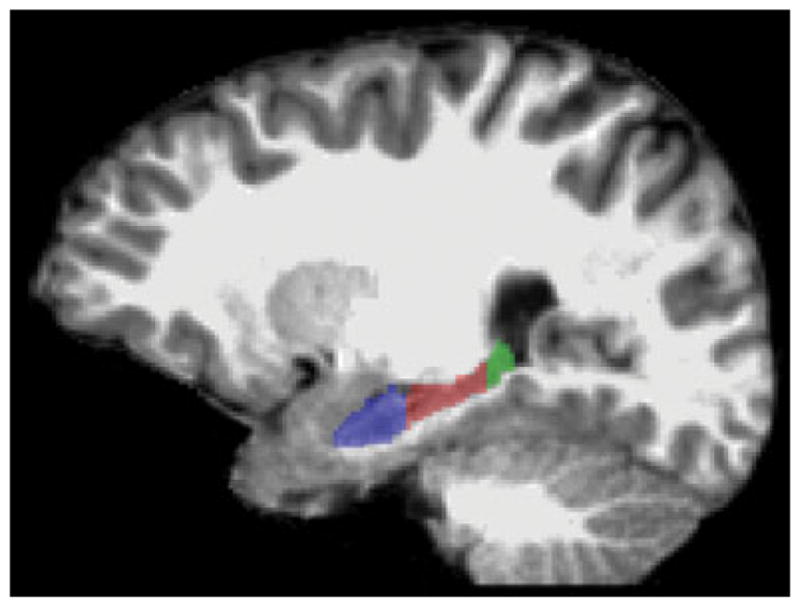
Sagittal oblique T1-weighted MRI demonstrating the hippocampal head, body, and tail.
Epilepsia © ILAE
Neuropsychological testing
All patients and controls underwent the same battery of neuropsychological testing. The Wechsler Abbreviated Scale of Intelligence20 was administered to obtain Full Scale Intelligence Quotients (IQs). The Children’s Auditory Verbal Learning Test-II21 was used to measure verbal memory (learning, interference, immediate, and delayed recall), and the Face Recognition subtest of the Children’s Memory Scale22 was used to measure nonverbal memory (immediate and delayed recognition). The tests selected allow for administration of materials across the age range included in the study. The raw verbal and nonverbal memory test scores were converted to z-scores, and the mean z-scores across the dependent variables of each measure were computed to yield a composite score of each of verbal and nonverbal memory.
Statistical analyses
Statistical analyses were performed with the Statistical Package for Social Sciences software (SPSS) version 18 (IBM SPSS, New York, US). Group differences in HH, HB, HT, and TotH volume of left and right lateralized epilepsy were compared to controls using multivariate analysis of covariance (MANCOVA) with age, sex, and intracranial volume as covariates. A p-value of <0.025 would be considered significant for the MANCOVA analyses to account for comparisons of left and right lateralized epilepsy relative to controls. Subgroup analyses of HH, HB, HT, and TotH volumes of left and right FLE and TLE were also compared to controls using MANCOVA with age, sex, and intracranial volume as covariates. Group differences in verbal and non-verbal memory between left and right lateralized epilepsy were compared to controls using t-test, with p-value of <0.025 considered significant. Subgroup analyses of verbal and nonverbal memory of left and right FLE and TLE compared to controls were also performed using t-test. Linear regression was performed between left hippocampal subregions and verbal memory, and between right hippocampal subregions and nonverbal memory, with IQ as a covariate in patients with left and right lateralized epilepsy and controls. To account for multiple comparisons in the regression analyses, a p-value of ≤0.002 was considered statistically significant. Subsequently, linear regression was done to assess the relations between hippocampal subregions and age, age at seizure onset, and seizure frequency in left and right lateralized epilepsy. Subgroup analysis was also done to assess the relations between left hippocampal subregions and verbal memory, and between right hippocampal subregions and nonverbal memory in patients with left and right FLE and left TLE using linear regression, with IQ as a covariate. Similarly the relations between hippocampal subregions and age, age at seizure onset, and seizure frequency were assessed using linear regression in patients with left and right FLE and left TLE. Regression analyses were not done in the group with right TLE due to the small number of subjects in this group (n = 4).
Results
Hippocampal volume in left-sided and right-sided epilepsy compared to controls
Left and right lateralized epilepsy
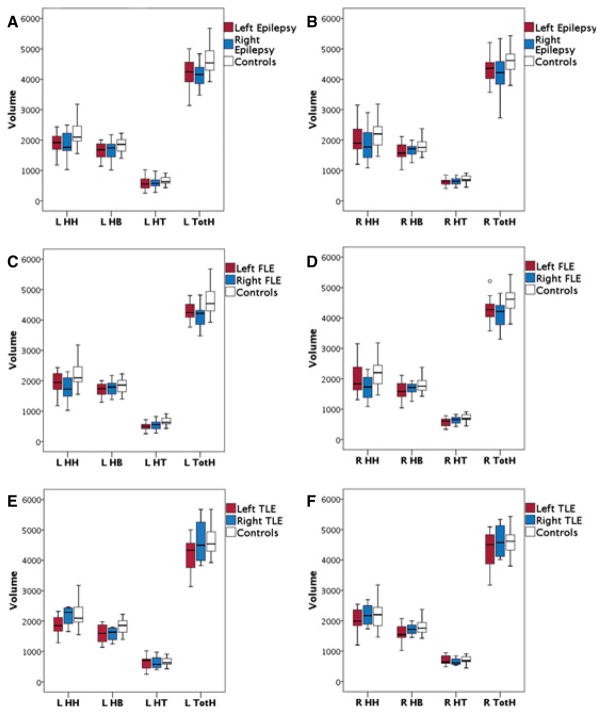
The HH, HB, HT, and TotH volume in left (n = 29) and right (n = 19) lateralized epilepsy and controls are shown in Fig. 2. In left lateralized epilepsy, there was a significant difference in overall hippocampal volume between patients and controls (F = 4.073, p = 0.001), with significantly smaller volume in left HH (p = 0.003), left HB (p = 0.012), right HB (p = 0.021), right HT (p = 0.015), and right TotH (p = 0.020) in patients. There was no significant difference in overall hippocampal volume between right lateralized epilepsy and controls (F = 2.167, p = 0.056); however, the right HT (p = 0.018) was significantly smaller in patients. The percentage difference in HH, HB, HT, and TotH of left and right lateralized epilepsy relative to controls is shown in Table 2.
Figure 2.
Boxplots showing (A, C, and E) left and (B, D, and F) right hippocampal head (HH), body (HB), tail (HT), and total (TotH) volume in children with (A and B) left and right lateralized epilepsy, (C and D), frontal lobe epilepsy (FLE), and (E and F) temporal lobe epilepsy (TLE), and controls. The box represents the 25th to the 75th percentiles and the bar represents the 50th percentile. In left lateralized epilepsy group, the left HH (p = 0.003), left HB (p = 0.012), right HB (p = 0.021), right HT (p = 0.015), and right TotH (p = 0.020) are smaller in patients. Patients with right lateralized epilepsy have smaller right HT (p = 0.018). In left FLE, the left HH (p = 0.016), left HT (p = 0.002), right HT (p = 0.003), and right TotH (p = 0.015) are smaller in patients. In right FLE patients, the left HH (p = 0.021) is smaller. Patients with left TLE have smaller left HH (p = 0.021) and left HB (p = 0.005).
Epilepsia © ILAE
Table 2.
Percentage difference in total hippocampus, hippocampal head, body, and tail volumes of left-sided and right-sided epilepsy relative to controls
| L/R | % Difference in left-sided epilepsy | % Difference in right-sided epilepsy | |
|---|---|---|---|
| Left and right lateralized epilepsy (n = 48) | |||
| Total hippocampal volume | R | 7.5 | 10.2 |
| L | 9.0 | 9.3 | |
| Hippocampal head volume | R | 9.4 | 15.2 |
| L | 15.8 | 15.8 | |
| Hippocampal body volume | R | 10.8 | 5.9 |
| L | 10.0 | 9.2 | |
| Hippocampal tail volume | R | 14.1 | 11.4 |
| L | 15.1 | 13.0 | |
| Frontal lobe epilepsy (n = 24) | |||
| Total hippocampal volume | R | 8.3 | 11.8 |
| L | 7.6 | 13.1 | |
| Hippocampal Head volume | R | 9.6 | 23.0 |
| L | 13.5 | 21.8 | |
| Hippocampal body volume | R | 11.6 | 3.4 |
| L | 7.0 | 6.3 | |
| Hippocampal tail volume | R | 19.4 | 11.9 |
| L | 25.8 | 19.2 | |
| Temporal lobe epilepsy (n = 18) | |||
| Total hippocampal volume | R | 7.2 | 0.7 |
| L | 11.0 | 0.6 | |
| Hippocampal head volume | R | 8.3 | 0.3 |
| L | 18.6 | 1.7 | |
| Hippocampal body volume | R | 11.3 | 4.3 |
| L | 13.2 | 13.7 | |
| Hippocampal tail volume | R | 8.4 | 9.4 |
| L | 6.1 | 4.9 | |
FLE
In left FLE (n = 13), there was a significant difference in overall hippocampal volume between FLE patients and controls (F = 5.524, p < 0.001), with smaller volume in left HH (p = 0.016), left HT (p = 0.002), right HT (p = 0.003), and right TotH (p = 0.015) in patients. In right FLE patients (n = 11), there was a significant difference in overall hippocampal volume between patients and controls (F = 3.065, p = 0.014), with smaller volume in left HH (p = 0.021) in patients.
TLE
In left TLE (n = 14), there was no significant difference in overall hippocampal volume between TLE patients and controls (F = 2.355, p = 0.044), but the left HH (p = 0.021) and left HB (p = 0.005) were smaller in patients. In right TLE (n = 4), there was no significant difference in overall hippocampal volume (F = 1.605, p = 0.189) or hippocampal subregions between patients and controls.
Neuropsychological testing
The mean IQ of patients was 92.6 (standard deviation [SD] 15.4). The mean IQ of controls was 113.2 (SD 9.5). Patients had significantly lower IQ than controls (p < 0.001).
Memory function
Left and right lateralized epilepsy
The mean z-scores of verbal memory in left and right lateralized epilepsy and controls were −0.338 (SD = 1.371), −0.451 (SD = 1.293), and 0.000 (SD = 0.976), respectively. The mean z-scores of nonverbal memory in left and right lateralized epilepsy and controls were −0.378 (SD = 1.138), −0.283 (SD = 0.926), and −0.001 (SD = 0.973), respectively. There was no significant difference between verbal (p = 0.296) and nonverbal (p = 0.212) memory in patients with left lateralized epilepsy relative to controls. Significantly reduced memory was defined as performance below two z-scores; using this criterion, four patients with left lateralized epilepsy had reduced verbal memory and three had reduced nonverbal memory. There was no significant difference between verbal (p = 0.184) and nonverbal (p = 0.337) memory in patients with right lateralized epilepsy relative to controls. In patients with right lateralized epilepsy, three had reduced verbal memory and one had reduced nonverbal memory.
FLE
There was no significant difference between verbal (p = 0.478) memory and a trend for lower nonverbal memory (p = 0.033) in patients with left FLE relative to controls. Of those with left FLE, three had reduced verbal memory and three had reduced nonverbal memory. There was no significant difference between verbal (p = 0.091) and nonverbal (p = 0.525) memory in patients with right FLE relative to controls. Of those with right FLE, one had reduced verbal memory and one had reduced nonverbal memory.
TLE
There was no significant difference between verbal (p = 0.237) and nonverbal (p = 0.423) memory in patients with left TLE relative to controls. Of those with left TLE, one had reduced verbal memory and none had reduced non-verbal memory. There was no significant difference between verbal (p = 0.373) and nonverbal (p = 0.566) memory in patients with right TLE relative to controls. None of the patients with right TLE had reduced verbal or nonverbal memory.
Relation between hippocampal volume and memory function
Left and right lateralized epilepsy
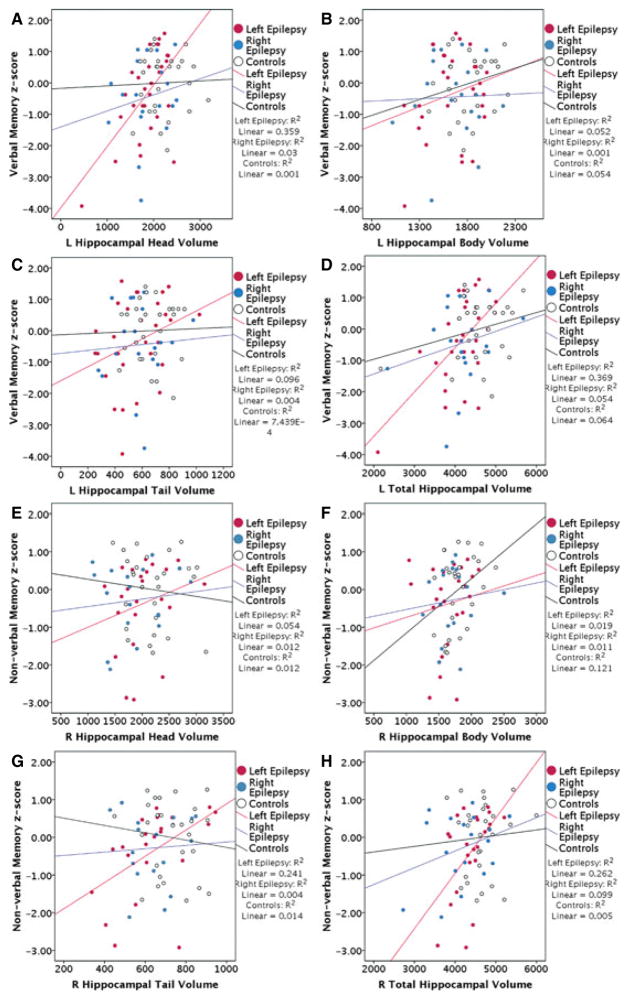
In patients with left lateralized epilepsy, there was a significant association between left HH (β = 0.492, p = 0.001) with verbal memory (Fig. 3), and weak associations between left TotH (β = 0.456, p = 0.003) with verbal memory and right TotH (β = 0.412, p = 0.012) with non-verbal memory.
Figure 3.
Scatterplots of left hippocampal (A) head, (B) body, (C) tail, and (D) total volume against verbal memory, and right hippocampal (E) head, (F) body, (G) tail, and (H) total volume against nonverbal memory in left and right lateralized epilepsy and controls. Patients with left lateralized epilepsy show significant association between left HH (p = 0.001) with verbal memory. In patients with right lateralized epilepsy and controls, there is no significant association between left hippocampal subregions and verbal memory, or right hippocampal subregions and nonverbal memory.
Epilepsia © ILAE
In patients with right lateralized epilepsy, there was no significant association between left hippocampal subregions with verbal memory, or between right hippocampal subregions with nonverbal memory (β = −0.003 to 0.288; p = 0.274–0.989).
In controls, there was also no significant association between left hippocampal subregions with verbal memory, or between right hippocampal subregions with nonverbal memory (β = −0.139 to 0.301, p = 0.150–0.875).
FLE
In patients with left FLE, there were weak associations between left HH (β = 0.434, p = 0.05) with verbal memory, and between right HT (β = 0.702, p = 0.017) with nonverbal memory. In right FLE, there was no significant association between left hippocampal subregions with verbal memory, or between right hippocampal subregions with nonverbal memory (β = −0.321 to 0.209, p = 0.412–0.953).
TLE
In patients with left TLE, there were weak associations between left HH (β = 0.645, p = 0.015) and left TotH (β = 0.799, p = 0.011) with verbal memory, and between right HT with nonverbal memory (β = 0.763, p = 0.043).
Relation between hippocampal volume and clinical parameters
Left and right lateralized epilepsy
In patients with left lateralized epilepsy, there was a weak association between left HB with age (β = 0.555, p = 0.009). In those with right lateralized epilepsy, there was no significant association between hippocampal subregions with age (β = −0.064 to 0.293, p = 0.238–0.800). There was also no significant association between hippocampal subregions with age at seizure onset (β = −0.001 to 0.403, p = 0.087–0.994) and seizure frequency (β = −0.289 to 0.253, p = 0.185–0.965) in patients with left and right lateralized epilepsy.
FLE
In patients with left and right FLE, there was no significant association between hippocampal subregions with age (β = −0.273 to 0.588, p = 0.057–0.872), age at seizure onset (β = −0.341 to 0.365, p = 0.254–0.964), and seizure frequency (β = −0.303 to 0.442, p = 0.130–0.917).
TLE
In patients with left TLE, there were weak associations between HB (β = 0.708, p = 0.049) and HT (β = 0.798, p = 0.018) with age, and no significant association between hippocampal subregions with age at seizure onset (β = −0.003 to 0.474, p = 0.087–0.991) and seizure frequency (β = −0.234 to 0.298, p = 0.271–0.910).
Discussion
We have found that children with nonlesional localization-related epilepsy have reduced hippocampal volume, affecting HH, HB, HT, and TotH. However, we did not identify a gradient in volume reduction along the hippocampal axis. The hippocampal volume reduction was detected not only in children with TLE, but also in children with seizures remote from the hippocampus, including FLE. The reduction in left HH volume was significantly associated with reduced verbal memory in left lateralized epilepsy. There was no significant association between the volume of hippocampal subregions and clinical parameters.
Volumetric studies of the hippocampus in adults with mesial TLE have shown ipsilateral and in some cases contralateral hippocampal volume reduction.14,23–25 However, there is no consensus on whether regional reduction in hippocampal volume occurs. Quigg et al.23 found that overall volume loss in hippocampal sclerosis is diffuse, neither favoring the head nor body-tail. Bernasconi et al.14 found the HH was more atrophic than the HB and HT in patients with mesial TLE. Others have found greater atrophy in the HB than in the HH or HT12,13 in patients with mesial TLE.
Most studies on pediatric epilepsies have assessed the whole hippocampus rather than subregions of the hippocampus.4–11 Previous studies have reported that children with localization-related epilepsy have unilateral4 or bilateral6 hippocampal atrophy. The reduction in hippocampal volume varied from 10% to 31%.5,6,10 One study assessed subregions of the hippocampus in 19 children with complex partial seizures and showed a significant reduction in the anterior hippocampal volume, and a trend for smaller posterior hippocampal volume,26 with a reduction of 21.7% in TotH volume, 51.5% in anterior, and 11.8% in posterior hippocampal volume. The lack of a statistically significant reduction in posterior hippocampal volume may have been due to the relatively small sample size. We have found that the reduction in TotH volume varied from 7.5 to 10.2%, which was within the range of volume loss described in the literature. However, the reduction in HH (9.4–15.8%) was less than the reduction in anterior hippocampal volume reported by Daley et al.26
We have assessed children with localization-related epilepsy and did not find a gradient in the severity of volume reduction along the hippocampal axis. Failure to identify regional differences along the hippocampal axis may be due to several reasons. First, we have used HH, HB, and HT to define the hippocampal subregions, which may not be differentially susceptible to seizure-related injury. Volumetric changes have been identified in different subfields of the cornu ammonis and dentate gyrus in adults with TLE with and without hippocampal sclerosis.27 Future study assessing volumetric changes within subfields of the cornu ammonis and dentate gyrus may shed light on the regions that are more susceptible to seizure-related injury in children with epilepsy. Second, volumetric measurement may not be sufficiently sensitive to identify subtle differences in hippocampal subregions. Further study using alternative technique such as shape analysis may uncover more subtle differences in hippocampal subregions. There were more subregions of the hippocampus that demonstrated volume reduction in left lateralized epilepsy than in right lateralized epilepsy, as well as in left FLE than right FLE. There were also several subregions that demonstrated volume reduction in left TLE, but not right TLE. The smaller number of patients in the right TLE subgroup may have confounded the findings, resulting in failure to detect volume changes in this subgroup. The greater number of subregions demonstrating volume reduction in left-sided epilepsy may be related to the involvement of the dominant hemisphere or different networks in left-sided epilepsy relative to right-sided epilepsy.
Previous study of children and young adults with seizures remote from the hippocampus has shown prolongation of T2 relaxation time in the hippocampus.28 We have found hippocampal volume reduction in children with focal seizures remote from the hippocampus. Together, these findings suggest that there is a network between the remote epileptogenic zone and hippocampus that facilitates spread of seizures to the hippocampus, thereby resulting in injury to the hippocampus. Histology from patients with extrahippocampal seizures has shown neuronal loss in the hippocampus.29–31 The reduction in hippocampal volume in children with extrahippocampal seizures in our study may be secondary to neuronal loss from seizures. We did not detect a significant association between TotH or hippocampal subregions with age, age at seizure onset, and seizure frequency. The reason for this lack of significant association between hippocampal volume and clinical parameters was not apparent. Eroglu et al.5 also did not identify an association between hippocampal volume and age at seizure onset and duration of epilepsy, despite a reduction in corrected TotH volume. Similarly, Daley et al.26 did not detect an association between anterior hippocampal volume with age at seizure onset, duration of epilepsy, seizure frequency, and number of antiepileptic medications; the TotH and posterior hippocampal volume was associated with duration of epilepsy, but not with the other clinical parameters.
Despite the lack of significant differences in verbal and nonverbal memory in children with left and right lateralized epilepsy relative to controls, we found mild volume reduction in subregions of the hippocampus that was detected by quantitative measures but was not apparent on visual inspection. There may be a threshold volume loss before significant impairment in verbal or nonverbal memory is manifested. We found a significant association between reduced left HH volume with reduced verbal memory in left lateralized epilepsy. Previous studies have shown that greater HH volume was associated with higher delayed recall test scores in nondemented elderly subjects32 and elevated diffusivity in the left HH was significantly associated with verbal episodic memory impairment in early Alzheimer’s disease.33 These data suggest that the left HH may play a greater role in verbal memory function than the rest of the hippocampus, both in children and adults. There was one prior study in children with TLE showing a correlation between left hippocampal volume and word recall, but paradoxically, the association was negative; there was no relation between story recall or visual memory with left or right hippocampal volume.4
There are several limitations to this study. We have defined the epileptogenic zone noninvasively using seizure semiology, video-EEG, magnetoencephalography, and FDG-PET scan. It is possible that in some patients with FLE, the epileptogenic zone may have been more extensive and involved the temporal lobe as well. Due to the relatively small number of TLE patients, we have not categorized these patients to mesial TLE and neocortical TLE. Patients with mesial TLE may have greater volume reduction in the hippocampus relative to those with neocortical TLE.
We have used automated segmentation of the hippocampus. Manual segmentation of the hippocampus is more sensitive in detecting hippocampal atrophy than automated segmentation,34–36 but it is time-consuming and prone to operator error. Automated segmentation of the hippocampus has the potential to provide rapid and accurate assessment of the hippocampal volume, and has been shown to be sensitive at detecting hippocampal atrophy.34,36–39 Automated segmentation using FreeSurfer has shown good correlation with expert manual segmentation (correlations ranging from 0.61 to 0.84).34,36,39
We have found hippocampal volume reduction in children with seizures remote from the hippocampus, suggesting that there may be an abnormal network that facilitates spread of seizures from the remote epileptogenic zone to the hippocampus, resulting in injury to the hippocampus. However, there was no gradient in the severity of volume reduction along the hippocampal axis. Further study evaluating the cornu ammonis subfields and dentate gyrus volume may shed light on the regions that are more susceptible to seizure-related injury. We have found a significant association between reduced left HH volume with reduced verbal memory in left lateralized epilepsy, suggesting that the left HH may play a greater role in verbal episodic memory than the remaining hippocampus. Longitudinal studies of adult patients with TLE have shown a decline in hippocampal volume with continuing seizures40,41 and cessation of seizures was associated with arrest in hippocampal volume loss.40 Longitudinal study is needed to clarify whether there is a differential rate of volume reduction along the axis of the hippocampus, in the cornu ammonis subfields and dentate gyrus in children with continuing seizures.
Biography
Dr Widjaja is a neuroradiologist interested in studying how epilepsy impacts the developing brain.
Footnotes
Disclosure
EW received funding from Sickkids Foundation/CIHR Institute of Human Development, Child and Youth Health, and GE-AUR. None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Gogtay N, Nugent TF, III, Herman DH, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- 2.Ghetti S, DeMaster DM, Yonelinas AP, et al. Developmental differences in medial temporal lobe function during memory encoding. J Neurosci. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demaster DM, Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2012;49:1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes CA, Bonilha L, Franzon RC, et al. Distribution of regional gray matter abnormalities in a pediatric population with temporal lobe epilepsy and correlation with neuropsychological performance. Epilepsy Behav. 2007;11:558–566. doi: 10.1016/j.yebeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Eroglu B, Kurul S, Cakmakci H, et al. The correlation of seizure characteristics and hippocampal volumetric magnetic resonance imaging findings in children with idiopathic partial epilepsy. J Child Neurol. 2007;22:348–353. doi: 10.1177/0883073807301916. [DOI] [PubMed] [Google Scholar]
- 6.Wu WC, Huang CC, Chung HW, et al. Hippocampal alterations in children with temporal lobe epilepsy with or without a history of febrile convulsions: evaluations with MR volumetry and proton MR spectroscopy. AJNR Am J Neuroradiol. 2005;26:1270–1275. [PMC free article] [PubMed] [Google Scholar]
- 7.Major P, Decarie JC, Nadeau A, et al. Clinical significance of isolated hippocampal volume asymmetry in childhood epilepsy. Neurology. 2004;63:1503–1506. doi: 10.1212/01.wnl.0000142079.79612.cb. [DOI] [PubMed] [Google Scholar]
- 8.Lawson JA, Vogrin S, Bleasel AF, et al. Predictors of hippocampal, cerebral, and cerebellar volume reduction in childhood epilepsy. Epilepsia. 2000;41:1540–1545. doi: 10.1111/j.1499-1654.2000.001540.x. [DOI] [PubMed] [Google Scholar]
- 9.Cormack F, Gadian DG, Vargha-Khadem F, et al. Extra-hippocampal grey matter density abnormalities in paediatric mesial temporal sclerosis. Neuroimage. 2005;27:635–643. doi: 10.1016/j.neuroimage.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Varho T, Komu M, Sonninen P, et al. Quantitative HMRS and MRI volumetry indicate neuronal damage in the hippocampus of children with focal epilepsy and infrequent seizures. Epilepsia. 2005;46:696–703. doi: 10.1111/j.1528-1167.2005.30804.x. [DOI] [PubMed] [Google Scholar]
- 11.Berg AT, Pardoe HR, Fulbright RK, et al. Hippocampal size anomalies in a community-based cohort with childhood-onset epilepsy. Neurology. 2011;76:1415–1421. doi: 10.1212/WNL.0b013e318216712b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronen RA, Fulbright RK, Kim JH, et al. Regional distribution of MR findings in hippocampal sclerosis. AJNR Am J Neuroradiol. 1995;16:1193–1200. [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzniecky RI, Burgard S, Bilir E, et al. Qualitative MRI segmentation in mesial temporal sclerosis: clinical correlations. Epilepsia. 1996;37:433–439. doi: 10.1111/j.1528-1157.1996.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernasconi N, Bernasconi A, Caramanos Z, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 15.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Fischl B. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Trans Med Imaging. 2007;26:479–486. doi: 10.1109/TMI.2007.893282. [DOI] [PubMed] [Google Scholar]
- 17.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Berlin, Heidelberg, Germany: Springer-Verlag; 2005. [Google Scholar]
- 19.Malykhin NV, Bouchard TP, Ogilvie CJ, et al. Three-dimensional volumetric analysis and reconstruction of amygdala and hippocampal head, body and tail. Psychiatry Res. 2007;155:155–165. doi: 10.1016/j.pscychresns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D, editor. Wechsler Abbreviated Scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 21.Talley JL, editor. Children’s auditory verbal learning test-2. Odessa, Florida: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- 22.Cohen MJ, editor. Children’s memory scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 23.Quigg M, Bertram EH, Jackson T. Longitudinal distribution of hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsy Res. 1997;27:101–110. doi: 10.1016/s0920-1211(97)01026-7. [DOI] [PubMed] [Google Scholar]
- 24.Lambert MV, Brierley B, Al-Sarraj S, et al. Quantitative magnetic resonance imaging of the amygdala in temporal lobe epilepsy-clinico-pathological correlations (a pilot study) Epilepsy Res. 2003;53:39–46. doi: 10.1016/s0920-1211(02)00253-x. [DOI] [PubMed] [Google Scholar]
- 25.Bonilha L, Rorden C, Castellano G, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol. 2004;61:1379–1384. doi: 10.1001/archneur.61.9.1379. [DOI] [PubMed] [Google Scholar]
- 26.Daley M, Ott D, Blanton R, et al. Hippocampal volume in childhood complex partial seizures. Epilepsy Res. 2006;72:57–66. doi: 10.1016/j.eplepsyres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SG, Laxer KD, Scanlon C, et al. Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Hum Brain Mapp. 2012;33:489–499. doi: 10.1002/hbm.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott RC, Cross JH, Gadian DG, et al. Abnormalities in hippocampi remote from the seizure focus: a T2 relaxometry study. Brain. 2003;126:1968–1974. doi: 10.1093/brain/awg199. [DOI] [PubMed] [Google Scholar]
- 29.Babb TL, Brown WJ. Neuronal, dendritic, and vascular profiles of human temporal lobe epilepsy correlated with cellular physiology in vivo. Adv Neurol. 1986;44:949–966. [PubMed] [Google Scholar]
- 30.Levesque MF, Nakasato N, Vinters HV, et al. Surgical treatment of limbic epilepsy associated with extrahippocampal lesions: the problem of dual pathology. J Neurosurg. 1991;75:364–370. doi: 10.3171/jns.1991.75.3.0364. [DOI] [PubMed] [Google Scholar]
- 31.Fried I, Kim JH, Spencer DD. Hippocampal pathology in patients with intractable seizures and temporal lobe masses. J Neurosurg. 1992;76:735–740. doi: 10.3171/jns.1992.76.5.0735. [DOI] [PubMed] [Google Scholar]
- 32.Hackert VH, den Heijer T, Oudkerk M, et al. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- 33.Yakushev I, Muller MJ, Lorscheider M, et al. Increased hippocampal head diffusivity predicts impaired episodic memory performance in early Alzheimer’s disease. Neuropsychologia. 2010;48:1447–1453. doi: 10.1016/j.neuropsychologia.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Pardoe HR, Pell GS, Abbott DF, et al. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation? Epilepsia. 2009;50:2586–2592. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doring TM, Kubo TT, Cruz LC, Jr, et al. Evaluation of hippocampal volume based on MR imaging in patients with bipolar affective disorder applying manual and automatic segmentation techniques. J Magn Reson Imaging. 2011;33:565–572. doi: 10.1002/jmri.22473. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Benavides G, Gomez-Anson B, Sainz A, et al. Manual validation of FreeSurfer’s automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiatry Res. 2010;181:219–225. doi: 10.1016/j.pscychresns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Bonilha L, Halford JJ, Rorden C, et al. Automated MRI analysis for identification of hippocampal atrophy in temporal lobe epilepsy. Epilepsia. 2009;50:228–233. doi: 10.1111/j.1528-1167.2008.01768.x. [DOI] [PubMed] [Google Scholar]
- 38.Akhondi-Asl A, Jafari-Khouzani K, Elisevich K, et al. Hippocampal volumetry for lateralization of temporal lobe epilepsy: automated versus manual methods. Neuroimage. 2011;54(Suppl 1):S218–S226. doi: 10.1016/j.neuroimage.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cherbuin N, Anstey KJ, Reglade-Meslin C, et al. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. PLoS ONE. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuerst D, Shah J, Shah A, et al. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–416. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- 41.Briellmann RS, Berkovic SF, Syngeniotis A, et al. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51:641–644. doi: 10.1002/ana.10171. [DOI] [PubMed] [Google Scholar]