Abstract
Plant hemoglobins are ubiquitous molecules involved in several aspects of plant development and stress responses. Studies on the functional aspects of plant hemoglobins at the cellular level in these processes are limited, despite their ability to scavenge nitric oxide (NO), an important signal molecule interfering with hormone synthesis and sensitivity. This mini-review summarizes current knowledge on plant hemoglobins, analyzes their participation in plant pathogen interaction and embryogenesis and proposes a possible model centering on jasmonic acid (JA) as a downstream component of hemoglobin responses.
Keywords: hemoglobin, embryogenesis, jasmonic acid, pathogen, auxin
Hemoglobins (Hbs) are heme-containing proteins, examples of which have been found in all nucleated organisms. The first plant Hbs isolated, leghemoglobins, were involved in nitrogen fixation in legumes, facilitating oxygen movement in nodules of nitrogen-fixing species, thus, preventing inactivation of nitrogenise.1 Other plant Hbs that were not involved in symbiotic relationships were later isolated.2 Based on their oxygen binding properties, amino acid sequences and expression patterns, Hbs have been classified into three distinct classes, with members of class-1 and class-2 sharing a 3-on-3 α-helical loop around the hemoglobin moiety,3 and members of class-3 resembling truncated bacterial globins.4 Due to their involvement in developmental processes, such as embryo formation,5 seed storage accumulation,6 overall plant architecture7 and biotic and abiotic stress-related events,2 the mode of action of Hbs has received attention especially in nitric oxide (NO) scavenging mechanisms. Hbs are avid scavengers of NO, an important signal molecule in both animals and plants.8 NO is a component of the signal transduction pathway of several growth regulators, including jasmonic acid (JA)9 that is involved in morphogenesis and plant pathogen interaction.10 The aim of this mini-review is to summarize the ability of Hbs to modulate cellular NO and influence JA-mediated responses.
Hbs and NO Regulation
The majority of the research in this area has been on class-1 and class-2 Hbs. Members of class-1 and -2 Hbs are upregulated by applications of abscisic acid and cytokinin,11,12 whereas hypoxia induces only class-1 Hbs expression.13,14 Class-1 and class-2 Hbs bind oxygen very tightly,15,16 making it unlikely that they function as oxygen carriers, stores or sensors.17 Oxyhemoglobin reacts very rapidly with NO and regulating cellular NO levels is a more plausible function of these Hbs.2 The Hb-mediated alterations of cellular NO interfere with hormone signaling as NO acts as a messenger in hormone synthesis and response. There are results of several published studies that could be interpreted in this fashion. The time of bolting in Arabidopsis is effected by the expression of Hbs, with a downregulation causing a delay and an upregulation accelerating bolting.18 These effects were ascribed to the ability of Hbs to scavenge NO, the level of which affects flowering time. NO suppresses floral transition.19 Cultured Alfalfa roots with altered Hb levels exhibited changes in NO content consistent with the NO-scavenging ability of Hbs.21
NO as a Regulator of Jasmonic Acid
There is an interesting dichotomy between NO and jasmonic acid (JA) signaling and accumulation in plants. NO accumulates rapidly in Arabidopsis tissues treated with exogenous JA,22 while JA levels increase in these same plants upon treatment with NO donors as a result of the induction of three biosynthetic genes: lipoxygenase (LOX2), allene oxide synthase (AOS) and 12-oxophytodienoate reductase (OPRT3).22 S-nitrosoglutathione (GSNO), an important mobile bioactive reserve of NO, rapidly accumulates and is uniformly observed in directly wounded Arabidopsis leaves, moving through the vascular system into systemic tissues.23 The JA-dependent wound responses were strongly regulated by GSNO, whereas the JA-independent gene responses were unaffected.23,24 When salicylic acid (SA) was removed from NO-treated plants through the use of a SA degradation transgenic line (NahG), JA levels and expression of responsive genes increased.22 Increasing NO levels in Arabidopsis leaves by suppressing expression of Hb1also resulted in JA accumulation.25 Apparently contrasting responses were observed in tomato plants, where NO application was found to be inhibitory toward the JA mediated wound responses.26 Many of the responses due to NO accumulation have been explained through S-nitrosylation of cysteine residues.27 Nitrosylation of regulatory proteins can lead to alterations in enzyme function (activation/inactivation), localization, altered protein-protein interactions and decreased protein stability.28 The Nonexpressor of Pathogenesis Related (NPR1), a key master control transcription factor regulating many of the responses separating the SA and JA dependent pathways, has differing effects depending upon its intracellular location. NPR1 is translocated to the nucleus if it is S-nitrosylated where it causes activation of SA responsive elements.29 NPR1, in the absence of nitrosylation, remains in the cytosol and serves to regulate JA responsive genes. Hb modulation of NO levels can, therefore, change the outcomes of complex transcription factors, such as NPR1, altering hormone levels and cellular responses.
Hb-Mediated Regulation of JA Modulates Plant Pathogen Interaction and Embryogenesis
Regulation of pathogen-related defense pathways in plants is complex, however, most of the active defense regulation is through SA-dependent or JA/ethylene (ET) dependent pathways.30 NO modulation on SA pathways has been covered extensively; here we will focus primarily on Hbs modulation of NO leading to altered JA levels and responses.28 Cotton Hb1 (GhHb1) was discovered to be induced through challenge by either the necrotrophic fungal pathogen, Verticillum dhaliae or through exogenous application of phytohormones including JA and ET.31 Constitutively overexpressing GhHb1in Arabidopsis increased expression of PDF1.2 and PR1, classic defense marker genes for both SA and JA/ET responses, leading to elevated resistance to V. dhaliae.32 Similarly, overexpression of alfalfa Hb in Arabidopsis resulted in constitutive upregulation of genes involved in JA biosynthesis.33 Altering endogenous Hb1 in transgenic Arabidopsis, affected the disease response toward different pathogens including the strong necrotrophic fungus Botrytis cinerea.34 Interestingly increased disease symptoms were observed in both up and downregulating lines, compared with controls. The increase in symptoms due to downregulation of Hb1 was accompanied with an increase in NO and H2O2, followed by a rise in JA. Strongly necrotrophic pathogens including B. cinerea have demonstrated the ability to induce programmed cell death (PCD) in susceptible hosts in order to infect more rapidly.35 Increased cellular degradation caused by NO accumulation likely facilitated the rapid progression of the pathogen. In contrast, lines overexpressing Hb1 showed reduced levels of NO.36
The role of JA during embryo development is poorly characterized, although JA signaling plays an active role during reproductive development by interfering with PCD mechanisms.37,38 Jasmonic acid biosynthetic and signaling mutants are male sterile,39,40 while tomato JA insensitive mutants have reduced pollen viability.41 Levels of JA are high in reproductive tissue, especially flowers42 and in initial stages of embryo development, reaching a peak as early as 5 d after pollination.43 The role of JA during embryo development is unknown although, in some in vitro embryogenic systems, JA enhances the frequency of somatic embryos.44 This enhancement may occur through the regulation of auxin, which controls embryo patterning in vivo45 and embryogenic competence in vitro.46 Auxin application during in vitro embryogenesis is required for the initiation of the embryogenic process. However, the exact role of auxin is unclear with several hypotheses existing including auxin acting as a “stress signal,”47 by regulating DNA methylation48 or abscisic acid synthesis.49 JA promotes auxin biosynthesis50,51 and also alters auxin distribution and signaling.52 Applications of JA induce the expression of the auxin biosynthetic gene Anthranilate Synthase α1 (ASA1)51 and affects the expression of transcription factors participating in auxin response such as Plethora1 and 2 (PLT1 and 2).53 Most importantly JA suppresses MYC254 a repressor of auxin during embryogenesis.5 Hbs, by modulating NO within the cell, may alter the NO-influenced JA signal transduction pathway and, consequently, regulate embryogenesis. Suppression of Hb2increases Arabidopsis somatic embryo production by increasing cellular NO, a repressor of MYC2.5 JA levels increase in HB2-suppressing cells or cells with elevated NO content. Low levels of MYC2 induce transcription of several IAA biosynthetic genes, including ASA1 and AMIDASE 1 (AMI1) resulting in localized IAA maxima in specific domains of the explants thus promoting embryogenic competence.5 The inductive effects of class-2 Hbs on somatic embryogenesis are not restricted to Arabidopsis as similar results were observed in maize (our lab, unpublished data). While the interaction between JA and IAA during embryogenesis requires further studies, this model is a valid framework for additional experiments.
Conclusions
By controlling NO levels through their scavenging ability, plant Hbs have emerged as important regulators of plant growth and development. The Hbs-modulation of NO content in specific cellular domains is perceived as a signal interfering with hormone synthesis and action and triggering several responses. Indirect and direct evidence places JA downstream in the Hbs-mediated signaling (Fig. 1). Besides the well-established role in modulating defense responses during plant pathogen interaction JA might play a role during embryogenesis by influencing the biosynthesis of the inductive signal auxin. While additional studies are needed to confirm this regulation in embryogenic tissue, this model opens interesting avenues for dissecting HBs signaling in plants.
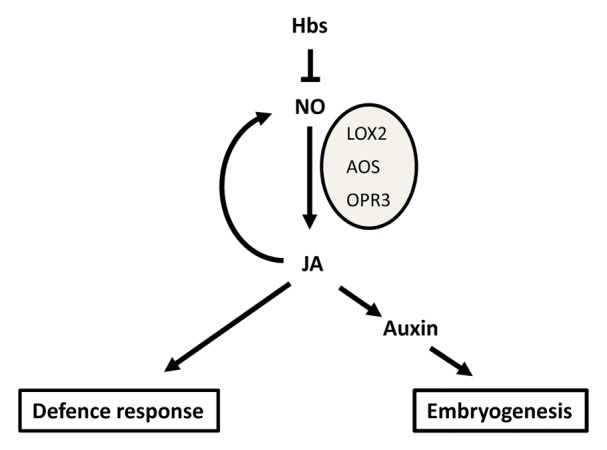
Figure 1. Hemoglobin (Hb) regulation of defense responses and embryogenesis through nitric oxide (NO) and jasmonic acid (JA). Known JA biosynthetic genes upregulated by NO are lipoxygenase (LOX2), allene oxide synthase (AOS) and 12-oxophytodienoate reductase (OPRT3).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25264
References
- 1.Smagghe BJ, Hoy JA, Percifield R, Kundu S, Hargrove MS, Sarath G, et al. Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers. 2009;91:1083–96. doi: 10.1002/bip.21256. [DOI] [PubMed] [Google Scholar]
- 2.Hill RD. Non-symbiotic haemoglobins-What’s happening beyond nitric oxide scavenging? AoB Plants. 2012;2012:pls004. doi: 10.1093/aobpla/pls004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargrove MS, Brucker EA, Stec B, Sarath G, Arredondo-Peter R, Klucas RV, et al. Crystal structure of a nonsymbiotic plant hemoglobin. Structure. 2000;8:1005–14. doi: 10.1016/S0969-2126(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 4.Watts RA, Hunt PW, Hvitved AN, Hargrove MS, Peacock WJ, Dennis ES. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc Natl Acad Sci USA. 2001;98:10119–24. doi: 10.1073/pnas.191349198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, et al. Function of type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J. 2013 doi: 10.1111/tpj.12181. In press. [DOI] [PubMed] [Google Scholar]
- 6.Vigeolas H, Hühn D, Geigenberger P. Nonsymbiotic hemoglobin-2 leads to an elevated energy state and to a combined increase in polyunsaturated fatty acids and total oil content when overexpressed in developing seeds of transgenic Arabidopsis plants. Plant Physiol. 2011;155:1435–44. doi: 10.1104/pp.110.166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebelstrup KH, Hunt P, Dennis ES, Jensen SB, Jensen EØ. Hemoglobin is essential for normal growth of Arabidopsis organs. Physiol Plant. 2006;127:157–66. doi: 10.1111/j.1399-3054.2006.00653.x. [DOI] [Google Scholar]
- 8.Wendehenne D, Pugin A, Klessig DF, Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001;6:177–83. doi: 10.1016/S1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- 9.Wang JW, Wu JY. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005;46:923–30. doi: 10.1093/pcp/pci098. [DOI] [PubMed] [Google Scholar]
- 10.Chehab EW, Yao C, Henderson Z, Kim S, Braam J. Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol. 2012;22:701–6. doi: 10.1016/j.cub.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, et al. Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol. 2001;47:677–92. doi: 10.1023/A:1012440926982. [DOI] [PubMed] [Google Scholar]
- 12.Bustos-Sanmamed P, Tovar-Méndez A, Crespi M, Sato S, Tabata S, Becana M. Regulation of nonsymbiotic and truncated hemoglobin genes of Lotus japonicus in plant organs and in response to nitric oxide and hormones. New Phytol. 2011;189:765–76. doi: 10.1111/j.1469-8137.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor ER, Nie XZ, MacGregor AW, Hill RD. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol. 1994;24:853–62. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- 14.Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, et al. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe Interact. 2008;21:1175–83. doi: 10.1094/MPMI-21-9-1175. [DOI] [PubMed] [Google Scholar]
- 15.Dordas C. Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Sci. 2009;176:433–40. doi: 10.1016/j.plantsci.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Hoy JA, Hargrove MS. The structure and function of plant hemoglobins. Plant Physiol Biochem. 2008;46:371–9. doi: 10.1016/j.plaphy.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Hill RD. What are hemoglobins doing in plants? Can J Bot. 1998;76:707–12. [Google Scholar]
- 18.Hebelstrup KH, Jensen EO. Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta. 2008;227:917–27. doi: 10.1007/s00425-007-0667-z. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–71. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 20.Cantrel C, Vazquez T, Puyaubert J, Rezé N, Lesch M, Kaiser WM, et al. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189:415–27. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 21.Igamberdiev AU, Stoimenova M, Seregélyes C, Hill RD. Class-1 hemoglobin and antioxidant metabolism in alfalfa roots. Planta. 2006;223:1041–6. doi: 10.1007/s00425-005-0145-4. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218:938–46. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- 23.Espunya MC, De Michele R, Gómez-Cadenas A, Martínez MC. S-Nitrosoglutathione is a component of wound- and salicylic acid-induced systemic responses in Arabidopsis thaliana. J Exp Bot. 2012;63:3219–27. doi: 10.1093/jxb/ers043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wünsche H, Baldwin IT, Wu JQ. S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata. J Exp Bot. 2011;62:4605–16. doi: 10.1093/jxb/err171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mur LAJ, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH. Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot. 2012;63:4375–87. doi: 10.1093/jxb/ers116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orozco-Cárdenas ML, Ryan CA. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002;130:487–93. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M. NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol. 2009;12:451–8. doi: 10.1016/j.pbi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Bellin D, Asai S, Delledonne M, Yoshioka H. Nitric oxide as a mediator for defense responses. Mol Plant Microbe Interact. 2013;26:271–7. doi: 10.1094/MPMI-09-12-0214-CR. [DOI] [PubMed] [Google Scholar]
- 29.Lindermayr C, Sell S, Müller B, Leister D, Durner J. Redox regulation of the NPR1-TGA1 system of Arabidopsis thaliana by nitric oxide. Plant Cell. 2010;22:2894–907. doi: 10.1105/tpc.109.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–16. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 31.Qu ZL, Wang HY, Xia GX. GhHb1: a nonsymbiotic hemoglobin gene of cotton responsive to infection by Verticillium dahliae. Biochim Biophys Acta. 2005;1730:103–13. doi: 10.1016/j.bbaexp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Qu ZL, Zhong NQ, Wang HY, Chen AP, Jian GL, Xia GX. Ectopic expression of the cotton non-symbiotic hemoglobin gene GhHbd1 triggers defense responses and increases disease tolerance in Arabidopsis. Plant Cell Physiol. 2006;47:1058–68. doi: 10.1093/pcp/pcj076. [DOI] [PubMed] [Google Scholar]
- 33.Maassen A, Hennig J. Effect of Medicago sativa Mhb1gene expression on defense response of Arabidopsis thaliana plants. Acta Biochim Pol. 2011;58:427–32. [PubMed] [Google Scholar]
- 34.Mur LA, Sivakumaran A, Mandon J, Cristescu SM, Harren FJ, Hebelstrup KH. Haemoglobin modulates salicylate and jasmonate/ethylene-mediated resistance mechanisms against pathogens. J Exp Bot. 2012;63:4375–87. doi: 10.1093/jxb/ers116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–7. doi: 10.1016/S0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 36.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–27. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 37.Sreenivasulu N, Radchuk V, Strickert M, Miersch O, Weschke W, Wobus U. Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA- regulated maturation in developing barley seeds. Plant J. 2006;47:310–27. doi: 10.1111/j.1365-313X.2006.02789.x. [DOI] [PubMed] [Google Scholar]
- 38.Maccarrone M, Melino G, Finazzi-Agrò A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001;8:776–84. doi: 10.1038/sj.cdd.4400908. [DOI] [PubMed] [Google Scholar]
- 39.Browse J. Jasmonate: preventing the maize tassel from getting in touch with his feminine side. Sci Signal. 2009;2:pe9. doi: 10.1126/scisignal.259pe9. [DOI] [PubMed] [Google Scholar]
- 40.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–97. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–43. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–9. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimitrov MI, Donchev AA, Kolev KG, Christov CD. Jasmonic acid levels and seed development in sunflower (Helianthus annus L.) Gen Appl Plant Physiol. 2005;31:135–42. [Google Scholar]
- 44.Teixeira da Silva JA. Impact of methyl jasmonate on PLB formation of hybrid Cymbidium (Orchidaceae) J Plant Develop. 2012;19:47–52. [Google Scholar]
- 45.Möller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. 2009;1:a001545. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, et al. The Role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002;129:1807–19. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fehér A, Pasternak TP, Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org. 2003;74:201–28. doi: 10.1023/A:1024033216561. [DOI] [Google Scholar]
- 48.Phillips RL, Kaeppler SM, Olhoft P. Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA. 1994;91:5222–6. doi: 10.1073/pnas.91.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woeste KE, Ye C, Kieber JJ. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–30. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–45. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell. 2009;21:1495–511. doi: 10.1105/tpc.108.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Q, Sun J, Zhai Q, Zhou W, Qi L, Xu L, et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell. 2011;23:3335–52. doi: 10.1105/tpc.111.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–4. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 54.Kazan K, Manners JM. Jasmonate signaling: toward an integrated view. Plant Physiol. 2008;146:1459–68. doi: 10.1104/pp.107.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]


