Abstract
3′,5′-cyclic guanosine monophosphate (cGMP) and hydrogen peroxide (H2O2) function as the important signaling molecule which promote the lateral root development of Arabidopsis thaliana. In this study, interestingly, application of 8-Br-cGMP (the membrane permeable cGMP analog) promoted the endogenous H2O2 production. In addition, the decrease of endogenous H2O2 also inhibited the effect of cGMP on the lateral root development. Thus, H2O2 maybe act as a downstream signaling of cGMP molecule which is involved in the lateral root development of Arabidopsis. We further found that H2O2 affected cGMP modulating polar auxin transport. When the endogenous H2O2 level was inhibited, the effect of cGMP on the acropetal auxin transport and the basipetal auxin transport was removed. Moreover, pin2 was insensitive for cGMP and H2O2 suggesting that PIN2 protein plays an important role in cGMP and H2O2 modulating the lateral root development of Arabidopsis.
Keywords: cGMP, H2O2, Arabidopsis lateral root, polar auxin transport
The main determinant of root systems structure is the lateral root formation.1 Therefore, researching the mechanism of lateral root formation and growth is very important in agricultural production. Arabidopsis thaliana as an important model plant, the molecular mechanism of Arabidopsis lateral root development has been extensively studied. In Arabidopsis, lateral root development can be divided into three stages. The lateral root initiates the pericycle cells of the xylem.1 Division and expansion of pericycle cells form the lateral root primordium.2 At the stage of emergence, the primordium forms an active meristem and becomes a new lateral root.2
Auxin plays a key role in lateral root development. It is proposed that indole acetic acid (IAA) is necessary at lateral root primordia formation and lateral root development.3 Laskowski et al. have suggested that IAA is required for isolated lateral root primordia which promote the rapidly division of the pericycle cells.4 However, developing lateral root primordia obtain IAA via polar auxin transport.5 In the root, IAA is polarly transported, from shoot to the root apex (acropetal, rootward) and toward the root-shoot junction (basipetal, shootward).5
In the past many years, hydrogen peroxide (H2O2) is considered to be a toxic cellular metabolite in the environmental stress. Recently, many reports suggest that H2O2 functions is an important signaling molecule in plants.6 The production of H2O2 is regulated by PM-bound NADPH oxidase complex in plants and the inhibitor of the NADPH oxidase can decrease endogenous H2O2 level in both mammals and plants.7 In plants, a lot of physiological and biochemical processes are regulates by H2O2 signal, including programmed cell death, stomatal closure, hypersensitive response, the development of cell wall and pollen development.8-10 Recently, there are many reports demonstrate that H2O2 is involved in mung bean adventitious rooting development.11 Our work found that the density of Arabidopsis lateral root markedly increased after 10 μM H2O2 treatment for 5 d (Fig. 1A). In addition, application of diphenylene iodonium (DPI, a PM NADPH oxidase inhibitor) inhabited the density of lateral root in Arabidopsis (Fig. 1A). These data suggest that H2O2 maybe act as an essential signaling molecule which is involved in the lateral root development of Arabidopsis.
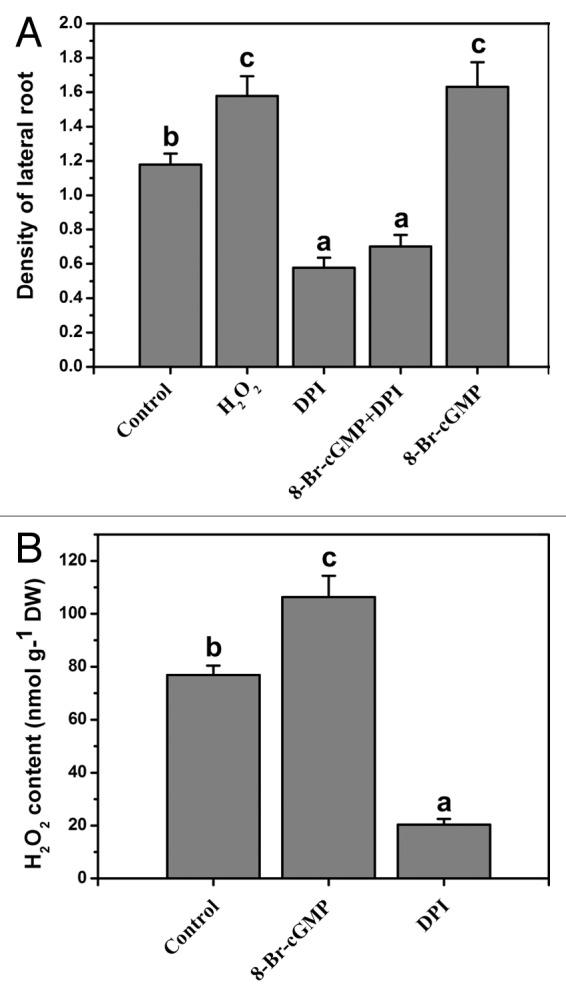
Figure 1. (A) Effects of 8-Br-cGMP and H2O2 on the density of lateral root in wild-type plant. Five days old seedlings to various treatments for 5 d. (B) Effects of 8-Br-cGMP on the endogenous H2O2 level in wild-type Arabidopsis roots. Seven-day- old seedlings to various treatments for 6 h. Ten μM H2O2, 40 μM 8-Br-cGMP and 5 μM DPI were used for various treatments. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters were significantly different at the p < 0.01 level.
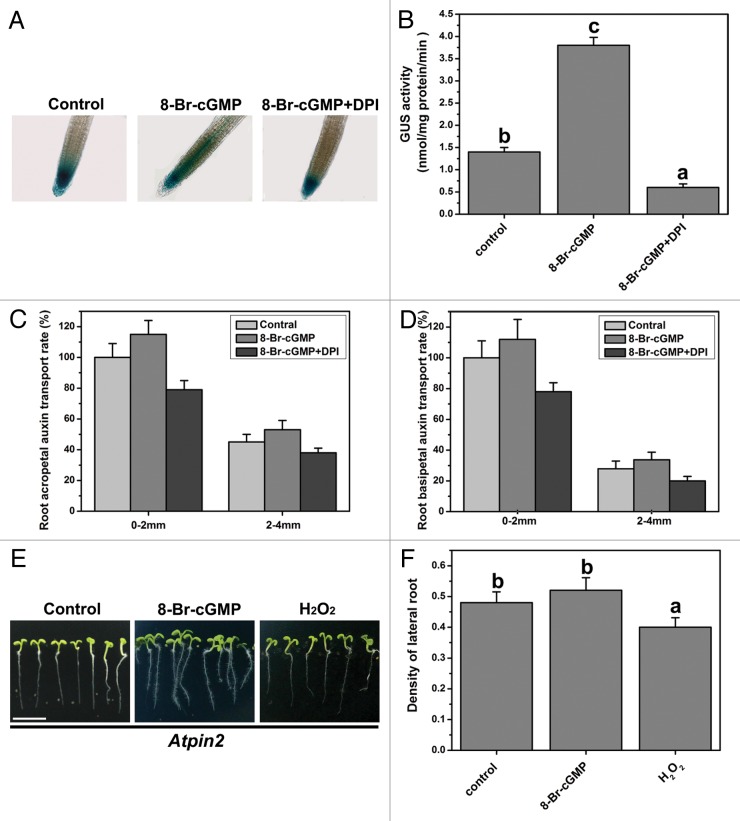
3′, 5′-cyclic guanosine monophosphate (cGMP) is an important secondary messenger, it is involved in various developmental processes in plants, such as chloroplast development, seed germination, pollen germination and stomatal closure.12-14 Recently, our study demonstrates that cGMP can regulate polar auxin transport in the root of Arabidopsis and promotes Arabidopsis lateral root development.15 However, when the endogenous H2O2 level was inhibited by DPI, the effect of cGMP on the lateral root was removed (Fig. 1A). Interestingly, In this study, we also found that application of 8-Br-cGMP (the membrane permeable cGMP analog) induced the endogenous H2O2 accumulation (Fig. 1B). Application of exogenous H2O2 also could promote the density of lateral root in Arabidopsis (Fig. 1A). Thus, the function of H2O2 seen to be involved in cGMP modulating the lateral root development of Arabidopsis. We further understand the interaction between cGMP and H2O2 on polar auxin transport in Arabidopsis roots. We has demonstrated that the activity and expression of DR5::GUS can be increased in root tips by 8-Br-cGMP. However, in this work we found that when the endogenous H2O2 level was inhibited by DPI, the effect of cGMP on the activity and expression of DR5::GUS was removed (Fig. 2A and B). cGMP could enhance the acropetal auxin transport and the basipetal auxin transport in Arabidopsis roots. However cGMP modulating polar auxin transport was also inhibited by DPI (Fig. 2C and D). Therefore, we suggest that H2O2 and cGMP are necessary signaling molecule which respond for the lateral root development of Arabidopsis and H2O2 maybe act as a downstream signaling molecule involved in cGMP modulating polar auxin transport.
Figure 2. Effects of 8-Br-cGMP and H2O2 on polar auxin transport in Arabidopsis root. (A) Histochemical GUS staining patterns of DR5::GUS after various treatments for 3 h in apical zone of primary root. (B) GUS activity assays of DR5::GUS transgenic root after various treatments for 3 h. (Cand D) Root acropetal auxin transport and basipetal auxin transport in wild-type Arabidopsis were assayed after various treatments for 12 h. (E and F) Effects of 8-Br-cGMP and H2O2 on the density of lateral root in pin2 mutant plant. Five-days-old seedlings to various treatments for 5 d. Ten μM H2O2, 40 μM 8-Br-cGMP 5 μM DPI and Ly83583 were used for various treatments. Bar = 1 cm. Mean values and SE were calculated from three replicates. Within each set of experiments, bars with different letters were significantly different at the p < 0.01 level.
In plants, a substantial amount of data demonstrate that PIN family proteins act as the auxin efflux carriers which are the essential facilitators of polar auxin transport.16,17 PIN proteins bind at the plasma membrane and show asymmetric subcellular localization. PIN proteins regulate polar auxin transport in roots and thus the local auxin gradients build up that promote the lateral root formation. Previous studies have shown that mutation of PINs cause the phenotypic changes of root in Arabidopsis.16,18 In pin1,2 double mutant, the root cannot elongation and the root meristem is inhibited.19 In different developmental processes, PIN proteins can be regulated by various signaling molecule, such as cGMP.3,15 cGMP functions as a signaling molecule to regulate the PIN family gene expression (PIN1, PIN2, PIN3 and PIN7) and affects polar distribution of PIN protein.11 H2O2 is involved in cGMP modulating polar auxin transport in Arabidopsis roots. We have also analyzed the effect of cGMP and H2O2 on pin2 mutant plant.20 The obtained results showed that pin2 was insensitive for cGMP and H2O2 (Fig. 2E and F). These data suggest that PIN2 protein plays an important role in cGMP and H2O2 modulating the lateral root development of Arabidopsis.
In conclusion, both cGMP and H2O2 are necessary signaling molecule which respond for the lateral root development. The relationship between cGMP and H2O2 shed a new light on the complex signaling mechanisms during the lateral root development in Arabidopsis. H2O2 functions as a downstream signaling molecule involved in cGMP modulating the lateral root development of Arabidopsis.
Acknowledgments
Support for this work is from the Northwest A&F University doctor start-up Foundation (Z109021116).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25052
References
- 1.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–52. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Mol Biol. 2006;60:871–87. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- 3.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 4.Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–10. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- 5.Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA. 2008;105:8790–4. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Wang MJ, Ding MQ, Deng SR, Liu MQ, Lu CF, et al. H2O2 and cytosolic Ca2+ signals triggered by the PM H-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010;33:943–58. doi: 10.1111/j.1365-3040.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Chen G, Wang X, Zhang Y, Jia H, Bi Y. Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol Plant. 2011;141:239–50. doi: 10.1111/j.1399-3054.2010.01429.x. [DOI] [PubMed] [Google Scholar]
- 8.Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol. 2006;170:501–12. doi: 10.1111/j.1469-8137.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 9.Hung KT, Hsu YT, Kao CH. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Physiol Plant. 2006;127:293–303. doi: 10.1111/j.1399-3054.2006.00662.x. [DOI] [Google Scholar]
- 10.Love AJ, Yun BW, Laval V, Loake GJ, Milner JJ. Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol. 2005;139:935–48. doi: 10.1104/pp.105.066803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li SW, Xue LG, Xu SJ, Feng HY, An LZ. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot. 2009;65:63–71. doi: 10.1016/j.envexpbot.2008.06.004. [DOI] [Google Scholar]
- 12.Li J, Wang X, Zhang Y, Jia H, Bi Y. cGMP regulates hydrogen peroxide accumulation in calcium-dependent salt resistance pathway in Arabidopsis thaliana roots. Planta. 2011;234:709–22. doi: 10.1007/s00425-011-1439-3. [DOI] [PubMed] [Google Scholar]
- 13.Teng Y, Xu WZ, Ma M. cGMP is required for seed germination in Arabidopsis thaliana. J Plant Physiol. 2010;167:885–9. doi: 10.1016/j.jplph.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Dubovskaya LV, Bakakina YS, Kolesneva EV, Sodel DL, McAinsh MR, Hetherington AM, et al. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol. 2011;191:57–69. doi: 10.1111/j.1469-8137.2011.03661.x. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Jia H. cGMP modulates Arabidopsis lateral root formation through regulation of polar auxin transport. Plant Physiol Biochem. 2013;66:105–17. doi: 10.1016/j.plaphy.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Friml J, Palme K. Polar auxin transport--old questions and new concepts? Plant Mol Biol. 2002;49:273–84. doi: 10.1023/A:1015248926412. [DOI] [PubMed] [Google Scholar]
- 17.Sauer M, Robert S, Kleine-Vehn J. Auxin: simply complicated. J Exp Bot. 2013 doi: 10.1093/jxb/ert139. [DOI] [PubMed] [Google Scholar]
- 18.Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–9. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 19.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 20.Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–11. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]