Abstract
Molecular interaction between powdery mildew fungi and Arabidopsis has been widely used as a model system to study plant immunity. Arabidopsis EDR2 (enhanced disease resistance 2) is a well characterized negative regulator in powdery mildew resistance and mildew-induced cell death. Recently, we showed that a mutation in BSK1 (br-signaling kinase 1), suppressed edr2-mediated disease resistance.1 And the bsk1-1 single mutant displayed enhanced susceptibility to multiple pathogens, indicating that BSK1 plays important roles in plant immunity. BSK1 is a receptor-like cytoplasmic kinase and localizes on plasma membrane; loss of the membrane localization signaling disrupts BSK1 functions in edr2-mediated resistance. Significantly, BSK1 physically associates with the PAMP receptor FLS2 (flagellin sensing 2) and is required by FLS2-mediated ROS burst.1 Here we show that disruption of BSK1 membrane localization affects the BSK1-FLS2 interactions, suggesting the membrane association of BSK1 is important for both edr2-mediated signaling and the BSK1-FLS2 complex formation. Previously, it was shown that BSK1 is a substrate of the brassinosteroid (BR) receptor BRI1 (brassinosteroid insensitive 1) and plays critical roles in BR signaling.2 Further exploration of signaling transductions downstream of BSK1-FLS2 complex will not only shed new light on how BSK1 regulates plant immunity, but may also help to dissect the connections between plant growth and defense.
Keywords: powdery mildew, disease resistance, BR, BSK1, FLS2
Powdery mildew fungi are biotrophic pathogens that infect many plant species, such as wheat, barley and tomato, causing significant economic losses.3 Powdery mildew fungi also infect model plant Arabidopsis and a number of Arabidopsis genes involved in powdery mildew resistance have been identified through mutant screen in recent years. For instance, edr1 (enhanced disease resistance 1), edr2 and edr3 mutants all exhibit enhanced disease resistance to powdery mildew Golovinomyces cichoracearum.4-8 The resistance correlates with a more rapid activation of host defenses compared with wild type plants, such as enhanced PR (pathogenesis related) genes induction and accompanied by mildew-induced cell death. EDR2 encodes a protein that localizes mainly on endomembrane system.8 edr2-mediated resistance is dependent on salicylic acid, but not jasmonic acid or ethylene.6,8 In an edr2 suppressor screening, we recently identified a mutation in BSK1, that suppressed the powdery mildew resistance and programmed cell death caused by edr2. The bsk1-1 single mutant displayed enhanced susceptibility to a number of pathogens and accumulated lower levels of SA and defense marker genes upon infection with G. cichoracearum and Pseudomonas syringae. BSK1 is also involved in edr1, mlo2 (mildew resistance locusO2) and pmr4 (powdery mildew resistance 4)-mediated powdery mildew resistance. These data indicate that BSK1 plays important roles in plant immunity.
BSK1 belongs to a receptor-like cytoplasmic kinase subfamily RLCK-XII and previously was shown to be a substrate of the brassinosteroid (BR) receptor BRI1 (brassinosteroid insensitive 1).2 BSK1 displays kinase activity in vitro and this kinase activity is required for its function.1 BSK1 has a N-terminal myristoylation site, which is a potential membrane localization signal.9 The disruption of myristoylation site (G2A) affects BSK1 membrane association and, in contrast to wild type BSK1, the mutant form of BSK1 (BSK1 G2A) was unable to complement the bsk1-1 phenotype, indicating membrane localization is essential for BSK1 function. Further co-immunoprecipitation assays indicate that BSK1 physically associates with the PAMP receptor FLS2 both in Arabidopsis and in N. benthamiana.1
To further explore the role of BSK1 membrane association, we examined the physical association of FLS2 and BSK1 G2A, a mutant form of BSK1 that lost the membrane localization. We performed co-immunoprecipitation assay by expressing BSK1 G2A-GFP and FLS2-FLAG in Arabidopsis protoplasts. We co-expressed BSK1-GFP and FLS2-FLAG as a positive control and expressed FLS2-FLAG alone as a negative control. Total proteins were extracted from protoplasts and BSK1 G2A protein was immunoprecipitated with GFP antibody. In the precipitate, we were unable to detect any FLS2 protein with FLAG antibody, whether or not treated with flg22, which was in contrast to the strong interactions detected between the wild type BSK1 and FLS2 proteins (Fig. 1), indicating that membrane association is important for BSK1 and FLS2 complex formation in Arabidopsis. In the Co-IP assay, we repeatedly saw that the association of BSK1 and FLS2 was reduced upon flg22 treatment (Fig. 1), which suggests that BSK1 maybe released from FLS2 receptor complex to transduce flg22 signaling to downstream upon flg22 elicitation.
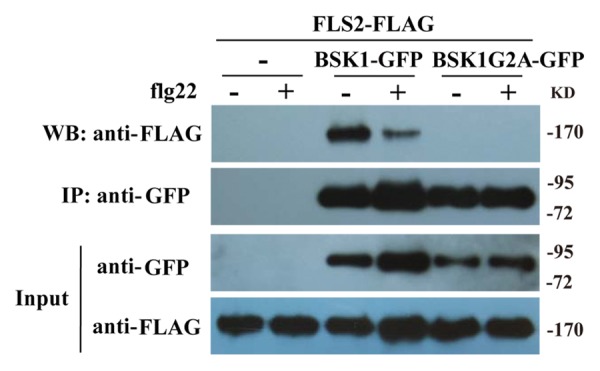
Figure 1. Disruption of BSK1 membrane localization affects BSK1 and FLS2 association in Arabidopsis protoplasts. Co-immunoprecipitation of BSK1 G2A and FLS2 from Arabidopsis protoplasts transiently expressing BSK1 G2A-GFP and FLS2-FLAG before (−) or 10 min after (+) elicitation with 1 µM flg22 as indicated. FLS2-FLAG alone was used as a negative control. Total protein was subjected to immunoprecipitation with GFP antibody, followed by immunoblot analysis with anti-FLAG antibody. These experiments were repeated three times with similar results.
Together with previous finding,1,2 BSK1 has dual roles in both BR signaling and plant immunity. Several lines of evidence suggest there is crosstalk between BR signaling and plant immunity.10-12 For instance, BAK1, an important component in BR signaling, physically associates with both BRI1 and FLS2; disruption of BAK1 compromises basal defense in plants.13-18 Recently, it was shown that FLS2-mediated immune signaling was inhibited by activation of BR signaling, in contrast, flg22 has no effect on BR-induced responses, suggesting a unidirectional inhibition of PTI by BR.0.11,12 Increased BR signaling led to enhanced susceptibility to oomycete H. a. Noco2 in the presence of BAK1, but enhanced resistance to the same pathogen at the absence of BAK1, suggesting that BR can affect plant immunity by BAK1 dependent and independent mechanisms.11
It was shown that the amino acid Ser230 of BSK1 is a major site that is phosphorylated by BRI.2 However, the S230A mutation fully restored edr2 bsk1-1 to edr2-like phenotypes in response to powdery mildew infection,1 suggesting that the phosphorylation of Ser230 is dispensable for BSK1 function in edr2-mediated defense responses.1 One possibility is that BSK1 has dual functions in both BR signaling and defense responses and these functions could be uncoupled. Interestingly, recently it was shown that a mutant allele of bak1-5, has strong defects in FLS2 and EFR-mediated PTI, but is not impaired in BR signaling, indicating the BR signaling and immune responses are differentially regulated.18 Considering that the bsk1-1 mutant displays enhanced susceptibility to multiple pathogens, and BSK1 is a substrate of the BR receptor BRI1, it would be very interesting to dissect whether BSK1 is a point of crosstalk between BR signaling and defense pathways and what is the relationship between BSK1 and BAK1 in both BR signaling and immune response.
Acknowledgments
This work was supported by grants from National Basic Research Program of China (2011CB100700 and 2009CB118306), the National Natural Science Foundation of China (31271296).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/24996
References
- 1.Shi H, Shen Q, Qi Y, Yan H, Nie H, Chen Y, et al. BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. Plant Cell. 2013;25:1143–57. doi: 10.1105/tpc.112.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang W, Kim TW, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, et al. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–60. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze-Lefert P, Vogel J. Closing the ranks to attack by powdery mildew. Trends Plant Sci. 2000;5:343–8. doi: 10.1016/S1360-1385(00)01683-6. [DOI] [PubMed] [Google Scholar]
- 4.Frye CA, Tang D, Innes RW. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA. 2001;98:373–8. doi: 10.1073/pnas.98.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frye CA, Innes RW. An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell. 1998;10:947–56. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang D, Ade J, Frye CA, Innes RW. Regulation of plant defense responses in Arabidopsis by EDR2, a PH and START domain-containing protein. Plant J. 2005;44:245–57. doi: 10.1111/j.1365-313X.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang D, Ade J, Frye CA, Innes RW. A mutation in the GTP hydrolysis site of Arabidopsis dynamin-related protein 1E confers enhanced cell death in response to powdery mildew infection. Plant J. 2006;47:75–84. doi: 10.1111/j.1365-313X.2006.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorwerk S, Schiff C, Santamaria M, Koh S, Nishimura M, Vogel J, et al. EDR2 negatively regulates salicylic acid-based defenses and cell death during powdery mildew infections of Arabidopsis thaliana. BMC Plant Biol. 2007;7:35. doi: 10.1186/1471-2229-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson GA, Jr., Okuyama H. Lipid-linked proteins of plants. Prog Lipid Res. 2000;39:19–39. doi: 10.1016/S0163-7827(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZY. Brassinosteroids modulate plant immunity at multiple levels. Proc Natl Acad Sci USA. 2012;109:7–8. doi: 10.1073/pnas.1118600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belkhadir Y, Jaillais Y, Epple P, Balsemão-Pires E, Dangl JL, Chory J. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc Natl Acad Sci USA. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, et al. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc Natl Acad Sci USA. 2012;109:303–8. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 14.Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–12. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–22. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 16.Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–22. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–55. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, et al. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]


