Abstract
Palmitoylation is the post-translational addition of lipids to proteins though thioester bonds and acts to promote association with membranes. Palmitoylation also acts to target proteins to specific membrane compartments, control residence in and movement between membrane microdomains and regulate protein conformation and activity. Palmitoylation is unique among lipid modifications of proteins as it is reversible, allowing for dynamic control over all palmitoylation dependent processes. Palmitoylation cannot be predicted from protein sequence and as a result is understudied when compared with other post-translational modifications. We recently published a proteomic analysis of palmitoylation in plants and increased the number of proposed palmitoylated proteins in plants from ~30 to over 500. The wide range of identified proteins indicates that palmitoylation is likely important for a variety of different functions in plants. Many supposedly well characterized proteins were identified as palmitoylated and our new data provides novel insight into regulatory mechanisms and potential explanations for observed phenomena. These data represent a new resource for plant biologist and will allow the study of palmitoylated proteins in plants to expand and move forward.
Keywords: palmitoylation, S-acylation, proteomics, microdomain, lipid raft, membrane
Palmitoylation, more correctly known as S-acylation, is a reversible post-translational modification of proteins involving the addition of fatty acids to cysteine residues through thioester bonds. S-acylation promotes strong association of proteins with membranes but can also promote or suppress association of proteins with specific membrane lipids. There is increasing evidence that membranes are not homogeneous structures, instead specific proteins and lipids form distinct and sometimes transient proteolipid complexes that have been termed microdomains (ref. 1). Given its reversible nature and ability to alter affinity for different lipids S-acylation is considered to be one of the main mechanisms providing dynamic control over proteins entering or being excluded from specific microdomains and regulating protein complex formation in response to stimuli.
In recent years S-acylation in plants has begun to come of age with the enzymes responsible for S-acylation being identified and characterized,2-4 in-depth analysis of the role of S-acylation in the function of ROP GTPases,5-8 many other S-acylated proteins recently being described9–12 and tools to analyze S-acylation being developed.7,13 Despite the important properties of S-acylation and increased interest in its function, it is still very difficult to predict whether a protein is S-acylated. Each study published to date has therefore had to rely on empirical determination for each suspected S-acylated protein. Recently however, advances in proteomics methods have allowed the S-acyl proteome of plants to begin to be elucidated and the number of proposed S-acylated proteins in plants has increased from ~30 to over 500.14 Through this proteomics study we identified known S-acylated proteins such as the heterotrimeric G-protein α-subunit GPA1, the RPM1 interacting protein RIN4 and many calcium dependant protein kinases (CPKs) and calcineurin-B like proteins (CBLs), thereby validating our approach. Novel proteins identified included receptor-like kinases, cell wall synthesis proteins, various classes of membrane transporter, various types of ATPase, SNAREs, putative membrane microdomain organizing and stabilizing proteins (Band7, Remorin, Tetraspanin and NHL proteins) and Raf-like MAP kinases. These novel data, derived solely from root callus culture, highlight the fact that S-acylation affects many more proteins in plants than previously thought. Given the limited nature of the source material it is also likely that S-acylation affects many more proteins than just those reported. Interestingly, using TMHMM15 to identify likely transmembrane regions, the majority of the proteins identified as S-acylated (59%) are predicted to have no transmembrane helices and would likely be annotated as soluble based on their sequence. 20% are predicted to have a single transmembrane helix while the remaining 21% have two or more predicted transmembrane helices. These data indicate that S-acylation is likely to be very important for recruiting many otherwise soluble proteins to membranes.
One of the largest families of proteins we identified as being S-acylated was the receptor-like kinase (RLKs) superfamily. RLKs represent one of the largest gene families in plants and their S-acylation had hitherto not been suspected. We defined potential S-acylated residues using a multiple alignment strategy and identified conserved cysteine residues in all of the RLK family members in our data set. We subsequently demonstrated that the leucine-rich repeat RLK bacterial flagellin receptor FLS2 is S-acylated and confirmed that the cysteine residues identified through multiple alignments are the sites of S-acylation.14 These data on FLS2 provide a possible mechanism for the observed change in FLS2 partitioning between detergent soluble and detergent insoluble membrane fractions upon perception of flagellin.16 Many other RLK family members also contain these conserved cysteine residues, suggesting that S-acylation of this family of proteins may be a common occurrence. These data illustrate the utility of this large new data set for identifying potential S-acylation sites in other proteins.
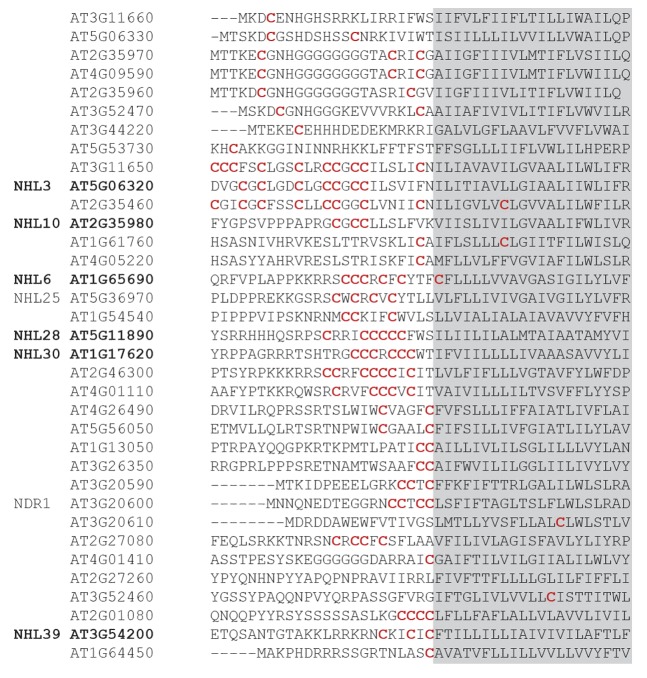
We also demonstrated that the NDR1/HIN1-like NHL family of proteins is S-acylated. NHL-family proteins are induced in response to a variety of biotic and abiotic stresses but no molecular function has yet been ascribed to them. NHL proteins appear to be glycosylated17 and we demonstrated that the glycosylated form of NHL3 is also the predominant S-acylated form. Although the order of glycosylation and S-acylation in NHL3 processing has yet to be determined, S-acylation may well act as an export or plasma membrane targeting signal for mature glycosylated NHL3. Using multiple alignments of the 6 NHL proteins that were identified through our proteomics approach we have identified a common cysteine rich region (Fig. 1) N-terminal to the most highly supported predicted transmembrane domain.17All other cysteine residues conserved between the NHL-family members were in the predicted glycosylated extracellular domain and therefore unlikely to be sites of S-acylation.
Figure 1. Multiple alignment of NHL stress-induced proteins from Arabidopsis. NHL proteins identified as being S-acylated through proteomics work are indicated in bold. These data identify a conserved region of cysteine residues (indicated in red) adjacent to the transmembrane domain (shaded in gray) as the likely site for S-acylation.
With these novel proteomic data14 providing a sound starting point and the tools required to address S-acylation in plants now being available7,13 our understanding of the function of S-acylation in plants is likely to rapidly increase.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25209
References
- 1.Malinsky J, Opekarová M, Grossmann G, Tanner W. Membrane microdomains, rafts, and detergent-resistant membranes in plants and fungi. Annu Rev Plant Biol. 2013;64:501–29. doi: 10.1146/annurev-arplant-050312-120103. [DOI] [PubMed] [Google Scholar]
- 2.Hemsley PA, Kemp AC, Grierson CS. The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Plant Cell. 2005;17:2554–63. doi: 10.1105/tpc.105.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou LZ, Li S, Feng QN, Zhang YL, Zhao X, Zeng YL, et al. PROTEIN S-ACYL TRANSFERASE10 Is Critical for Development and Salt Tolerance in Arabidopsis. Plant Cell. 2013;25:1093–107. doi: 10.1105/tpc.112.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batistic O. Genomics and localization of the Arabidopsis DHHC-cysteine-rich domain S-acyltransferase protein family. Plant Physiol. 2012;160:1597–612. doi: 10.1104/pp.112.203968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavy M, Bracha-Drori K, Sternberg H, Yalovsky S. A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell. 2002;14:2431–50. doi: 10.1105/tpc.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavy M, Yalovsky S. Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J. 2006;46:934–47. doi: 10.1111/j.1365-313X.2006.02749.x. [DOI] [PubMed] [Google Scholar]
- 7.Sorek N, Poraty L, Sternberg H, Bar E, Lewinsohn E, Yalovsky S. Activation status-coupled transient S acylation determines membrane partitioning of a plant Rho-related GTPase. Mol Cell Biol. 2007;27:2144–54. doi: 10.1128/MCB.02347-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Sorek N, Gutman O, Bar E, Abu-Abied M, Feng X, Running MP, et al. Differential effects of prenylation and s-acylation on type I and II ROPS membrane interaction and function. Plant Physiol. 2011;155:706–20. doi: 10.1104/pp.110.166850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traverso JA, Micalella C, Martinez A, Brown SC, Satiat-Jeunemaître B, Meinnel T, et al. Roles of N-terminal fatty acid acylations in membrane compartment partitioning: Arabidopsis h-type thioredoxins as a case study. Plant Cell. 2013;25:1056–77. doi: 10.1105/tpc.112.106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leśniewicz K, Poręba E, Smolarkiewicz M, Wolff N, Stanisławski S, Wojtaszek P. Plant plasma membrane-bound staphylococcal-like DNases as a novel class of eukaryotic nucleases. BMC Plant Biol. 2012;12:195. doi: 10.1186/1471-2229-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng Q, Wang X, Running MP. Dual lipid modification of Arabidopsis Ggamma-subunits is required for efficient plasma membrane targeting. Plant Physiol. 2007;143:1119–31. doi: 10.1104/pp.106.093583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batistic O, Sorek N, Schültke S, Yalovsky S, Kudla J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell. 2008;20:1346–62. doi: 10.1105/tpc.108.058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemsley PA, Taylor L, Grierson CS. Assaying protein palmitoylation in plants. Plant Methods. 2008;4:2. doi: 10.1186/1746-4811-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemsley PA, Weimar T, Lilley KS, Dupree P, Grierson CS. A proteomic approach identifies many novel palmitoylated proteins in Arabidopsis. New Phytol. 2013;197:805–14. doi: 10.1111/nph.12077. [DOI] [PubMed] [Google Scholar]
- 15.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. In Glagow J et al (eds), Proc 6th Int Conf Int Syst Mol Biol, 1998; 6:175-82. [PubMed] [Google Scholar]
- 16.Keinath NF, et al. PAMP-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem. 2010;285:39140–9. doi: 10.1074/jbc.M110.160531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varet A, Hause B, Hause G, Scheel D, Lee J. The Arabidopsis NHL3 gene encodes a plasma membrane protein and its overexpression correlates with increased resistance to Pseudomonas syringae pv. tomato DC3000. Plant Physiol. 2003;132:2023–33. doi: 10.1104/pp.103.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]