Abstract
Light plays a vital role in seedling de-etiolation during which it remarkably inhibits hypocotyl growth and promotes cotyledon opening and the synthesis of chlorophyll and anthocyanin. After light perception, photoreceptors act to repress two main branches of the light signaling, PIFs and COP1-HY5. We recently identified PKL/EPP1, a chromatin remodeling factor, as a new component in regulating light-mediated hypocotyl growth. In this study, we found that EPP1 acts additively with SPA1 to repress seedling de-etiolation. Moreover, the expression of EPP1 is downregulated specifically in the hypocotyl region of the cop1 mutant compared with that of the wild type. We further found that EPP1 drastically inhibits both the protein and transcript levels of HY5, but not vice versa, indicating that HY5 acts downstream of EPP1. We thus propose a model in which EPP1 defines a new repressor and mediates a distinct signaling pathway of photomorphogenesis.
Light provides plants important environmental information for variety of growth and developmental processes, including seedling de-etiolation (also termed photomorphogenesis) in the early stage of their life cycle. Plants have thus evolved an array of photoreceptors, including phytochromes and cryptochromes, to monitor the light signals, which are then transduced to regulate physiological responses through the signaling transduction pathway.1 An increasing body of evidence has established a primary network composed of both positive and negative components in regulating seedling de-etiolation in the model plant Arabidopsis thaliana.1-3 COP1 (for constitutive photomorphogenic 1), a RING-type E3 ubiquitin ligase, functions as a central repressor controlling photomorphogenesis downstream of all photoreceptors.4 A group of bHLH transcription factors, designated as PIFs, physically interact with phytochromes and repress photomorphogenesis under red and far-red light conditions.5 Recently, we identified the chromatin remodeling factor EPP1 (enhanced photomorphogenic 1) PKL (pickle) as a new negative regulator in the light signaling pathway.6 PKL/EPP1 is an ATP-dependent chromatin remodeling enzyme that plays vital role in plant development.7 Genetic studies had also discovered a large number of photomorphogenesis-promoting factors. For example, HY5 (elongated hypocotyl 5) is one of the most important positive transcription factors that are targeted by COP1 and thereafter degraded through the 26S proteasome pathway.8,9 The negative and positive factors work together to fine-tune the downstream gene expression and modulate physiological responses that allows plants to adapt to the light environments properly.
SPA1 and PKL/EPP1 Additively Regulate Seedling De-etiolation
Previous genetic experiments identified SPA1 (suppressor of phytochrome A 1) as a negative component in the phyA signaling pathway.10 It has been well documented that SPA1 physically interacts with COP1 to form E3 ligase complex and represses photomorphogenesis through destabilizing positive transcription factors, such as HY5, in Arabidopsis.11,12 Our previous study revealed that EPP1 acts in parallel with COP1 in repressing photomorphogenesis.6
To study the relationship between EPP1 and SPA1, we generated epp1 spa1 double mutant by genetic crossing of epp1-1 and spa1 mutants. When grown under far-red light, the epp1 spa1 double mutant seedlings showed much shorter hypocotyls and accumulated more anthocyanin than their single parent mutants (Fig. 1). The double mutant also displayed reduced hypocotyl growth in blue and red light conditions (Fig. 1C). These results indicate that EPP1 functions additively with SPA1, similar to with COP1, in modulating seedling de-etiolation process.
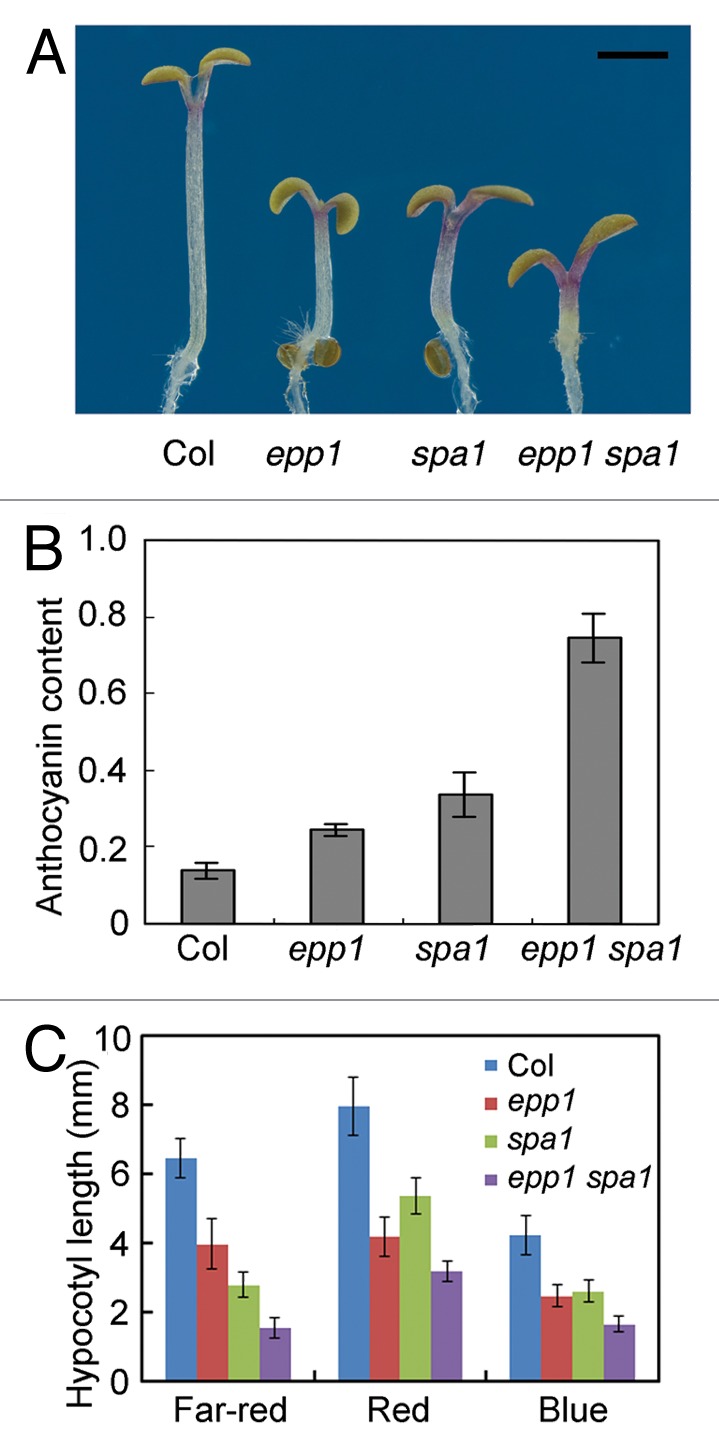
Figure 1. SPA1 and EPP1 additively regulate seedling de-etiolation. (A and B) Seedling phenotype (A) and anthocyanin content [(A530-0.25 × A657)/100 seedlings] (B) of wild-type and mutant seedlings in far-red light for 5 d. Bar in (A) denotes 2 mm. (C) Hypocotyl length of 5-d-old seedlings. Data in (B and C) represent the mean ± SD of triplicate assays.
COP1 Activates PKL/EPP1 Expression
Consistent with the role of EPP1 in repressing light-inhibited hypocotyl elongation, the expression of EPP1 transcript is downregulated by light specifically in the hypocotyl regions and this inhibition is mediated by both phytochromes and cryptochromes.6 To examine a possible regulation by COP1, we performed a reverse-transcription with quantitative PCR assay and found that the transcript level of EPP1 was remarkably reduced in the cop1-4 mutant compared with Col wild type (Fig. 2A). Furthermore, by visualizing the expression of EPP1 using a GUS reporter gene driven by the EPP1 promoter sequence (ProPKL:GUS), we observed that GUS staining was greatly decreased in the hypocotyl of cop1 related to that of the wild-type seedlings (Fig. 2B). However, no pronounced difference was observed in the root region between cop1 and the wild type. These data suggest that COP1 promotes EPP1 gene expression in the hypocotyl. On the contrary, COP1 expression was upregulated in the epp1 mutant (Fig. 2C), suggesting a negative feedback role of EPP1.
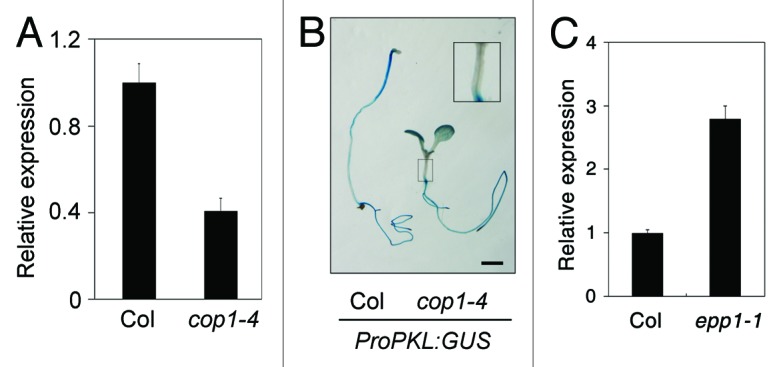
Figure 2.EPP1 expression is downregulated by COP1 mutation. (A) A quantitative RT-PCR assay of EPP1 transcript levels in cop1-4 and wild-type seedlings grown in darkness for 5 d. (B) GUS staining of ProPKL:GUS transgenic seedlings grown in darkness for 5 d. The enlarged image of cop1 hypocotyl is shown up-right. Bar, 2 mm. (C) COP1 transcript level in 5-d-old light-grown wild type and epp1 mutant. In (B and C), relative expression was normalized to that of UBQ1 and data represent mean ± SD of three biological replicates.
PKL/EPP1 Negatively Regulates HY5 Activity
HY5 defines a master point of signaling converged from multiple photoreceptors and itself is regulated mainly at the post-translational level. In the dark, HY5 is targeted by COP1 and further degraded, whereas light triggers nuclear export of COP1, thus stabilizing HY5.8 On the other hand, HY5 possesses transcriptional repression activity toward downstream genes involved in cell elongation to promote photomorphogenesis. However, the recruitment of EPP1 through interaction is proposed to dampen the activity of HY5 from over entry into photomorphogenesis, providing an additional regulatory mechanism to fine-tune the light signaling pathway.6 Using an immunobloting assay against HY5 antibody, we also detected a significant increase of HY5 protein in the epp1 mutant alleles compared with the wild type (Fig. 3A). This HY5 repression by EPP1 is modulated, at least in part, at the transcriptional level as HY5 expression was greatly increased in the epp1 mutant (Fig. 3B). However, mutation in HY5 does not affect the protein level of EPP1 (Fig. 3C). Thus, EPP1 plays a dual role on HY5, de-repressing the transcriptional activity of HY5 toward target loci and inhibiting the transcriptional level of HY5 as well.
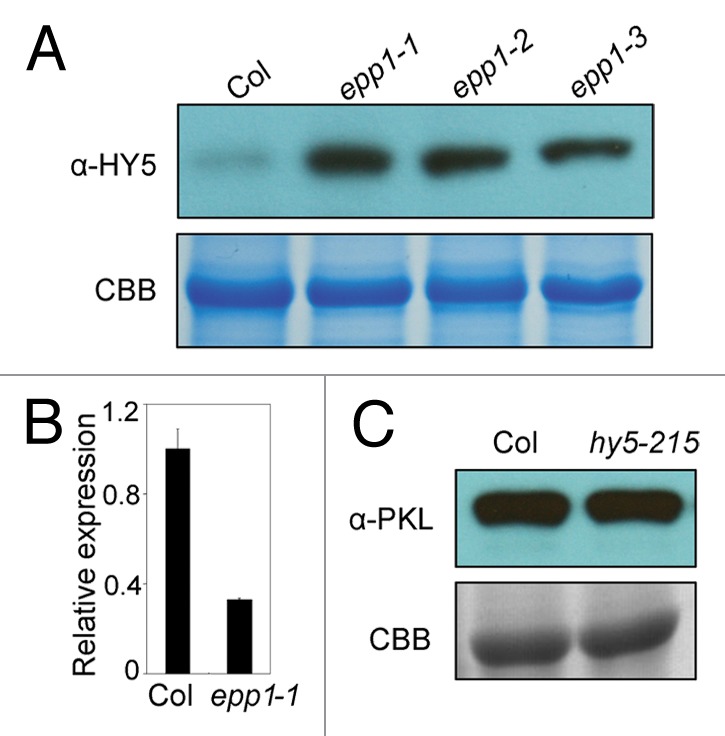
Figure 3. EPP1 inhibits the mRNA and protein levels of HY5. (A) Immunobloting assay showing HY5 protein level in the epp1 mutants and Col wild type. (B) HY5 expression level by qRT-PCR. Relative expression was normalized to UBQ1 and data represent mean ± SD of three biological replicates. (C) EPP1 protein level in hy5 mutant and Col wild type. Seedlings were grown in the dark for 5 d in all assays. Staining with coomassie brilliant blue of the gel serves as a loading control in (A and C).
The light signaling pathway composes of different layers of components, ranging from early photoreceptors, central negative regulators and positive transcription factors, to downstream responsive genes (Fig. 4). Although COP1, PIFs (including PIF1, PIF3, PIF4 and PIF5) and EPP1 all act as repressors in regulating photomorphogenesis, their functions and the underlying regulatory mechanisms are distinct from each other due to their divergent biochemical features. COP1 is an E3 ubiquitin ligase targeting positive factor for degradation, while PIF proteins bind to and regulate the expression of downstream genes in phy-mediated pathway.5,9 Consistently, PIF4 directly activates the expression of a set of genes involved in hypocotyl cell elongation.13 The stability or activity of HY5 is negatively regulated by COP1 and EPP1, respectively.
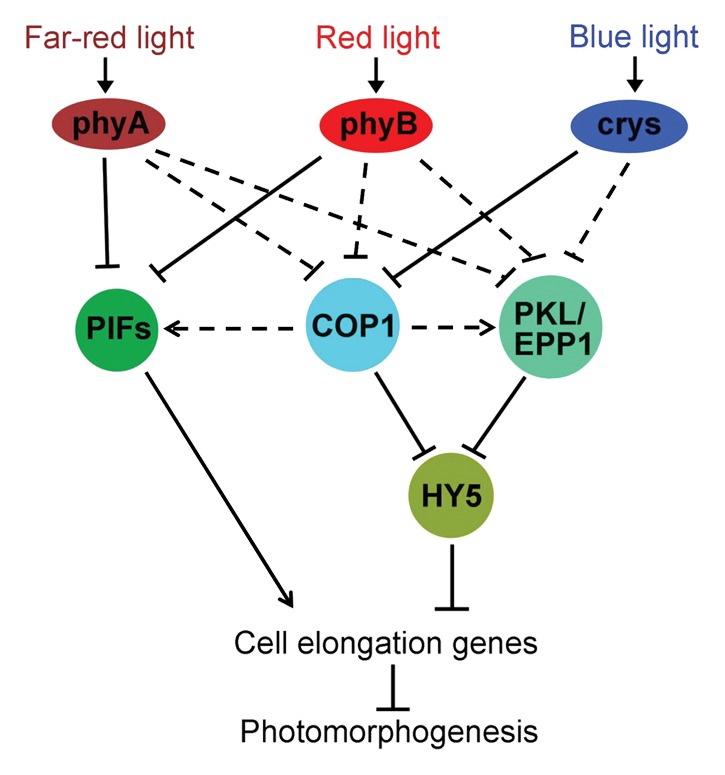
Figure 4. A simplified model of photomorphogenic pathway in Arabidopsis. Different spectrum of light are absorbed by the corresponding photoreceptors (phytochromes and cryptochromes) and the light signals are then transduced to suppress three main types of negative factors, including COP1, PIFs and PKL/EPP1. COP1 represses HY5 through mediating its protein turnover, while EPP1 inhibits the transcriptional activity of HY5. PIFs (including PIF1, PIF3, PIF4 and PIF5) and HY5 are transcription factors that promote or suppress the transcription of cell elongation genes, respectively. Solid lines indicate a direct regulation, while dotted lines denote an indirect effect. Modified from Lau and Deng.9
Chromatin remodeling plays critical roles in specifying gene expression and maintaining transcriptional states in yeast, plants and animals.14 Strikingly, the identification of EPP1 uncovers the chromatin remodeling-mediated regulatory mechanism by which plants precisely respond to light environment in a temporal and/or spatial manner. Future efforts on identifying more factors and elucidating various histone modification mechanism involved in epigenetic controlling of light signaling are highly desired. Previous studies suggest that light integrates hormonal signaling pathways to modulate diverse growth and development and that EPP1 is involved in regulating multiple processes.9,15 It will be interesting to investigate whether and how EPP1 integrates multiple signalings to modulate a particular developmental program.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (31000530) to Y.J. and (31170221) to R.L.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25026
References
- 1.Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117. doi: 10.1146/annurev.genet.38.072902.092259. [DOI] [PubMed] [Google Scholar]
- 2.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–30. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 3.Lau OS, Deng XW. Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol. 2010;13:571–7. doi: 10.1016/j.pbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yi C, Deng XW. COP1 - from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–25. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, et al. Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell. 2013;25:242–56. doi: 10.1105/tpc.112.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogas J, Kaufmann S, Henderson J, Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:13839–44. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–6. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 9.Lau OS, Deng XW. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 2012;17:584–93. doi: 10.1016/j.tplants.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Hoecker U, Tepperman JM, Quail PH. SPA1, a WD-repeat protein specific to phytochrome A signal transduction. Science. 1999;284:496–9. doi: 10.1126/science.284.5413.496. [DOI] [PubMed] [Google Scholar]
- 11.Hoecker U, Quail PH. The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signaling in Arabidopsis. J Biol Chem. 2001;276:38173–8. doi: 10.1074/jbc.M103140200. [DOI] [PubMed] [Google Scholar]
- 12.Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, et al. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17:2642–7. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh E, Zhu J-Y, Wang Z-Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol. 2012;14:802–9. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Bishop B, Ringenberg W, Muir WM, Ogas J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 2012;159:418–32. doi: 10.1104/pp.112.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]


