Abstract
Cooper questions one specific technical aspect of our study—the site of cleavage in EIN2—and suggests that cleavage of EIN2 likely occurs elsewhere. Here, we explain how our immunoblotting, mass spectrometry and genetic mutation studies justify our conclusions.
We recently reported1 that the Arabidopsis thaliana ethylene signaling pathway component EIN2 is located in the ER and upon perception of the gaseous plant hormone ethylene, CTR1 dependent phosphorylation of EIN2 is prevented, resulting in cleavage of the EIN2 C-terminus and movement of the cleavage product (EIN2 C′) to the nucleus with concomitant activation of ethylene responses. Since the publication of our study, two other groups have independently confirmed these major conclusions.2,3 In his “Perspective,” Cooper4 questions one specific aspect of our study which was not reported in either of the two other studies–the site of cleavage in EIN2. His concerns center on results we provided in three areas: (1) our observed size of the EIN2 C′ fragment, (2) the results of our mass spectrometry methods for detection of the EIN2 cleavage site and (3) the conclusions of genetic mutational analysis of the site of EIN2 cleavage (S645). Below we address his concerns for each of these experiments.
Regarding the first concern, we used sodium dodecyl sulfate PAGE (SDS-PAGE), followed by immunoblotting using EIN2 antibodies to measure the size of the cleavage product. Our studies indicate an approximate size (80 kDa) for EIN2-C′ which Cooper points out is different from the expected size based on conceptual translation of DNA sequence (70 kDa). In our view, this minor difference is not unexpected since it is well known that SDSPAGE is only able to approximate the size of many proteins but specific proteins can often show aberrant mobility (see http://en.wikipedia.org/wiki/SDS-PAGE). One of the well-understood reasons for such size discrepancies is differential levels of SDS binding to proteins. The amino acid composition of each protein is unique and the different amino acid side chains cause each protein to bind SDS with varying affinity. Different proteins can bind from 1.1–2.2 g of SDS per gram of protein. These differences in SDS binding can cause significant differences in the observed mobility of the protein compared with the predicted protein size. Different buffer systems can cause small differences in the migration of proteins; in particular, integral membrane proteins like EIN2 may show aberrant migration in SDS PAGE. Finally, addition of posttranslational modifications might alter the electrophoretic mobility of EIN2. However, while we described multiple sites of phosphorylation in EIN2, we have not explored any of the other possible post-translational modifications that could affect the migration mobility of the protein.
The second concern is that the mass spectrometry (MS) data reported in our study for EIN2 does not agree with MS data for EIN2 from his laboratory.5 From our unpublished studies of the proteome of ethylene-treated plants, we observed that EIN2 ranks low in overall abundance ~1,200 out of 5,000 Arabidopsis leaf and root proteins and also the background of the protein/peptide extract is complex. Thus, we believe that the disputed 2-fold difference in peptide level compared with his published results is not significant enough to draw any conclusions. Cooper asserts that we should have observed the EIN2 630-645 peptide by whole proteome profiling but there are several possible reasons that it was missed. First, although modern MS based proteomics has advanced dramatically, it is not yet a comprehensive tool able to detect or identify all proteins/peptides in a complex biological sample. It is very rare to have full protein sequence coverage from a complex sample, even for abundant proteins. In addition, different peptides have different ionization and fragmentation efficiencies, which determine their signal intensity. Basic amino acids (K or R) usually enhance the ionization efficiency. So it is reasonable to predict that the EIN2 630-645 peptide has lower ionization efficiency than the fully tryptic 630-647 peptide and, thus, is less detectable. Second, it is possible that only a fraction of the EIN2 protein molecules in a cell undergo de-phosphorylation/cleavage/translocation at any given time. Thus, it is not difficult to understand our inability to identify the EIN2 630-645 peptide from global MS protein profiling data. Therefore, as reported in our study, to confirm the precise site of cleavage in EIN2, we employed a more sensitive targeted method (pMRM), which allowed us to detect and quantify the EIN2 630-645 peptide with higher confidence1 (Fig. 3C in reference 1).
Regarding Cooper’s third concern, we provided genetic evidence showing that S645 is essential for cleavage of EIN2.1 Specifically we created two versions of EIN2 using genetic mutations (S646A and S645E), to test whether genetic alteration of this residue would affect cleavage of EIN2, but only one of these mutations is mentioned by Cooper. The S645A mutation prevents phosphorylation, whereas the S645E mutation mimics phosphorylation. In our study, each mutated EIN2 protein along with the wild type EIN2 was fused to YFP and the CaMV promoter and each version of EIN2 was expressed in ein2 mutant plants. We used the CaMV 35S promoter for expression in plants since results from our previous studies,6,7 and our current study1 (Fig. 4C and D in reference 1) show expression of EIN2 with this promoter produces strong ethylene response phenotypes which are dependent on the nuclear localization of EIN21 (Fig S1 in reference 1). This then allows a direct comparison of the effect of these two mutations (S645A and S645E) on (1) nuclear localization of the C′ fragment using fluorescence microscopy and biochemically, using nuclear purification followed by immunoblotting for EIN2 and (2) observe and compare visible phenotypes of the EIN2S645A plants with those of the EIN2S645E plants. As we reported, the EIN2 S645A mutation resulted in constitutive cleavage1 (Fig. 4G in reference 1), nuclear localization1 (Fig. 4F in reference 1) and visible ethylene phenotypes in the absence of ethylene1 (Fig. 4C and D in reference 1). Importantly, the sizes of the EIN2 C′ protein observed in wild type and in the constitutive mutant EIN2S645A were identical1 (Fig. 4H in reference 1), indicating that the substitution at position 645 by alanine does not affect the cleavage profile of the protein in contrast to Cooper's suggestion.
It is argued by Cooper that these conditions (CaMV 35S expression of EIN2) caused greater than normal levels of this fragment, calling into question the “importance” of the S645 site relative to other reported phosphorylation sites in EIN2.2 This argument misses a key point and the purpose for conducting this experiment, which is not mentioned in Cooper’s technical comment. Our aim was to test the requirement of EIN2 S645 for cleavage by directly comparing the effect of these two mutations (S645A and S645E) on subcellular trafficking of EIN2. We observed that the phosphomimic mutant (S645E) plants (also expressed using the CaMV 35S promoter), showed no ethylene response phenotypes1 (Fig. 4C and D in reference 1), no nuclear translocation1 (Fig. 4F in reference 1) and no EIN2 cleavage product even in the presence of ethylene1 (Fig. 4G in reference 1) providing strong genetic evidence for the requirement of this single site (S645) for cleavage. This contradicts with Cooper’s hypothesis that simply overexpressing EIN2 (S645E or S645A) would mimic S924A to produce the ethylene response phenotype.
We are aware that the EIN2 S924A mutation also causes strong ethylene response phenotypes.2 However, this result doesn’t conflict with our results. We would argue that one site is not “more important” than the other as suggested by Cooper but, rather, each plays a unique role in the ethylene response. While evidence for a functional role of the S924 site was not reported2 we suspect that phosphorylation at this residue may affect protein stability. This hypothesis is based on our earlier studies which identified two F-box proteins (ETP1/ETP2) that play important roles in EIN2 stability.7 Thus, we propose that in plants expressing the EIN2 S924A mutation, even at native levels there is a greater amount of EIN2 protein accumulated, thus mimicking the ethylene environment. However, S924A alone is not sufficient for EIN2 processing because in the EIN2 S645E mutant, no nuclear localization of EIN2 YFP occurs.1
Lastly, although not presented in our paper, we have observed that EIN2 is an unstable protein. If the protein sample is treated using harsh conditions, such as boiling at 100 degrees for 10 min, multiple EIN2 bands are detected, as reported in reference 3. However, if the protein samples are treated using mild conditions, the majority of these extraneous bands do not appear. For example, Figure 1 shows protein extracts of EIN2-C1-YFP-HA transgenic plants where multiple smaller bands are detected after the sample is treated at 100 degrees for 10 min (Fig. 1A, gel 1). However most of the smaller EIN2 bands are missing from the samples treated at 65 degrees for 10 min (Fig. 1A, gel 2). Similarly, as shown in Figure 1B, protein extracts of EIN2S645A-YFP-HA transgenic plants treated at 100 degrees for 10 min also show multiple smaller EIN2 fragments (Fig. 1B, lane 1). However, all of these smaller bands are missing in the samples treated at 65 degrees for 10 min, with a concomitant accumulation of full length EIN2-C′ (Fig. 1B, lane 2). Additionally, we found that in experiments conducted using fresh tissue (not stored at −80 degrees), the lower EIN2 fragments were missing (data not shown). Therefore, we conclude that the multiple smaller EIN2 fragments are mostly likely artifacts created by degradation of the larger EIN2 C′ or full length EIN2 proteins.
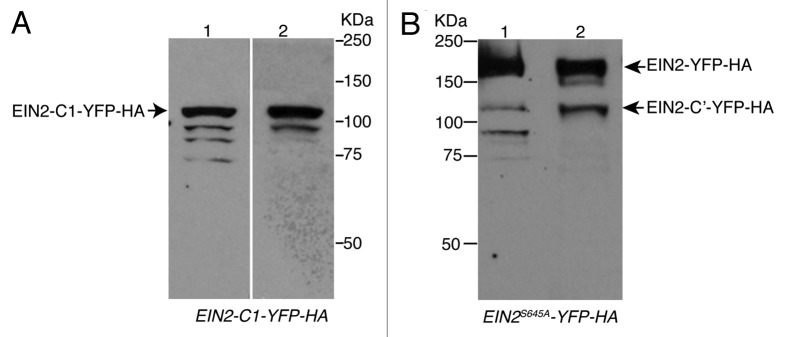
Figure 1. Comparison of EIN2 protein mobility in samples treated with different conditions. (A) Western blot of protein extracts prepared from EIN2-C1-YFP-HA tissues using the sample treated with 100 degree for 10 min (track 1) or treated with 65 degree for 10 min (track 2). (B) Western blot of protein extracts prepared from EIN2-YFP-HA and EIN2-C′-YFP-HA using the sample treated with 100 degree for 10 min (lane 1) or treated with 65 degree for 10 min (lane 2).
In summary, we believe that the combined biochemical, genetic and cell biological results reported in our study justify our conclusion that EIN2 is cleaved at position S645 in response to ethylene.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25037
References
- 1.Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, et al. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science. 2012;338:390–3. doi: 10.1126/science.1225974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA. 2012;109:19486–91. doi: 10.1073/pnas.1214848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, et al. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res. 2012;22:1613–6. doi: 10.1038/cr.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper B. Separation anxiety: An analysis of ethylene-induced cleavage of EIN2. Plant Signal Behav. 2013;8:e24721. doi: 10.4161/psb.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Binder BM, Garrett WM, Tucker ML, Chang C, Cooper B. Proteomic responses in Arabidopsis thaliana seedlings treated with ethylene. Mol Biosyst. 2011;7:2637–50. doi: 10.1039/c1mb05159h. [DOI] [PubMed] [Google Scholar]
- 6.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–52. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 7.Qiao H, Chang KN, Yazaki J, Ecker JR. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 2009;23:512–21. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]


