Abstract
Studies on the basic mechanisms that regulate vacuolar delivering of proteins synthesized in the endoplasmic reticulum (ER) have a great importance in plant cell biology. Indeed, many aspects of plant physiology are affected by this intracellular traffic, for example, germination or reaction to biotic stresses due to the accumulation of storage proteins in seeds or enzymes in vegetative tissues, respectively. Up to now, the Golgi complex has been considered the main hub in the sorting of vacuolar secretory proteins; those polypeptides able to reach their final destination without the aid of this organelle are regarded as exceptions to an established route. This mini-review aims to emphasize the existence of several Golgi-independent pathways involved in the trafficking of different types of vacuolar proteins.
The secretory pathway is an important and fascinating process that regulates key functions of cell biology in eukaryotes by exporting proteins from the endoplasmic reticulum (ER) to their target compartments. In plant cells, among all of the routes that form this varied and integrated system, the most studied is the one which transfers proteins from the ER to the vacuole. The importance of this route is based on the fact that the most consistent food source for human beings arises from secretory proteins accumulated in the vacuoles of edible plant seeds. Recently, protein transfer to the vacuole has been extensively characterized and, in many cases, it has been observed that vacuolar proteins reach the vacuole by endomembrane progression through the Golgi using secretory vesicles.1-4 Generally, those pathways that start from the ER and reach the vacuole bypassing the Golgi complex have been reported to as exceptions to the classical Golgi path. These alternative transport mechanisms are usually activated in particular cellular situations, with time limit because they occur during plant development or in response to stress, or with tissue specificity due to their occurrence only in particular cell types. One of the key factors that appear to characterize these alternative pathways is related to the formation of protein aggregates,5 but soluble or tonoplast membrane proteins can be also directly delivered to the vacuole. However, the classical Golgi pathway turns out to be only one route of a complex system of pathways which deliver proteins to the vacuoles, thus it is not the predominant mechanism for vacuolar protein transport.6-8 Here we want to give a brief overview of the direct ER-vacuole transport of secretory proteins.
Unconventional Vacuolar Deliver of Tonoplast Membrane Proteins
Tonoplast transporters and membrane channels play an important role in determining and regulating vacuolar functions such as the maintenance of cellular turgor, the accumulation of reserve and defense substances, the regulation of intracellular signaling. Soluble proteins that enter the secretory pathway are secreted by default unless they contain sorting signals that would direct them to a specific cellular compartment.9 What about membrane proteins? In the absence of a specific sorting signal, membrane proteins are targeted to plasma membrane regarding it as the default membrane in plants. Therefore, specific signals identified in the cytosolic N- or C-terminal domains of the protein itself are needed to reach the tonoplast.10 Transport mechanisms in plants are further complicated by the presence of two types of vacuoles: one is the lytic vacuole (LV), analogous to the lysosome of animal cells, which occupies most of the cellular volume and is found mainly in vegetative tissues; the other is the protein storage vacuole (PSV), used for the storage of nutrients and mostly present in reproductive tissues such as seeds. These two types of vacuoles can also coexist within the same cell in particular types of tissue (root tips for example) or into protoplasts.11The discovery that the soluble vacuolar proteins and the tonoplast ones could be targeted to the vacuole by two different mechanisms was made 20 years ago, based on the cellular traffic of two vacuolar bean proteins expressed in tobacco protoplasts: the tonoplast α-TIP aquaporin and the soluble phytohemagglutinin (PHA).12 In that study the addition of the brefeldin A (BFA) drug in the cell medium, a fungal toxin that negatively affects Golgi-mediated protein traffic,13 blocked the transport of PHA to the vacuolar lumen whereas produced no effect on the α-TIP transport to the tonoplast, thus showing a Golgi-independent, BFA-insensitive traffic for this aquaporin. In the following years, studies have highlighted, through the use of chimerical constructs14 and more recently thanks to a novel inhibitory chemical affecting the BFA-insensitive pathway,15 that the same tonoplast aquaporins may reach their final destination by using different mechanisms, one is Golgi-dependent (γ-TIP) and the other is Golgi-independent (α-TIP). Similarly, in a recent study on the rice two-pore K+ channels (TPK), it is demonstrated that the TpKa isoform present in the LV reaches the tonoplast in a Golgi-dependent manner. Conversely, the TpKb isoform, located on the surface of PSV-like compartments, traffics in a BFA-insensitive manner indicating a Golgi-independent transport.16 In addition, Isayenkov and colleagues identified three C-terminal amino acids that are heavily involved in determining the TPK proteins addressing to the lytic or storage vacuoles probably thanks to post-translational modifications, such as phosphorylation. The use of mutants that inhibit the formation of the COPII vesicles, capable of mediating the outgoing traffic from the ER to the Golgi complex, has evidenced their involvement even in the Golgi-independent route to the tonoplast.10 One exception is represented by the calcineurin B-like protein 6 (CBL6): this protein, localized in the tonoplast of tobacco protoplasts and leaves, traffics to the vacuole in a COPII-independent manner. However, CBL6 does not show clear addressing signals to the ER, so perhaps it reaches the vacuolar membrane directly from the cytosol.17,18In plant cell, the existence of at least two different paths involved in protein trafficking from the ER to the tonoplast is now well established. It remains to be determined if the pathway type (Golgi-mediated or Golgi-independent and BFA-sensitive or insensitive) is related to the vacuole type that the protein have to reach (LV or PSV, respectively).
ER-Bodies Mediate Golgi-Independent Protein Transport to the Vacuole
In plant cells, the existence of alternative pathways to the main vacuolar sorting mechanism which proceeds through the Golgi apparatus is well documented. For example, there are some proteins that, independently from their own solubilization characteristics, originate aggregates in the ER named ER bodies which are then directly transported to the vacuole.6,19 Generally, these ER bodies are larger in diameter than the secretory vesicles like the Golgi-derived dense vesicles (DVs, diameter of ~0.1 µm) or the clathrin-coated vesicles (CCVs, diameter of 0.05 to 0.07 µm). Indeed, two main types of proteins have been reported to follow this route: storage proteins and enzymes. The first type of proteins form spherical aggregates which range in size from 0.2 to 1.8 µm and are detected mainly in cereal seed tissues. These aggregates are formed during storage protein accumulation, above all from middle to late stages of seed maturation. Seed storage proteins can be transported directly from the ER to protein storage vacuoles (PSVs), apparently by atypical autophagic processes, as reported for γ-gliadins in wheat endosperm,20 glutelins and α-globulins in rice endosperm,21 glycinin (11S) in soybean cotyledons,22 zeins, α-globulin and legumin-1 in maize aleurone.23 The authors suggested differerent names for the observed ER bodies like protein bodies (PBs)20 or prevacuolar compartments (PVCs).23 Takahashi and colleagues21 adopted the name PAC-like vesicles, because rice vesicles are similar to the ER bodies containing precursors of 2S storage proteins described in pumpkin seeds and designated precursor-accumulating (PAC) vesicles.24 This route which bypasses the Golgi system seems to be linked to the specific transport of proteins that form large aggregates, which are then directly fused with the vacuolar compartment during seed maturation.5 Golgi-independent pathways can be cell-specific as shown in maize seeds for zeins, which accumulate in PBs inside the ER in the endosperm but are sequestered into complex PVCs and then delivered to the PSVs in the aleurone cells.23 In this work it is suggested that Golgi-independent pathways of seed storage proteins can represent special cases of selective autophagy. The second type of proteins, mainly cysteine proteases, accumulate in precursor protease vesicles (PPVs) and have been described both in the epidermis of Arabidopsis thaliana vegetative tissues25,26 and seedlings of both Vigna mungo and Ricinus communis.27,28 These vacuolar enzymes are stored as precursors in spindle-shaped ER-bodies with a length up to 5–10 µm. In the epidermis of A. thaliana seedlings, the cysteine proteases RD21 and the vacuolar processing enzyme-γ (VPEγ) are present in PPVs, which fused to vacuoles in presence of salt stress.25 VPEγ is involved in the maturation of the vacuolar protease carboxypeptidase Y (CPY) and in the degradation of invertase AtFruct4 in vacuoles.26 Therefore, it was suggested that PPVs fusion with the acidic vacuolar lumen activate processes related to cell death, stresses, protein processing and degradation. Indeed, in V. mungo the cysteine proteinase SH-EP is responsible for the degradation of storage proteins and in germinated seeds accumulates in PPVs sorted to PSVs.27 The ER-derived protein accretions, initially observed in A. thaliana by Hayashi and colleagues,25 have been given a more general term: ER body/fusiform body.29 The reason is that these bodies accumulate not only proteases but also high amounts of β-glucosidases and are likely to be involved in the defense against pathogens and herbivores. During aggregate formation of abundant β-glucosidases in the ER, RD21 and VPEγ may be trapped into the ER bodies and then directly delivered to the vacuole via ER-bodies.30 Recently, several specific membrane proteins have been described in A. thaliana both in ER bodies (MEB1 and MEB2)31 and in ER-compartments morphologically distinct from spindle-shaped bodies (ATI1 and ATI2).7 Deep studies of such membrane proteins can help us to identify the multiple functions that ER bodies appear to have in plants.
Delivery of Soluble Proteins Directly from ER to the Vacuole, an Exception Which May Become Routine
We have already described how some membrane proteins and protein aggregates are delivered from ER to the vacuole bypassing the Golgi complex, but this type of transport is also used by some soluble proteins. The first evidence that the classical secretory pathway was not sufficient to explain the traffic of all the vacuolar proteins in plant, was found by Gomord and colleagues.32 They showed that replacement of the vacuolar sorting signal of sporamin with the ER retention signal HDEL led to an accumulation of this protein in the ER, but a detectable amount of the protein was still delivered to the vacuole. Unfortunately, the authors did not indicate the protein transport route used by recombinant sporamin to reach the vacuole. In a study performed later by other researchers, the same ER retention signal was fused to another vacuolar glycoprotein (phaseolin), which ultimately produced the same result obtained with sporamin. However, in this case, the vacuolar phaseolin fraction showed no the typical glycan modification induced by the trans-Golgi enzymes, suggesting that phaseolin-KDEL used an alternative sorting mechanism.33 A third study has shown that different ER resident proteins containing the HDEL domain reach the vacuole without passing through the Golgi complex.34 ER resident proteins appear to be sent to the vacuole for their degradation, using paths that may or may not involve the Golgi complex.34,35 The presence of an alternative, Golgi-independent, sorting system to the lysosome has been demonstrated also in mammalian cells for the soluble proteins EDEM1 and OS-9 which are key regulators of ER-associated degradation machinery (ERAD). These proteins do not contain the classic ER retention signal (K/HDEL) and their escape from the ER seems to be spatially restricted to specific exit sites.36,37 In this case, vacuolar sorting of EDEM1 and OS-9 modulates their ER accumulation and consequently their activities, suggesting that EDEM1 and OS-9 vacuolar deliver is not merely a degradative process. Therefore, the secretory pathway in eukaryotic cells seems to be a dynamic system that involves the use of different sorting solutions according to the requirements of the cell during a given time.38 Recently, it has been suggested that the sorting of certain vacuolar proteins that follow the classic secretory pathway does not occur at the Golgi complex level, but it has been decided earlier, perhaps already at the ER level.39,40 It is quite reasonable to think that this ER characteristic in protein sorting could be also used in the vacuolar deliver of proteins that bypass the Golgi apparatus. Observing the behavior of a human lysosomal enzyme (α-mannosidase) expressed in plant cells, a new Golgi-independent vacuolar deliver of a soluble protein has been revealed.8 In this paper it has been demonstrated that this glycoprotein is completely transported to the vacuole in a BFA-insensitive manner. Moreover, the processed α-mannosidase vacuolar fragments are not modified by Golgi enzymes, suggesting that this protein uses an alternative route which bypasses this compartment. This study demonstrates that α-mannosidase vacuolar transport is an unconventional route of a functionally active enzyme in plant cells and it is not due to a sorting mechanism for defective proteins. In addition, α-mannosidase appears to use an N-terminal amino acidic signal able to redirect, to the same alternative pathway, a protein that is normally secreted in the extracellular space. This last result indicates that, in plants, may exist ER located receptors able to interact to peptide sequences of several soluble proteins directly transported to the vacuole. A similar receptor that binds 2S albumin in maturing pumpkin seeds, called PV72, has been described by Shimada and collegues.41 PV72 is a BP80-related isomer present in membrane of PAC vesicles, which bud off the ER and fuse directly with the vacuole, thus bypassing the Golgi apparatus.24
Conclusions
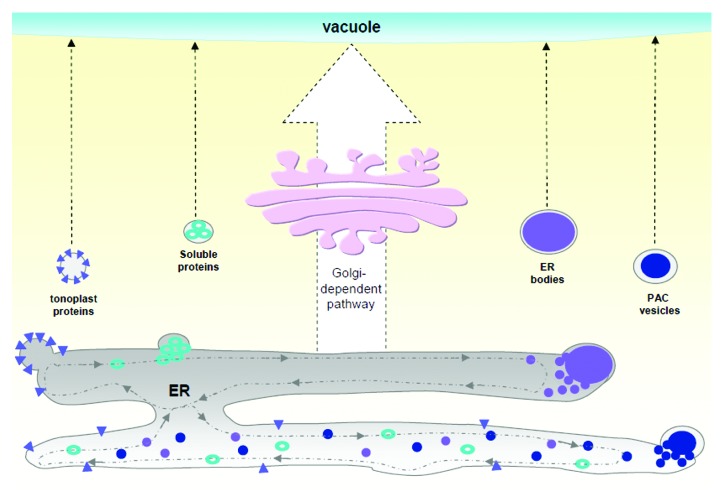
Once imported in the ER, secretory vacuolar proteins undergo an allocation process mediated by several factors related to their chemical/physical characteristics or due to interactions with other proteins. The spatial segregation along the ER sub-domains of these vacuolar polypeptides is likely determined during this selective process and this segregation can also affect the kind of pathways used by proteins to reach the vacuole (Fig. 1).5,36 Therefore, the ER seems to play a major role in sorting vacuolar proteins to their final destinations. The secretory routes to transfer vacuolar proteins can be divided between those which contemplate endomembrane progression through the Golgi (classical Golgi route) and those which bypass the Golgi and directly transport secretory proteins from ER to the vacuole (unconventional route). This last mechanism works with certain types of polypeptides in some physiological situations or particular groups of plant cells. The arising question is why some proteins reach the vacuole without using the classical Golgi route? A possible answer is that Golgi-independent pathways can be very fast or very efficient in transferring proteins and therefore are preferable in situations where the cell has to upregulate protein expression.38 This could be an efficient system which the cell can use in special situations such as in response to biotic or abiotic stresses,25,29 as well as during storage protein accumulation in seeds.5,23 Another hypothesis is that several vacuolar enzymes are imported in the ER and then transported directly to the vacuole, in order to avoid eventual harmfulness to other proteins during the transfer process. For example, bypassing the Golgi in tobacco cells can be important for human α-mannosidase, because in this way it is prevented from possible alterations to the oligosaccharides of the glycosylated proteins present in the Golgi.8 In conclusion, even if the determinants that regulate this unconventional route are not yet well characterized, we think that its importance for the regulation of cell functionality in eukaryotes is becoming more and more evident. Figure 1
Figure 1. . Schematic representation of Golgi-independent pathways involved in the transport of different types of vacuolar proteins. The Golgi-dependent pathway is only one route for delivering proteins to the vacuole. Alternatively, some types of proteins are transported directly to the vacuole, probably after an allocation process that takes place at the ER level.
Acknowledgments
We are grateful to Dr Frantisek Baluska for kindly inviting this review. This work has been partially supported by Fondazione Cassa di Risparmio di Perugia, project “2012.0197.021 Ricerca scientifica e tecnologica,” and by the COST Action FA0804, Molecular Farming: Plants as a Production Platform for High Value Proteins.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25129
References
- 1.Vitale A, Hinz G. Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 2005;10:316–23. doi: 10.1016/j.tplants.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Hwang I. Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol. 2008;148:673–83. doi: 10.1104/pp.108.124925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Rogers JC, Jiang L. Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. FEBS J. 2011;278:59–68. doi: 10.1111/j.1742-4658.2010.07923.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiang L, Etxeberria E, Van den Ende W. Vacuolar protein sorting mechanisms in plants. FEBS J. 2013;280:979–93. doi: 10.1111/febs.12092. [DOI] [PubMed] [Google Scholar]
- 5.Herman EM. Endoplasmic reticulum bodies: solving the insoluble. Curr Opin Plant Biol. 2008;11:672–9. doi: 10.1016/j.pbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Herman EM, Schmidt M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol. 2004;136:3440–6. doi: 10.1104/pp.104.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honig A, Avin-Wittenberg T, Ufaz S, Galili G. A new type of compartment, defined by plant-specific Atg8-interacting proteins, is induced upon exposure of Arabidopsis plants to carbon starvation. Plant Cell. 2012;24:288–303. doi: 10.1105/tpc.111.093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Marchis F, Bellucci M, Pompa A. Traffic of human α-mannosidase in plant cells suggests the presence of a new endoplasmic reticulum-to-vacuole pathway without involving the Golgi complex. Plant Physiol. 2013;161:1769–82. doi: 10.1104/pp.113.214536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrispeels MJ. Sorting of proteins in the secretory system. Annual Review of Plant Physiology and lant. Mol Biol. 1991;42:21–53. [Google Scholar]
- 10.Pedrazzini E, Komarova NY, Rentsch D, Vitale A. Traffic routes and signals for the tonoplast. Traffic. 2013;14:622–8. doi: 10.1111/tra.12051. [DOI] [PubMed] [Google Scholar]
- 11.Paris N, Stanley CM, Jones RL, Rogers JC. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–72. doi: 10.1016/S0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- 12.Gomez L, Chrispeels MJ. Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell. 1993;5:1113–24. doi: 10.1105/tpc.5.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, et al. Protein quality control along the route to the plant vacuole. Plant Cell. 1997;9:1869–80. doi: 10.1105/tpc.9.10.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Rogers JC. Integral membrane protein sorting to vacuoles in plant cells: evidence for two pathways. J Cell Biol. 1998;143:1183–99. doi: 10.1083/jcb.143.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Serrano EE, Rodriguez-Welsh MF, Hicks GR, Rojas-Pierce M. A small molecule inhibitor partitions two distinct pathways for trafficking of tonoplast intrinsic proteins in Arabidopsis. PLoS ONE. 2012;7:e44735. doi: 10.1371/journal.pone.0044735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isayenkov S, Isner JC, Maathuis FJ. Rice two-pore K+ channels are expressed in different types of vacuoles. Plant Cell. 2011;23:756–68. doi: 10.1105/tpc.110.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batistic O, Waadt R, Steinhorst L, Held K, Kudla J. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J. 2010;61:211–22. doi: 10.1111/j.1365-313X.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 18.Bottanelli F, Foresti O, Hanton S, Denecke J. Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell. 2011;23:3007–25. doi: 10.1105/tpc.111.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrispeels MJ, Herman EM. Endoplasmic reticulum-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles. Plant Physiol. 2000;123:1227–34. doi: 10.1104/pp.123.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levanony H, Rubin R, Altschuler Y, Galili G. Evidence for a novel route of wheat storage proteins to vacuoles. J Cell Biol. 1992;119:1117–28. doi: 10.1083/jcb.119.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K. A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol. 2005;46:245–9. doi: 10.1093/pcp/pci019. [DOI] [PubMed] [Google Scholar]
- 22.Mori T, Maruyama N, Nishizawa K, Higasa T, Yagasaki K, Ishimoto M, et al. The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins. Plant J. 2004;40:238–49. doi: 10.1111/j.1365-313X.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 23.Reyes FC, Chung T, Holding D, Jung R, Vierstra R, Otegui MS. Delivery of prolamins to the protein storage vacuole in maize aleurone cells. Plant Cell. 2011;23:769–84. doi: 10.1105/tpc.110.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M. Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell. 1998;10:825–36. doi: 10.1105/tpc.10.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, et al. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42:894–9. doi: 10.1093/pcp/pce144. [DOI] [PubMed] [Google Scholar]
- 26.Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV. A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2003;100:7389–94. doi: 10.1073/pnas.1230987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyooka K, Okamoto T, Minamikawa T. Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J Cell Biol. 2000;148:453–64. doi: 10.1083/jcb.148.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C. The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2001;98:5353–8. doi: 10.1073/pnas.061038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada K, Hara-Nishimura I, Nishimura M. Unique defense strategy by the endoplasmic reticulum body in plants. Plant Cell Physiol. 2011;52:2039–49. doi: 10.1093/pcp/pcr156. [DOI] [PubMed] [Google Scholar]
- 30.Hara-Nishimura I, Matsushima R, Shimada T, Nishimura M. Diversity and formation of endoplasmic reticulum-derived compartments in plants. Are these compartments specific to plant cells? Plant Physiol. 2004;136:3435–9. doi: 10.1104/pp.104.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada K, Nagano AJ, Nishina M, Hara-Nishimura I, Nishimura M. Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol. 2013;161:108–20. doi: 10.1104/pp.112.207654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomord V, Denmat LA, Fitchette-Lainé AC, Satiat-Jeunemaitre B, Hawes C, Faye L. The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 1997;11:313–25. doi: 10.1046/j.1365-313X.1997.11020313.x. [DOI] [PubMed] [Google Scholar]
- 33.Frigerio L, Pastres A, Prada A, Vitale A. Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell. 2001;13:1109–26. doi: 10.1105/tpc.13.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K, Yamada K, Shimada T, Hara-Nishimura I. Endoplasmic reticulum-resident proteins are constitutively transported to vacuoles for degradation. Plant J. 2004;39:393–402. doi: 10.1111/j.1365-313X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- 35.Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J. Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell. 2006;18:198–211. doi: 10.1105/tpc.105.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuber C, Cormier JH, Guhl B, Santimaria R, Hebert DN, Roth J. EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites. Proc Natl Acad Sci USA. 2007;104:4407–12. doi: 10.1073/pnas.0700154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calì T, Galli C, Olivari S, Molinari M. Segregation and rapid turnover of EDEM1 by an autophagy-like mechanism modulates standard ERAD and folding activities. Biochem Biophys Res Commun. 2008;371:405–10. doi: 10.1016/j.bbrc.2008.04.098. [DOI] [PubMed] [Google Scholar]
- 38.Grieve AG, Rabouille C. Golgi bypass: skirting around the heart of classical secretion. Cold Spring Harb Perspect Biol. 2011;3:a005298. doi: 10.1101/cshperspect.a005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pompa A, De Marchis F, Vitale A, Arcioni S, Bellucci M. An engineered C-terminal disulfide bond can partially replace the phaseolin vacuolar sorting signal. Plant J. 2010;61:782–91. doi: 10.1111/j.1365-313X.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- 40.Niemes S, Labs M, Scheuring D, Krueger F, Langhans M, Jesenofsky B, et al. Sorting of plant vacuolar proteins is initiated in the ER. Plant J. 2010;62:601–14. doi: 10.1111/j.1365-313X.2010.04171.x. [DOI] [PubMed] [Google Scholar]
- 41.Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I. A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 1997;38:1414–20. doi: 10.1093/oxfordjournals.pcp.a029138. [DOI] [PubMed] [Google Scholar]