Abstract
Background
The medicinal leech is considered as a complementary and appropriate model to study immune functions in the central nervous system (CNS). In a context in which an injured leech’s CNS can naturally restore normal synaptic connections, the accumulation of microglia (immune cells of the CNS that are exclusively resident in leeches) has been shown to be essential at the lesion to engage the axonal sprouting. HmC1q (Hm for Hirudo medicinalis) possesses chemotactic properties that are important in the microglial cell recruitment by recognizing at least a C1q binding protein (HmC1qBP alias gC1qR).
Material/Methods
Recombinant forms of C1q were used in affinity purification and in vitro chemotaxis assays. Anti-calreticulin antibodies were used to neutralize C1q-mediated chemotaxis and locate the production of calreticulin in leech CNS.
Results
A newly characterized leech calreticulin (HmCalR) has been shown to interact with C1q and participate to the HmC1q-dependent microglia accumulation. HmCalR, which has been detected in only some microglial cells, is consequently a second binding protein for HmC1q, allowing the chemoattraction of resident microglia in the nerve repair process.
Conclusions
These data give new insight into calreticulin/C1q interaction in an immune function of neuroprotection, suggesting another molecular target to use in investigation of microglia reactivity in a model of CNS injury.
MeSH Keywords: Head Injuries, Closed; Complement C1q; Calreticulin; Microglia
Background
Microglial cells constitute the resident immune cells, maintaining the integrity of the nervous system by constantly surveying the environment [1,2]. Under pathological conditions, they rapidly change and turn to amoeboid activated microglia in order to migrate over long distances and accumulate at lesions [3].
The microglia in the leech Hirudo medicinalis were the first cells to be named “microglia” by del Rio-Hortega using his silver carbonate method [4,5] and are considered as the first microglia model [6]. Leech microglial cells represent the only cell population capable of moving through the nerve cord. Otherwise, they cannot be mistaken with other glial cells that have a larger size and a different shape. Because individual sensory cells regenerate new synaptic connections with a high degree of specificity after a section [7,8], the involvement of microglia was investigated in that natural nerve repair process. The leech nerve cord is tubular and is not vascularized within the blood sinus [9,10]. In this context, by using an isolated and injured segment of nerve cord maintained in tissue culture, some authors reported that synapse regeneration is possible following the accumulation of resident microglia at the crush site and without any blood cell [11]. Even in the presence of blood sinus, a very low infiltration of blood cells is observed in injured CNS, which highlights the importance of the resident microglia at the lesions. Their accumulation has been shown to be essential for the usual sprouting of injured axons [12]. The mobility of activated resident microglia has been associated with morphological changes from stellate to rounded shape, comparable to those of activated microglia in mammals [13], and is dependent on specific chemotactic signals [10,14–16]. Among these chemotactic factors, we demonstrated the role of HmC1q (for Hirudo medicinalis) in the microglial cell accumulation after leech CNS injury [17].
In mammals, it is well established that C1q recognizes the C1qR(P) receptor to contribute to the phagocytosis of debris by microglia [18,19]. Some authors showed the importance of C1q in the microglial activation [20] and a neuroprotective role for C1q in early inflammatory response [21], but the C1q-dependent recruitment of mammalian microglia at lesions is still poorly understood. The chemotactic properties of C1q have been shown towards blood immune cells [22–24] and some authors specified that such chemotactic activity occurs through the recognition of both gC1qR (alias C1qBP) and calreticulin (also known as cC1qR, CalR, or CRT) receptors on peripheral dendritic cells [25].
In the leech CNS, HmC1q contributes to the microglial recruitment by interacting with a recently characterized receptor, named HmC1qBP, which is homologous to mammalian gC1qR [26]. The present report shows the involvement of a second receptor in the C1q-dependent microglial activation. A molecule homologous to known calreticulins was characterized and shown to interact with HmC1q. This report establishes that the presence of HmCalR in the leech microglia represents a part of the C1q-dependent reactivity. It also contributes to new insight into C1q/calreticulin functions in the CNS.
Material and Methods
Leech CNS and microglial cell preparation
H. medicinalis adult leeches were obtained from Ricarimpex (Eysines, France). The leech nerve cord (CNS) is constituted by the head ganglion, 21 body ganglia, and 7 fused tail ganglia. The ganglia are joined by structures, called connectives, containing the axonal processes and glial cells [9]. After anesthesia in 10% ethanol at 4°C for 15 min, animal CNS were dissected out in a sterile Ringer solution (115 mM NaCl, 1.8 mM CaCl2, 4 mM KCl, 10 mM Tris maleate, pH 7.4) under a laminar flow hood. After isolation, samples were placed in 3 successive baths of antibiotics (10 UI/ml penicillin, 10 μg/ml streptomycin, and 10 μg/ml gentamicin) for 15 min and further incubated in Leibovitz L-15 medium (Invitrogen, USA) containing 2 mM L-glutamin, 0.6% glucose, and 10 mM Hepes (complete medium). The experimental injury was performed by crushing the connectives between the third and fourth ganglia of an isolated fragment. Nerve cords were used for fluorescence in situ hybridization, whole mount immunohistochemistry, or nerve cell preparation.
For microglial cell isolations, nerve cords treated as indicated above were placed in 35-mm Petri dishes with 200 μl of complete L-15 medium. Each ganglion was carefully decapsulated by removing, with micro-scissors, the collagen layer enveloping the nerve cord. Nerve cells, neurons, and microglial cells were mechanically dissociated by gentle scraping (total nerve cells). After a filtration through 7-μm nylon mesh as described [13,26], the enriched microglial cell population was then collected and centrifuged at 1000 × g for 10 min at RT. The cell pellet was resuspended in L-15 medium (100 μl per nerve cord) for migration assays.
Molecular characterization of HmCalR
The analysis of Hirudo medicinalis genome allowed in silico prediction of mRNA databases according to intro-exon boundaries. Based on the candidate sequence detected, forward and reverse primers were designed to frame the complete sequence of predicted mRNA. From total RNA extracted from leech nerve cord using TRIzol® reagent and according to the manufacturer’s procedure (Invitrogen, USA), cDNA were synthesized using an oligo(dT) priming. The calreticulin-related molecule was amplified by PCR using the specific forward (5′GGTAGCAATACGTGCAGTTTG3′) and reverse (5′GCAACCAAGAGTAGGCAACC3′) primers and the Platinum® Taq DNA Polymerase according to the manufacturer’s instructions (Invitrogen, USA). Selected PCR products were ligated into pGEM T-easy vector and cloned into JM109 cells according to the manufacturer’s instructions (Promega, USA). Finally, products were sequenced using BigDye Terminator v3.0 polymerization kit before detection on Genetic Analyzer (Applied Biosystems, USA). BLAST programs were used for sequence analysis in databases and comparison with initial predicted mRNA sequence [27,28]. Phylogenetic analysis was carried out by Geneious® Basic v5.6 software [29].
Fluorescent in situ hybridization (FISH)
Nerve cords were fixed for one hour at 4°C in 4% paraformaldehyde just after dissection. The 5′ biotin-labeled specific antisense probe and sense probe (negative control) were generated from the 842–1479 nucleotides sequence of hmcalr mRNA (Genbank Accession Number KF709537). After PCR amplification and the insertion of the product in pGEM-T easy vector system (Promega, USA), the RNA sequence of interest was obtained by in vitro transcription using DIG/Biotin RNA-labeling kit according to the manufacturer’s instructions (Roche, Switzerland). The hybridization protocol was performed as previously described [30]. Nerve cords were incubated with a secondary anti-biotin antibody conjugated to Alexa Fluor 488 (dilution 1: 5000 in PBS) (Invitrogen, USA). Samples were rinsed with PBS. Prior to mounting with Glycergel (Sigma Life Science, USA), the cell nuclei were counterstained by Hoechst 33342 fluorescent dye (1:1,000, Invitrogen USA) for 10 min. Slides were kept at 4°C in the dark until observation with a Zeiss LSM700 confocal microscope.
Immunohistochemistry
In experiments with anti-human calreticulin antibody, analyses were performed on nerve cords dissected out as described above. They were fixed for 1 hour at 4°C – immediately after dissection (T=0) or 24 h (T24h) after incubation in complete L-15 medium – in 4% paraformaldehyde, washed in PBS, permeabilized by a 24-h incubation at RT in 1% Triton X100 in PBS and pre-incubated for 8 h at RT in 1% Triton, 3% Normal Donkey Serum (NDS) and 1% ovalbumin in PBS. Samples were then incubated overnight at 4ºC with specific rabbit polyclonal anti-human calreticulin antibodies (Santa Cruz Biotechnology, USA) diluted in a PBS solution (1:250) containing 1% BSA, 0.05% Triton, 1% NDS, and 1% ovalbumin. The anti-calreticulin antibodies were directed against an antigen sequence corresponding to the Lys248-Leu417 region of human calreticulin, presenting 81% homology with leech calreticulin. After 3 washes with PBS, samples were incubated 1 h at room temperature with anti-rabbit donkey antibody (Invitrogen, USA) conjugated to Alexa Fluor 488 (1:2000) in a PBS solution containing 1% BSA, 0.05% Triton, 1% NDS, and 1% ovalbumin. Final rinsing and mounting steps for confocal microscopy observation were performed as described above. Prior to mounting, the cell nuclei were counterstained by Hoechst 33342 fluorescent dye (1: 1000, Invitrogen USA) for 10 min. Samples without the addition of primary antibody were used as negative control.
Human C1q biotinylation and streptavidin affinity purification
The biotinylation of the recombinant human C1q (Prospecbio, USA) was carried out by using the Sulfo-NHS-SS-Biotin kit (Pierce, USA) according to the manufacturer’s instructions. Unreacted Sulfo-NHS-SS-Biotin was removed using the Zeba Desalt Spin Columns (Pierce, USA).
Biotinylated human C1q was immediately fixed onto a streptavidin column (Pierce, USA), previously equilibrated with 5 volumes of PBS 0.1M. The interaction between biotin and streptavidin occurred at RT for 10 min. Microglia protein extract (800 μg) was added in the column, incubated overnight at 4°C, and rinsed 10 times with PBS 0.1M. Captured microglial cell proteins were eluted from the streptavidin-agarose with 5% 2-mercaptoethanol-PBS 0.1 M at 30°C for 30 min. Proteins were precipitated in 10% trichloroacetic acid/acetone at −20°C for 45 min and centrifuged at 13000 × g for 15 min. The protein pellet was washed in cold acetone, air dried and dissolved in Læmmli buffer. Two other columns were used for the negative controls: the first containing the biotinylated human C1q without any microglia protein extract, and the second containing only the microglia protein extract to evaluate the unspecific reaction between streptavidin and microglial cell components. Samples were loaded on a 12% SDS-PAGE. Separated proteins were transferred to Amersham™ Hybond™-ECL nitrocellulose (GE Healthcare, France). The membrane was incubated for 30 min at RT in blocking solution (0.05% Tween, milk powder 5% w/v in PBS) and then overnight at 4°C in rabbit polyclonal anti-human calreticulin antibodies (Santa Cruz Biotechnology, USA), diluted at 1:5000 in blocking solution containing 1% ovalbumin. After rinsing with PBS-0.05% Tween, the membrane was incubated for 1 h at RT in secondary goat anti-rabbit polyclonal antibodies conjugated with horseradish peroxidase (Jackson Immunoresearch, USA) (dilution 1:20000). The signal was revealed using the ECL Kit SuperSignal West Dura chemoluminescent substrate (Pierce, USA) on Kodak X-Omat LS film (Sigma-Aldrich, USA).
Chemotaxis assays
In vitro chemotaxis assays were performed by using the double-P assay as described by Köhidai et al. with minor modifications [31]. Thirty-five millimeter Petri dishes were filled with 1 ml of a 0.5% agar and 1% gelatin solution. After drying, two 6-mm diameter wells were done, each presenting a parallel individual channel. One well was filled with 50 μl of purified microglial cells (see above) and the other with chemotactic factor or negative controls reagents. A channel was further created perpendicularly to others using a coverslip. One hour later, cells in the well containing chemoattractant were collected. Either vehicle (non-transformed Pischia pastoris culture supernatant) or recombinant HmC1q (rHmC1q) produced in P. pastoris [17] alone or with soluble CalR were used (8 μl) as chemotactic factors. For inhibitory chemotactic experiments, cells were pre-incubated for 1 h at RT either with rabbit polyclonal anti-human calreticulin antibodies (1:1000) or with rabbit IgG isotype as negative control (1:1000). The number of migrating cells was counted on a hemocytometer (3 different counts) under an Axioskop microscope (Zeiss, Germany). Experiments were done in triplicate. The results are expressed as the mean cell number ±S.D. Comparisons between means were made using Student’s t-test. Statistical differences were considered to be significant if p was <0.01.
Results
Molecular characterization of HmCalR
In mammals, C1q exerts a chemotactic activity on dendritic cells through different receptors: a gC1qR recognizing the globular head of C1q and a cC1qR recognizing the collagen domain of C1q. The presence of a putative calreticulin (cC1qR) was investigated in the leech H. medicinalis. Based on the H. medicinalis genome analysis, a sequence homologous to known calreticulin sequences was detected. The design of specific primers then allowed the amplification of a full-length mRNA sequence for a calreticulin-related molecule, named HmCalR (for Hirudo medicinalis Calreticulin). Hmcalr mRNA (Genbank KF709537) encodes a 420-amino-acid sequence with a theoretical molecular weight of 48 610 Da. The protein sequence presents a putative signal peptide and a calreticulin domain in Met1-Ser19 and in Val23-Ile334 amino-acid regions, respectively (Figure 1A). In addition, the analysis of HmCalR protein using a BLAST-P program shows a high conservation with human calreticulin, particularly in different domains that are already described for mammalian calreticulins [32]. As presented in Figure 1B, HmCalR might possess a single disulfide bridge (Cys107-Cys139) and contains a single histidine residue (His172). These residues were also described in an N-domain of the human mature calreticulin in Cys88-Cys120 and His153 positions, respectively, (numerated from the signal peptide-free sequence) and were demonstrated as essential for the chaperoning function [33,34]. The HmCalR sequence is also Proline-rich and exhibits 2 repeated amino-acid sequences – repeats A (PxxIxDPDAxKPEDWDE) and B (GxWxPPxIxNPxYx) – as observed in the human form’s P-domain (Figure 1B). In leeches, some non-essential amino acids have been substituted with different ones having equivalent physicochemical properties (Glu214 instead of Asp in human repeat A; and Val268 instead of Ile in human repeat B). Finally, the high conservation of numerous acidic residues in HmCalR suggests the presence of a C-domain, as in mammals.
Figure 1.
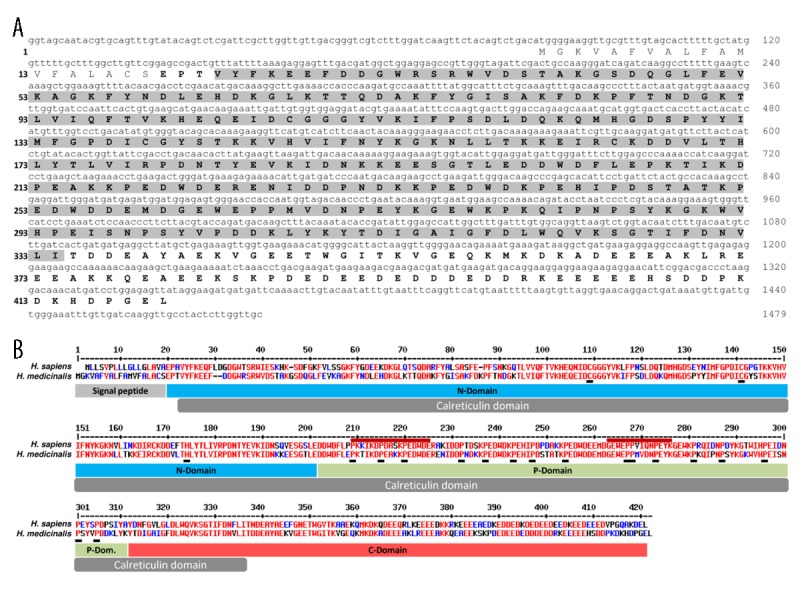
Characterization of a calreticulin-related molecule (HmCalR) in the medicinal leech. (A) Nucleotide and amino acid sequences of leech HmCalR. Numbers of nucleotides and amino acids are indicated on right and left of the sequence, respectively. The protein sequence contains a putative signal peptide (1–19) and a mature form (20–420) containing a Calreticulin-conserved domain (23–334) highlighted in light grey. (B) Sequence alignment with human calreticulin. High and low consensus homologies are represented by red and blue residues, respectively. Signal peptide and calreticulin domain are indicated by grey boxes. The 3 significant domains from human form (N-, P- and C-domains) are indicated by blue, green, and red boxes, respectively. Critical residues (Cysteines and Histidine in N-domain, and Proline in P-domain) are indicated by black boxes. Two amino-acid sequences, named repeats A (PxxIxDPDAxKPEDWDE) and B (GxWxPPxIxNPxYx) in human calreticulin are specified by the upper red lines.
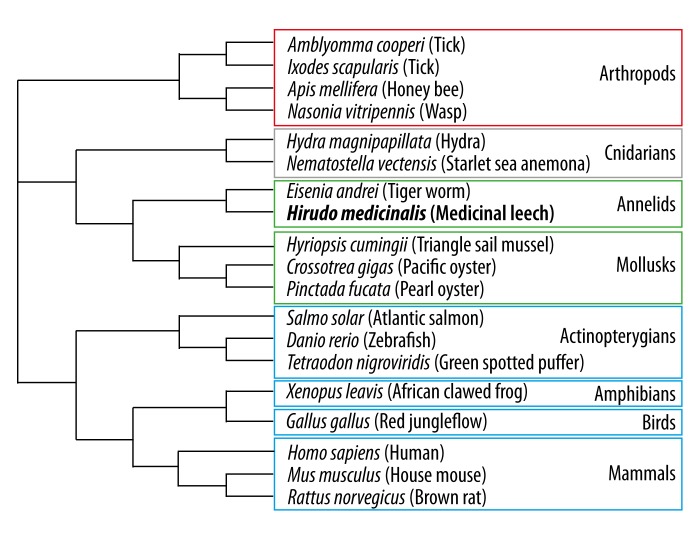
Thus HmCalR presents sequence similarities with mammalian calreticulins and also with other forms from vertebrate, protostomian, and diploblastic species, as revealed by a neighbor-joining phylogenetic tree using selected sequences among them (Figure 2). Depending on the 200 highest similarities between calreticulin molecules and HmCalR, some were chosen to check the evolutive position of HmCalR according to species. The distribution of sequences in the cladogram respects the species organization between vertebrates and protostomians. The neighbor-joining phylogenetic tree shows that leech calreticulin is positioned in lophotrochozoan organisms sharing the same origin with Eisenia andrei, another annelid organism, and also close to mollusks. Arthropods have calreticulins that form a distinct group in protostomians, which is relevant in their distinction from the lophotrochozoans. Otherwise, although diploblastic organisms, cnidarians present higher similarities in calreticulin sequences with lophotrochozoans than with those of arthropods. Finally, vertebrate calreticulins are positioned in a distinct clade.
Figure 2.
Neighbor-joining phylogenetic tree relating amino acid sequences of Hirudo medicinalis calreticulin (HmCalR) and calreticulins of selected species. Calreticulin amino acid sequences were chosen in the 200 highest similarities after a BLAST-P program obtained from the NCBI. The calreticulin sequences from the following species (including protein accession numbers) present a respective homology with HmCalR: Amblyomma cooperi (AAR29934) 70.5%, Ixodes scapularis (AAQ18696) 69.5%, Apis mellifera (XP_392689) 67.9%, Nasonia vitripennis (NP_001155151) 63.4%, Hydra magnipapillata (XP_002161300) 66.4%, Nematostella vectensis (XP_001640172) 77.1%, Eisenia andrei (ABI74618) 80%, Hyriopsis cumingii (AFR69202) 75.2%, Crassostrea gigas (BAF63639) 76.5%, Pinctada fucata (ABR68546) 75.7%, Salmo salar (ACI33338) 66.2%, Danio rerio (NP_956007) 68.8%, Tetraodon nigroviridis (CAG07986) 69.7%, Xenopus laevis (NP_001080765) 69.5%, Gallus gallus (AAS49610) 69.0%, Homo sapiens (NP_004334) 71.8%, Mus musculus (NP_031617) 72%, and Rattus norvegicus (NP_071794) 71.2%.
Localization of hmcalr mRNA and HmCalR protein in leech microglia
Cells expressing hmcalr transcripts in the leech nervous system were investigated by specific fluorescence in situ hybridization (FISH) on injured nerve cords after different times. The transcripts were detected in only some scattered microglial cells among all microglial cells (blue nuclei) in the connectives 24 h after lesion. The labeling appears as a punctuated signal (Figure 3A). No specific signal was detected with sense riboprobes used as negative control (Figure 3A’).
Figure 3.
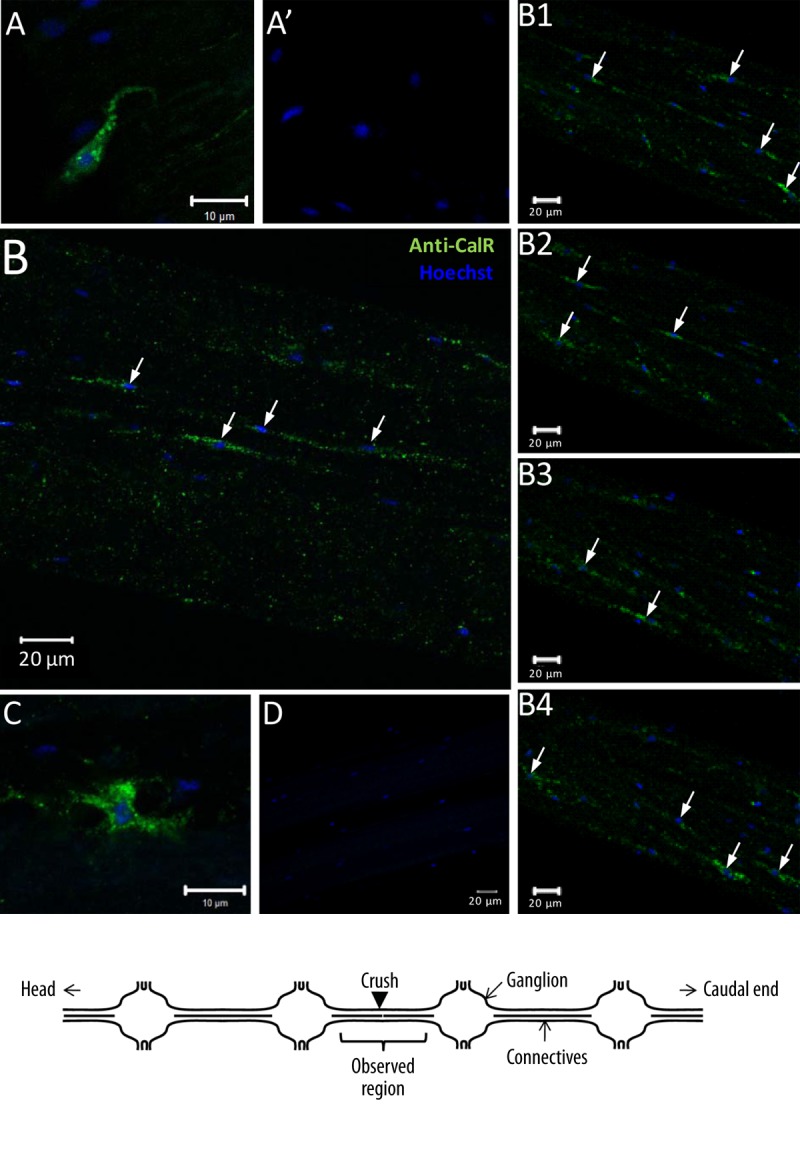
Hmcalr mRNA and HmCalR protein distribution in isolated segments of leech nervous system (see the diagram below). (A) Fluorescence in situ hybridization on leech connective. Confocal microscopy images showed hmcalr mRNA localization (green) in microglial cells using antisense riboprobes. (A’) Sense probes were used as negative controls. (B and C) Fluorescent immunostaining of HmCalR protein in leech CNS detected at T=0 (B) or T24h post-lesion (C) using rabbit polyclonal anti-human calreticulin antibodies showed immunopositive signal (green) for some microglial cells (arrows). (B1–B4) Immunostaining analyses of successive focal plans in the connective tissues at T=0 allowed the observation of more positive microglial cells (arrows). (D) No immunostaining was observed using secondary antibodies alone as negative control. In any experiment, microglial cell nuclei (blue) were counterstained with Hoechst fluorescent dye.
Cells containing calreticulin protein were investigated by immunohistochemistry with rabbit polyclonal anti-human calreticulin antibodies on whole mounted injured nerve cords immediately after dissection (Figure 3B–3B4) and 24 h after lesion (Figure 3C, 3D). The microglia nuclei were simultaneously counterstained by using a Hoechst dye (blue nuclei). Immediately following the crush (T=0), calreticulin staining (green) was detected in only some microglial cells in the connectives among the whole microglia (blue nuclei). The HmCalR-positive microglia appeared spindle-shaped and very elongated, suggesting cell mobility. The labeling appeared vesicular (Figure 3B). Only a few microglial cells in the connective seemed to be positive for calreticulin, but the analyses of successive focal plans in the connectives allowed the observation of more microglial cells among the whole connective (Figure 3B–3B4). Twenty-four hours after injury, when the acute microglia migration declines, the calreticulin staining was detected in some ramified microglial cells distributed in the injured connectives (Figure 3C). In addition, the labeling was mainly localized around the nucleus and seemed to be vesicular in the microglia cytoplasm (Figure 3C). Negative controls performed using the secondary antibodies alone did not show any signal (Figure 3D).
Binding properties between microglial HmCalR and C1q
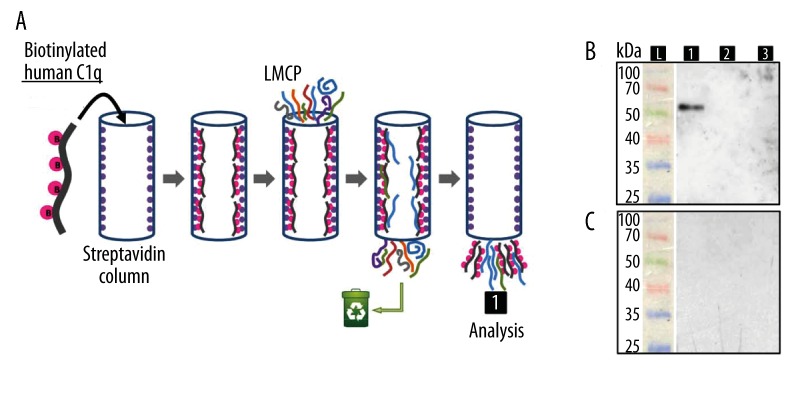
Calreticulin (alias cC1qR) and C1qBP (alias gC1qR) bind to the collagen domain and globular head, respectively, of C1q [35]. Because previous results demonstrated an interaction between HmC1q and HmC1qBP in the leech CNS, co-purification experiments were performed to assess the putative presence of HmCalR [26]. Once biotinylated, human C1q was immobilized on a streptavidin column and incubated with leech microglia protein extracts (Figure 4A). Following the elution, the interactants of C1q were analyzed by Western blotting using polyclonal anti-human CalR antibodies (Figure 4B) that specifically recognized a unique ~55 kDa molecule (Figure 4B, lane1), probably corresponding to HmCalR due to the 81% identity of both leech and human antigenic sequences. This analysis presents a shift between the observed size and the expected one (48 610 Da for HmCalR containing the signal peptide). This difference of migration in SDS-PAGE was already reported for other calreticulins [36–39] and is probably due to the high content of acidic residues in the C-terminal end of the sequence. In the first negative control, when microglia protein extracts were incubated on a streptavidin column alone (Figure 4B, lane 2), no signal was obtained, showing that anti-human CalR antibodies do not react with leech microglial proteins non-specifically captured by streptavidin column. In the second negative control, when the biotinylated human C1q was loaded alone on streptavidin column (Figure 4B, lane 3), no immunoreactivity was detected, indicating that the anti-human CalR antibodies do not react with the human C1q. No signal was observed in negative controls using only the secondary antibody (Figure 4C). These results gave evidence of a specific interaction between human C1q and HmCalR protein present in leech microglia protein extract.
Figure 4.
Interaction between C1q and HmCalR. (A) Biotinylated human C1q was fixed onto a streptavidin column. LMCP (Leech Microglial Cell Protein) extracts were then added in the column. Captured microglial cell proteins by C1q were eluted from the streptavidin-agarose and precipitated in trichloroacetic acid/acetone to be further analyzed by Western blotting. (B) The eluted samples were applied to SDS-PAGE and analyzed by Western blotting using rabbit polyclonal anti-human calreticulin antibodies. (C) Same samples were analyzed by Western blotting using only the secondary antibody as negative control. The complete procedure (1) was compared to a negative control using only LMCP extracts without C1q (2) and a negative control using biotinylated human C1q without any LMCP extract (3) to evaluate unspecific reactions between eluted molecules and anti-CalR. Molecular weight protein ladder (L).
Involvement of calreticulin/HmC1q interaction for in vitro microglia chemotaxis
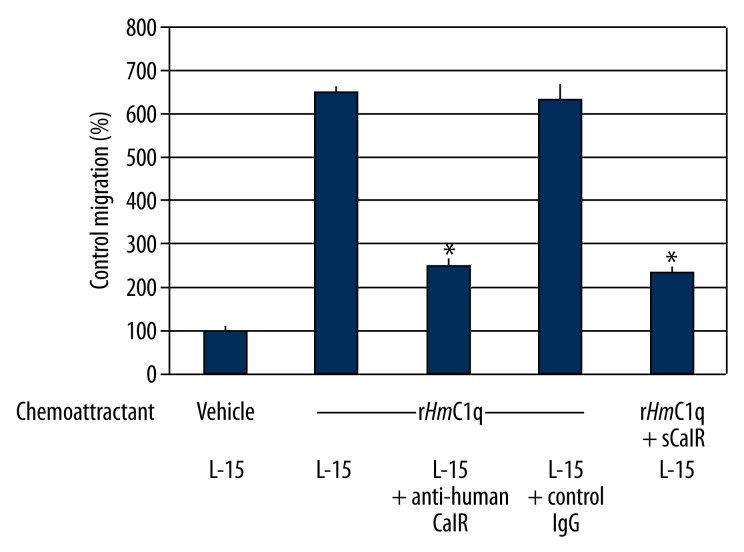
C1q has been shown to recruit leech microglial cells in a dose-dependent manner [17]. In the present study, leech microglial cells in L-15 medium were demonstrated to migrate towards the recombinant form of HmC1q (rHmC1q) about 6-fold more than towards vehicle (Figure 5). The pre-incubation of microglial cells with rabbit polyclonal anti-human calreticulin antibodies showed a significant decrease of the rHmC1q chemotactic effect (from 6-fold to 2.5-fold), whereas no significant inhibitory effect was detected with the rabbit IgG isotype as negative control. The microglial recruitment was also strongly reduced (from 6-fold to 2.3-fold) when rHmC1q was used in association with soluble CalR (sCalR) on cells in L-15 medium.
Figure 5.
Inhibitory effect of soluble CalR (sCalR) and rabbit polyclonal anti-human calreticulin antibodies on HmC1q-mediated leech microglia chemotaxis assays. From leech microglial cells incubated alone in Leibovitz medium (L-15), the vehicle (negative control migration =100%) was compared as a chemoattractant to recombinant rHmC1q (positive control) and to rHmC1q mixed with sCalR. The chemotactic effect of rHmC1q alone as chemoattractant was also evaluated on cells alone (L-15) or pre-incubated with either rabbit polyclonal anti-human CalR antibodies or rabbit IgG control. Asterisks denote that cell migrations of the indicated samples were significantly different (p<0.01) than those using rHmC1q alone on cells alone (L-15). All results were obtained from 3 independent experiments by condition.
Discussion
As we previously described in the medicinal leech, the C1q molecule called HmC1q is important for the recruitment of microglial cells within the hours following a lesion of the nerve cord [17]. We also reported that HmC1q exerts its chemotactic functions on leech microglia by recognizing at least one receptor homologous to human gC1qR (C1qBP) [26].
In mammals, the interaction between C1q and receptors such as gC1qR (C1qBP) and calreticulin (cC1qR) has so far been reported for dendritic cell chemoattraction [25] but never for microglia recruitment. We investigated in the leech CNS the existence of a calreticulin molecule and its involvement in microglial accumulation at the site of injury.
In the present report, the characterization of HmCalR in the medicinal leech showed high sequence similarities with other calreticulins as presented in the neighbor-joining phylogenetic tree of selected calreticulins. That corroborates the fact that calreticulin (CalR or CRT) is widely distributed in Metazoa. The result respects the actual phylogenetic systematics, except for the cnidarian sequences that appeared close to lophotrochozoan ones.
Then we focused on a comparison of the different domains between the leech and the human forms. Human calreticulin is a 46-kDa chaperone protein containing 3 structurally and functionally distinct domains (N-, P-, and C-domains) [40–42]. The N- and P-domains in mammalian calreticulins are associated to the lectin-like chaperoning function [33,43], whereas the C-domain is involved in Ca2+ storage in the endoplasmic reticulum through its highly acidic properties [44]. The alignment between leech and human sequences shows that they share several of these structural features [33,34]. The leech molecule HmCalR presents all the critical residues determining the 3 domains, including cysteines, probably forming a disulfide bridge, and the essential histidine responsible for the chaperoning function. Despite the lack of a structural analysis of HmCalR in this report, the high conservation of critical residues suggests some similarities with human calreticulin for domain-dependent functions. Among these functions, calreticulin plays an important role in immune responses by acting as a receptor for C1q, mannose-binding lectins, and ficolins [25,45,46].
The presence of hmcalr mRNA and HmCalR protein in only some microglial cells along the nerve cord led us to better understand the involvement of this molecule in neuro-immune processes. Beside the use of calreticulin in C1q-dependent chemoattraction of dendritic cells [25], immune functions for calreticulin have ever been reported in the literature. During a viral infection in crustaceans, for example, C1q receptors such as calreticulin or gC1qR are differentially produced in crustacean immune cells and help regulate the replication of viruses [47,48]. In mammals, the presence of calreticulin at the surface of cells can induce the attraction of macrophages and dendritic cells through a C1q-dependent interaction. Thus, the exposure of calreticulin can be used as a strong phagocytic signal leading to the recognition of calreticulin+ cells [49,50]. In this context, parasites such as Trypanosoma cruzi expose calreticulin to facilitate its recognition by host macrophages and optimize its internalization [51]. Those binding properties between calreticulin and C1q mainly concern peripheral immune mechanisms. Recently, they were also suggested on nerve cells where calreticulin exposure on neurons was required to allow their in vitro phagocytosis by BV2 microglia cell line [52].
In the leech CNS, even if HmCalR has not been detected in neurons so far, the affinity purification experiments from microglial protein extracts demonstrated the interaction of C1q with HmCalR through its specific detection using the polyclonal anti-human calreticulin antibodies. Then, both results in chemotaxis assays clearly indicated that this HmC1q/HmCalR interaction contributes to C1q-dependent chemotactic properties on microglia.
In this report, due to HmCalR staining in a few microglial cells observed at lesions, the use of such a molecule as a receptor for HmC1q can be restricted to a reactive microglia subpopulation. Thus, independently of the maturity of microglial cells, the limited exposure of HmCalR might be relevant to other known receptors. Indeed, we previously showed that the presence of leech gC1qR (HmC1qBP) concerned only a subpopulation of microglial cells accumulated at lesions [26]. Thus, HmC1q recognizes at least 2 receptors allowing the microglial recruitment. Our data have never permitted the observation of a dual localization of both HmC1qBP and HmCalR in the same microglial cells. Therefore, the action of HmC1q allows the chemoattraction of distinct microglia subtypes at the lesion site. Further studies will aim to specify their respective functions towards the damaged neurons.
In mammals, C1q is the first component of the classical complement pathway. In early brain development, it mediates the CNS synapse elimination during neurogenesis [53]. In adult CNS disorders, C1q is also released by activated microglia to maintain and regulate their activation [20,54,55]. Among the mediators expressed by microglial cells and neurons, C1q seems to be a key molecule in neuroinflammatory diseases [56,57] and in various neurodegenerative pathologies such as Alzheimer disease [58–62]. Importantly, some studies recently showed that C1q also has complement cascade-independent activities [63,64]. C1q exerts (independently of its interaction with C1r and C1s) a neuroprotective effect against Amyloid-β plaques [21,65,66]. Of interest, our data in the leech suggest the importance of C1q in a non-classical complement pathway because the analysis of H. medicinalis genome revealed neither C1r- nor C1s-related molecule. By taking into account the importance of microglia accumulation at a lesion to engage the axonal sprouting in the leech CNS [12], HmC1q might recruit specific microglial cells that lead to a neuroprotective process.
In addition, other chemotactic factors allow the microglial accumulation at a lesion in the leech CNS [13,67] and also use specific receptors. Thus, HmC1q, as well as HmIL-16 and HmEMAPII, allow the accumulation of microglia. So we must now consider the leech microglia as a mixture of reactive microglia subpopulations where distinct sets can be recruited through the influence of different chemotactic molecules. In this context, microglia probably contribute to different pro- and/or anti-inflammatory mechanisms by communicating with other glial cells and neurons. Further studies will investigate the discrimination of microglia subpopulations that are recruited to the lesions. They will specify the time course of the accumulation and the specific functions of subpopulations.
Conclusions
The complexity of immune responses following a CNS damage is enhanced by multiple cell origin and activation states of microglia [68]. In mammals, the resident microglia that result from the invasion processes during embryonic neurogenesis are helped by infiltrated bone marrow-derived cells and circulating monocytes during CNS diseases [69]. Nevertheless, despite in vitro cell analyses, morphological and/or histological in vivo studies do not permit discrimination of the resident and infiltrated cell types [70]. Besides the cell origin, different functional profiles can simultaneously act and exhibit pro-inflammatory features (classical activation, M1) or anti-inflammatory features (alternative activation, M2) depending on the recruitment of specific microglia subtypes at lesions [71]. In this context, comparative immune studies may help to distinguish specific microglial responses.
The medicinal leech as a model in nerve repair has been used for decades because it represents a new insight into study of the cell processes engaged during the axonal sprouting [72]. In addition, the leech microglia has been demonstrated to be essential for efficient nerve repair [12]. Because they are not supported by a significant infiltration of blood cells, microglial cells in the leech represent an alternate model of interest in comparative microglial studies. According to dynamic and functional properties, the microglia subpopulations may be actively involved in the repair capabilities. The impact of microglia recruitment under the influence of HmCalR/HmC1q interaction will be studied to specify the microglial contribution in CNS inflammatory regulation.
Acknowledgements
Authors would like to thank Dr. Elodie Richard of the CCMIC-Université Lille 1 (BICeL) and Pr Natalia Prevarskaya (INSERM U1003, Université Lille 1) for access to confocal microscopy facilities.
Footnotes
Source of support: This work was supported by grants from Le Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (MENESR, France) and from L’Agence Nationale de la Recherche (MIMIC, ANR-11-JSV4-005)
References
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–18. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 2.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 4.Del Rio-Hortega P. La microglia y su transformacion en células en bastoncito y cuerpos granulo-adiposos. Trab del Lab de Invest Biol. 1920;18:37. [in French] [Google Scholar]
- 5.Del Rio-Hortega P. Cytology and cellular pathology of the nervous system. In: Penfield W, editor. Microglia. P B Hoebaer; New York, NY: 1932. pp. 483–534. [Google Scholar]
- 6.Sieger D, Peri F. Animal models for studying microglia: The first, the popular, and the new. Glia. 2013;61:3–9. doi: 10.1002/glia.22385. [DOI] [PubMed] [Google Scholar]
- 7.Baylor DA, Nicholls JG. Patterns of regeneration between individual nerve cells in the central nervous system of the leech. Nature. 1971;232:268–70. doi: 10.1038/232268a0. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JK, Nicholls JG. Regeneration and changes in synaptic connections between individual nerve cells in the central nervous system of the leech. Proc Natl Acad Sci USA. 1972;69:636–39. doi: 10.1073/pnas.69.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggeshall RE, Fawcett DW. The Fine Structure of the Central Nervous System of the Leech, Hirudo Medicinalis. J Neurophysiol. 1964;27:229–89. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- 10.Le Marrec-Croq F, Drago F, Vizioli J, et al. The Leech Nervous System: A Valuable Model to Study the Microglia Involvement in Regenerative Processes. Clinical and Developmental Immunology. 2013;2013:12. doi: 10.1155/2013/274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgese VJ, Elliott EJ, Muller KJ. Microglial movement to sites of nerve lesion in the leech CNS. Brain Res. 1983;272:166–70. doi: 10.1016/0006-8993(83)90375-x. [DOI] [PubMed] [Google Scholar]
- 12.Ngu EM, Sahley CL, Muller KJ. Reduced axon sprouting after treatment that diminishes microglia accumulation at lesions in the leech CNS. J Comp Neurol. 2007;503:101–9. doi: 10.1002/cne.21386. [DOI] [PubMed] [Google Scholar]
- 13.Croq F, Vizioli J, Tuzova M, et al. A homologous form of human interleukin 16 is implicated in microglia recruitment following nervous system injury in leech Hirudo medicinalis. Glia. 2010;58:1649–62. doi: 10.1002/glia.21036. [DOI] [PubMed] [Google Scholar]
- 14.Arafah K, Croix D, Vizioli J, et al. Involvement of nitric oxide through endocannabinoids release in microglia activation during the course of CNS regeneration in the medicinal leech. Glia. 2013;61:636–49. doi: 10.1002/glia.22462. [DOI] [PubMed] [Google Scholar]
- 15.Duan Y, Sahley CL, Muller KJ. ATP and NO dually control migration of microglia to nerve lesions. Dev Neurobiol. 2009;69:60–72. doi: 10.1002/dneu.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuels SE, Lipitz JB, Dahl G, Muller KJ. Neuroglial ATP release through innexin channels controls microglial cell movement to a nerve injury. J Gen Physiol. 2010;136:425–42. doi: 10.1085/jgp.201010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahtouh M, Croq F, Vizioli J, et al. Evidence for a novel chemotactic C1q domain-containing factor in the leech nerve cord. Mol Immunol. 2009;46:523–31. doi: 10.1016/j.molimm.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR(P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–29. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 19.Webster SD, Yang AJ, Margol L, et al. Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol. 2000;161:127–38. doi: 10.1006/exnr.1999.7260. [DOI] [PubMed] [Google Scholar]
- 20.Farber K, Cheung G, Mitchell D, et al. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J Neurosci Res. 2009;87:644–52. doi: 10.1002/jnr.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–43. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leigh LE, Ghebrehiwet B, Perera TP, et al. C1q-mediated chemotaxis by human neutrophils: involvement of gClqR and G-protein signalling mechanisms. Biochem J. 1998;330(Pt.1):247–54. doi: 10.1042/bj3300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuna P, Iyer M, Peerschke EI, et al. Human C1q induces eosinophil migration. Clin Immunol Immunopathol. 1996;81:48–54. doi: 10.1006/clin.1996.0156. [DOI] [PubMed] [Google Scholar]
- 24.Ghebrehiwet B, Kew RR, Gruber BL, et al. Murine mast cells express two types of C1q receptors that are involved in the induction of chemotaxis and chemokinesis. J Immunol. 1995;155:2614–19. [PubMed] [Google Scholar]
- 25.Vegh Z, Kew RR, Gruber BL, Ghebrehiwet B. Chemotaxis of human monocyte-derived dendritic cells to complement component C1q is mediated by the receptors gC1qR and cC1qR. Mol Immunol. 2006;43:1402–7. doi: 10.1016/j.molimm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Tahtouh M, Garcon-Bocquet A, Croq F, et al. Interaction of HmC1q with leech microglial cells: involvement of C1qBP-related molecule in the induction of cell chemotaxis. J Neuroinflammation. 2012;9:37. doi: 10.1186/1742-2094-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlin S, Altschul SF. Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proc Natl Acad Sci USA. 1990;87:2264–68. doi: 10.1073/pnas.87.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearse M, Moir R, Wilson A, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–49. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nardelli-Haefliger D, Shankland M. Lox2, a putative leech segment identity gene, is expressed in the same segmental domain in different stem cell lineages. Development. 1992;116:697–710. doi: 10.1242/dev.116.3.697. [DOI] [PubMed] [Google Scholar]
- 31.Kohidai L. Method for determination of chemoattraction in Tetrahymena pyriformis. Curr Microbiol. 1995;30:251–53. doi: 10.1007/BF00293642. [DOI] [PubMed] [Google Scholar]
- 32.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–66. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Guo L, Groenendyk J, Papp S, et al. Identification of an N-domain histidine essential for chaperone function in calreticulin. J Biol Chem. 2003;278:50645–53. doi: 10.1074/jbc.M309497200. [DOI] [PubMed] [Google Scholar]
- 34.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–78. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 35.Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–70. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 36.Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem. 1991;266:21458–65. [PubMed] [Google Scholar]
- 37.Rokeach LA, Haselby JA, Hoch SO. High-level bacterial expression, purification and characterization of human calreticulin. Protein Eng. 1991;4:981–87. doi: 10.1093/protein/4.8.981. [DOI] [PubMed] [Google Scholar]
- 38.Krause KH. Ca(2+)-storage organelles. FEBS Lett. 1991;285:225–29. doi: 10.1016/0014-5793(91)80806-e. [DOI] [PubMed] [Google Scholar]
- 39.Milner RE, Baksh S, Shemanko C, et al. Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J Biol Chem. 1991;266:7155–65. [PubMed] [Google Scholar]
- 40.Ellgaard L, Riek R, Braun D, et al. Three-dimensional structure topology of the calreticulin P-domain based on NMR assignment. FEBS Lett. 2001;488:69–73. doi: 10.1016/s0014-5793(00)02382-6. [DOI] [PubMed] [Google Scholar]
- 41.Ellgaard L, Riek R, Herrmann T, et al. NMR structure of the calreticulin P-domain. Proc Natl Acad Sci USA. 2001;98:3133–38. doi: 10.1073/pnas.051630098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrag JD, Bergeron JJ, Li Y, et al. The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell. 2001;8:633–44. doi: 10.1016/s1097-2765(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 43.Michalak M, Corbett EF, Mesaeli N, et al. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344(Pt 2):281–92. [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Zuppini A, Arnaudeau S, et al. Functional specialization of calreticulin domains. J Cell Biol. 2001;154:961–72. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacroix M, Dumestre-Perard C, Schoehn G, et al. Residue Lys57 in the collagen-like region of human L-ficolin and its counterpart Lys47 in H-ficolin play a key role in the interaction with the mannan-binding lectin-associated serine proteases and the collectin receptor calreticulin. J Immunol. 2009;182:456–65. doi: 10.4049/jimmunol.182.1.456. [DOI] [PubMed] [Google Scholar]
- 46.Pagh R, Duus K, Laursen I, et al. The chaperone and potential mannan-binding lectin (MBL) co-receptor calreticulin interacts with MBL through the binding site for MBL-associated serine proteases. Febs J. 2008;275:515–26. doi: 10.1111/j.1742-4658.2007.06218.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang HC, Leu JH, Kou GH, et al. Protein expression profiling of the shrimp cellular response to white spot syndrome virus infection. Dev Comp Immunol. 2007;31:672–86. doi: 10.1016/j.dci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Watthanasurorot A, Jiravanichpaisal P, Soderhall I, Soderhall K. A gC1qR prevents white spot syndrome virus replication in the freshwater crayfish Pacifastacus leniusculus. J Virol. 2010;84:10844–51. doi: 10.1128/JVI.01045-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez G, Valck C, Molina MC, et al. Trypanosoma cruzi calreticulin: a novel virulence factor that binds complement C1 on the parasite surface and promotes infectivity. Immunobiology. 2011;216:265–73. doi: 10.1016/j.imbio.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Fricker M, Oliva-Martin MJ, Brown GC. Primary phagocytosis of viable neurons by microglia activated with LPS or Abeta is dependent on calreticulin/LRP phagocytic signalling. J Neuroinflammation. 2012;9:196. doi: 10.1186/1742-2094-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Lynch NJ, Willis CL, Nolan CC, et al. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol Immunol. 2004;40:709–16. doi: 10.1016/j.molimm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Mariani MM, Kielian T. Microglia in Infectious Diseases of the Central Nervous System. J Neuroimmune Pharmacol. 2009;4(4):448–61. doi: 10.1007/s11481-009-9170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen PC, Wang CR, Liu MF, et al. Correlation between the renal C1q deposition and serum anti-C1q antibody: a potential role of anti-C1q antibody in lupus nephritis. Asian Pac J Allergy Immunol. 2002;20:223–27. [PubMed] [Google Scholar]
- 57.Trendelenburg M. Antibodies against C1q in patients with systemic lupus erythematosus. Springer Semin Immunopathol. 2005;27:276–85. doi: 10.1007/s00281-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 58.Bergamaschini L, Donarini C, Gobbo G, et al. Activation of complement and contact system in Alzheimer’s disease. Mech Ageing Dev. 2001;122:1971–83. doi: 10.1016/s0047-6374(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 59.Tacnet-Delorme P, Chevallier S, Arlaud GJ. Beta-amyloid fibrils activate the C1 complex of complement under physiological conditions: evidence for a binding site for A beta on the C1q globular regions. J Immunol. 2001;167:6374–81. doi: 10.4049/jimmunol.167.11.6374. [DOI] [PubMed] [Google Scholar]
- 60.Fonseca MI, Kawas CH, Troncoso JC, Tenner AJ. Neuronal localization of C1q in preclinical Alzheimer’s disease. Neurobiol Dis. 2004;15:40–46. doi: 10.1016/j.nbd.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2004;24:6457–65. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephan AH, Madison DV, Mateos JM, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33:13460–74. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nayak A, Pednekar L, Reid KB, Kishore U. Complement and non-complement activating functions of C1q: a prototypical innate immune molecule. Innate Immun. 2012;18:350–63. doi: 10.1177/1753425910396252. [DOI] [PubMed] [Google Scholar]
- 64.Nayak A, Ferluga J, Tsolaki AG, Kishore U. The non-classical functions of the classical complement pathway recognition subcomponent C1q. Immunol Lett. 2010;131:139–50. doi: 10.1016/j.imlet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Benoit ME, Hernandez MX, Dinh ML, et al. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-beta neurotoxicity. J Biol Chem. 2013;288:654–65. doi: 10.1074/jbc.M112.400168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benoit ME, Tenner AJ. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci. 2011;31:3459–69. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schikorski D, Cuvillier-Hot V, Boidin-Wichlacz C, et al. Deciphering the immune function and regulation by a TLR of the cytokine EMAPII in the lesioned central nervous system using a leech model. J Immunol. 2009;183:7119–28. doi: 10.4049/jimmunol.0900538. [DOI] [PubMed] [Google Scholar]
- 68.Lively S, Schlichter LC. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J Neuroinflammation. 2013;10:75. doi: 10.1186/1742-2094-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 70.Ransohoff RM. Microgliosis: the questions shape the answers. Nat Neurosci. 2007;10:1507–9. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- 71.Prinz M, Mildner A. Microglia in the CNS: Immigrants from another world. Glia. 2011;59:177–87. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- 72.von Bernhardi R, Muller KJ. Repair of the central nervous system: lessons from lesions in leeches. J Neurobiol. 1995;27:353–66. doi: 10.1002/neu.480270308. [DOI] [PubMed] [Google Scholar]