Abstract
HOXA cluster antisense RNA 2 (HOXA-AS2) is a long non-coding RNA located between the HOXA3 and HOXA4 genes in the HOXA cluster. Its transcript is expressed in NB4 promyelocytic leukemia cells and human peripheral blood neutrophils, and expression is increased in NB4 cells treated with all trans retinoic acid (ATRA). Knockdown of HOXA-AS2 expression by transduced shRNA decreases the number of viable cells and increases the proportion of apoptotic cells, measured by annexin V binding and by activity and cleavage of caspases-3, -8, and -9. The increase in death of HOXA-AS2 knockdown cells was accompanied by an elevated TNF-related apoptosis-inducing ligand (TRAIL) levels, but ATRA-induced NB4 cells treated with TRAIL did show an increase in HOXA-AS2 expression. These results demonstrate that ATRA induction of HOXA-AS2 suppresses ATRA-induced apoptosis, possibly through a TRAIL-mediated pathway. HOXA-AS2-mediated negative regulation thus contributes to the fine-tuning of apoptosis during ATRA-induced myeloid differentiation in NB4 cells.
Keywords: myeloid, apoptosis, long intergenic non-coding RNA (lincRNA), non-coding RNA, Hox gene cluster
Long intergenic non-coding RNAs (lincRNAs) are non-coding transcripts longer than 200 nucleotides, transcribed from loci between coding genes and often from the opposite DNA strand (Cabili et al., 2011;Esteller, 2011). Most well-characterized lincRNAs have a functional role in the regulation of gene expression, typically at the transcriptional level, targeting genes that are genomically local (cis-regulation) or distant (trans-regulation)(Prensner and Chinnaiyan, 2011). In addition to long-recognized roles in X-inactivation and genetic imprinting, lincRNAs have recently been recognized to function in development and differentiation (Guttman et al., 2011;Rinn et al., 2008), including hematopoiesis (Hu et al., 2011;Zhang et al., 2009).
lincRNAs are particularly common within the four paralogous clusters of HOX genes (Derrien et al., 2012;Mainguy et al., 2007;Rinn et al., 2007), a set of highly homologous transcription factors that control not only embryological development (Duboule, 2007) but also hematopoietic lineage and differentiation (Eklund, 2011). Three functional lincRNAs have been identified in the HOXA cluster: HOTAIRM1, MISTRAL, and HOTTIP (Bertani et al., 2011;Wang et al., 2011;Zhang et al., 2009). HOTAIRM1, located at the 3′ end of the cluster, is a myeloid-specific lincRNA that appears to influence expression of both local and distant genes (Zhang et al., 2009). HOTTIP, located at the 5′ end, enhances expression of neighboring HOXA genes, most prominently HOXA13 (Wang et al., 2011). These observations distinguish HOTAIRM1 and HOTTIP, as most other lincRNAs have been associated with gene repression.
Acute promyelocytic leukemia (APL) is unique among myeloid leukemias because of its sensitivity to all trans retinoic acid (ATRA), due to the pathogenic t(15; 17) translocation that creates a fusion of the retinoic acid receptor α (RARα) to the promyelocytic leukemia (PML) proteins (Grimwade et al., 2010;Sanz and Lo-Coco, 2011). ATRA induces both cell cycle arrest and the maturation of APL cells which leads to the phenotype of terminally differentiated granulocyte-like cells characterized by limited lifespan and spontaneous, caspase-dependent apoptosis (Gianni et al., 2000). ATRA-induced apoptosis in NB4 cells, a human promyelocytic leukemia cell line derived from a patient with APL, is associated with the activation of various caspase proteases that lead to the execution phase of apoptosis (Gianni et al., 2000).
The current studies examined the expression and function in NB4 cells and peripheral blood neutrophils of HOXA cluster antisense RNA 2 (HOXA-AS2), a lincRNA located between and antisense to the human HOXA3 and HOXA4 genes. We identified and chose to study this RNA because of its proximity and similarity to the nearby lincRNA HOTAIRM1,(Zhang et al., 2009) and initially hypothesized that its function would be similar: that is, to regulate myeloid gene expression (Zhang et al., 2009) and cell cycle (unpublished data). The current results, however, indicate that HOXA-AS2 instead plays a role in regulation cell survival through suppression of apoptosis.
Materials and Methods
The BCA protein assay kit was purchased from Thermo Scientific; antibodies for cleaved caspase-3, cleaved caspase-8, cleaved caspase-9 and Bax, from Cell Signaling; TRAIL antibody, from BD Biosciences; and recombinant human TRAIL, from Invitrogen and BioVision.
Cell Culture
NB4, NB4R2 and K562 cell lines were obtained from American Type Culture Collection. NB4 cells, established from a patient with relapsed APL, terminally differentiates in response to ATRA;(Idres et al., 2001;Lanotte et al., 1991) whereas the derivative line NB4R2 is ATRA resistant.(Duprez et al., 2000) K562 is an ATRA non-responsive Philadelphia chromosome-positive chronic myelogenous leukemia cell line.(Lozzio and Lozzio, 1975)
Cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin 100 U/ml, and streptomycin 100 μg/ml. Human peripheral blood neutrophils were isolated from heparinized (10 U/ml) blood of healthy individuals by Ficoll-Paque PLUS gradient solution (GE Healthcare Bioscience AB, 17-1440-02). The purity of the neutrophils was determined by Wright-Giemsa solution staining and viable cells were counted by trypan blue dye exclusion. The neutrophils were washed three times with RPMI 1640 medium (GIBCO) and resuspended at 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin 100 U/ml, and streptomycin 100 μg/ml. After culture, cell viability was 99 % by trypan blue exclusion. Cultured neutrophils were treated with or without IFN-γ (1000U/ml) or TNF-α (1000U/ml) for 30 or 120 min.
High throughput RNA sequencing and data visualization
Cells were lysed in Tri-Reagent (Molecular Research Center, Inc.) and total RNA isolated as instructed by manufacturer and treated with TurboDNAse (Ambion). The integrity of the RNA was evaluated on an Agilent Bioanalyzer, and samples with sufficient RNA integrity were used to create libraries for deep sequencing. Samples were analyzed by paired-end sequencing on the Genome Analyzer Iix (Illumina) at the Yale Center for Genomics and Proteomics. Base calling and paired-end short reads (FASTQ format) were generated by Genome Analyzer Pipeline software at the sequencing facility. The resulting sequence reads were aligned to the human reference genome of Hg19 February 2009 GRCh37 build with bowtie and followed by exon junction detection with tophat (Trapnell et al., 2012). Reads coverage and detected junction tracks were visualized with the R package ‘ggbio’ of Bioconductor Project (Gentleman et al., 2004;Yin et al., 2012).
qRT-PCR
Total RNA was isolated by TRIzol (Invitrogen) reagent. First-strand cDNAs were reverse transcribed from total RNA using SuperScript III Reverse Transcriptase (Invitrogen). qRT-PCR assays using SYBR Green Master Mix (Applied Biosystems) were performed on an Applied Biosystems 7300 thermal cycler (Foster City, CA). Quantification was performed using the relative comparative CT Method and all measurements were performed in triplicate. Primer sequences are listed in the Supplementary Table 1.
HOXA-AS2 shRNA
Three MISSION® lentiviral particles that encode for shRNA against HOXA-AS2 were purchased from Sigma-Aldrich. The complementary target sequences on three different regions of human HOXA-AS2 genomic DNA (GenBank: AK311383) are presented in Supplementary Table 2. NB4 cells were transduced with MISSION lentiviral particles following the manufacturer's protocol, at final multiplicities of infection of 0.1, 0.5, 1.0, 5.0, or 10. Control NB4 cells were transduced with a “scramble” shRNA (Addgene plasmid 1864) in the same vector. Transduced cells were selected in culture medium containing puromycin 10 μg/ml. Visible clones were picked from the 96-well plates and expanded in 24-well tissue culture plates. The knockdown of HOXA-AS2 RNA was determined by quantitative (q)RT-PCR.
Annexin V binding
Cell apoptosis was analyzed on an LSR II flow cytometer (BD Biosciences) using an annexin V–FITC and propidium iodide apoptosis detection kit (BD Biosciences) according to the manufacturer's instructions. Data were analyzed using FlowJo software (Tree Star).
Caspase activity
Caspase activity assay kits (BioVision Research Products, Mountain View, CA) based on colorimetric detection of cleaved para-nitroaniline-labeled substrates specific for caspase-3 (acetyl-AspGlu-Val-Asp-p-nitroanilide), caspase-8 (acetyl-Ile–Glu–Thr–Asp–p-nitroanilide), and caspase-9 (acetyl-Leu-Glu-His-Asp-pnitroanilide were used to measure respective activities, according to manufacturer instructions, as sample absorbance at 405 nm in a spectrophotometer (NanoDrop).
Western blot analysis
Cells were lysed in chilled RIPA buffer (Cell Signaling) with 1 mM PMSF and boiled for 5 minutes. Protein was electrophoresed on 4–20% gradient SDS-PAGE gels (Invitrogen) and electro-transferred to PVDF membranes (Millipore). The blocked membranes were incubated with the primary antibody in TBST buffer containing 5% nonfat milk at 4°C overnight, followed by incubation with the species-appropriate HRP-conjugated secondary antibody according to manufacturer's instructions. Membranes were developed with Super-Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific).
Statistics
Descriptive statistics were performed and the results represented by bar graphs showing mean ± standard deviation (SD) values. ANOVA and Tukey's Multiple Comparison test were used for comparisons among groups; a p value <0.05 was considered significant.
Results
ATRA selectively induces HOXA-AS2 expression
lincRNAs are relatively common in the HOXA cluster (Derrien et al., 2012;Mainguy et al., 2007;Rinn et al., 2007), where they play regulatory roles in development and differentiation, including in myeloid cells (Zhang et al., 2009). In this study, we focus on the role of HOXA-AS2, a large intergenic lincRNA, on proliferation and apoptosis of ATRA-treated NB4 cells. HOXA-AS2 is located between HOXA3 and HOXA4 in the HOXA cluster in the human genome, and transcribed in the opposite direction (Fig. 1A). It is widely, but not universally, expressed and spliced into several isoforms, varying from 339 to 2045 nt (NCBI UniGene, 2012;Ensembl, 2012). As shown in Fig.1A, HOXA-AS2 is expressed and responsive to ATRA induction in NB4 cells and neutrophils as assessed by RNA deep-sequencing. Splice junction analysis by Tophat (green bars) demonstrates splicing and confirms transcription in both NB4 cells and primary neutrophils. The signal is not sufficiently precise in alignment to permit identification of which isoforms are expressed in these cells, perhaps because of a mix of isoforms and the known inefficiency of lincRNA splicing (Tilgner et al., 2012). qRT-PCR analysis confirmed progressive up-regulation in ATRA-treated NB4 cells (Fig. 1B). In contrast, ATRA had no significant effect on HOXA-AS2 expression in ATRA-resistant NB4R2 and K562 cells (Fig. 1B).
Figure 1.
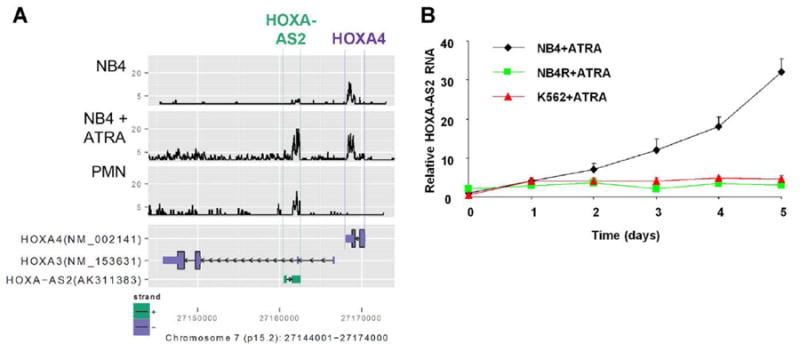
ATRA induction of HOXA-AS2 expression in NB4 cells. (A) lower panel: UCSC Genome Browser map of expressed sequences in the region between HOXA3 and HOXA4 in the HOXA cluster on chromosome 7. Upper panel: RNA-Seq signal from this region in RNA from NB4 cells with and without ATRA induction (1 μM for 5 days) and normal peripheral blood polymorphonuclear leukocytes (PMN). (B) HOXA-AS2 transcripts measured by qRT-PCR in NB4, NB4R2 (ATRA-resistant) or K562 cells were incubated with ATRA (1 μM) for the indicated number of days. Point and error bars represent the mean ± SD of four independent experiments.
HOXA-AS2 expression is induced by IFN-γ and TNF-α in neutrophils
As all other experiments were performed in the NB4 cell line, we sought to verify regulated expression of HOXA-AS2 in primary peripheral blood neutrophils as well. Gene expression was analyzed by qRT-PCR for early (30 min) and late (2 hr) responses (Fig. 2). HOXA-AS2 expression in response to IFN-γ was higher at the 30 min time point while TNF-α induced a higher level of gene expression at 2 hr.
Figure 2.
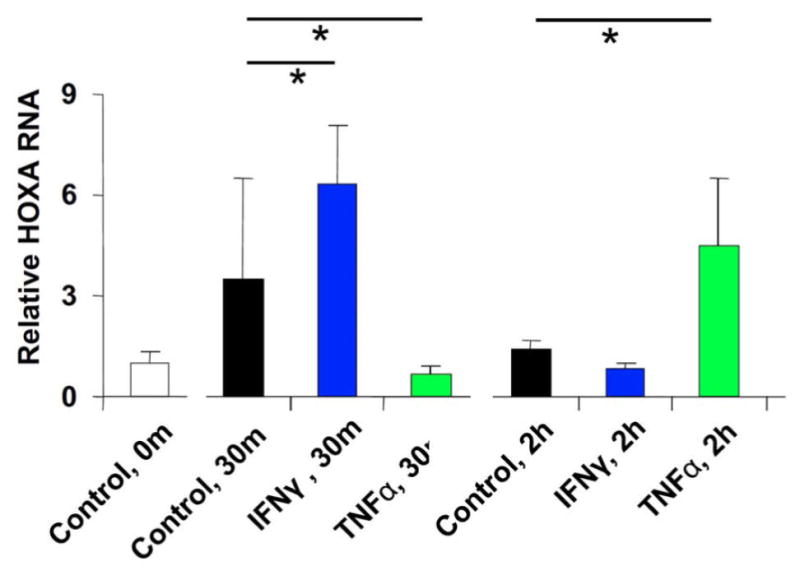
Effect of IFNγ and TNFα on HOXA-AS2 gene expression in PMN. Relative expression of (A) HOXA-AS2 and (B) TRAIL in normal PMN incubated with or without IFN-γ (1000U/ml), TNF-α (1000U/ml), or IFN-γ plus TNF-α for 30 min or 2 hours. Bars and error bars represent the mean ± SD of three measurements in a representative experiment, of three independent experiments with PMN from two donors. Horizontal bars with asterisks indicate comparisons that reached statistical significance at p<0.05.
HOXA-AS2 knockdown decreases cell viability
To investigate the role of HOXA-AS2 on myeloid cell proliferation and apoptosis, three different shRNA constructs targeting HOXA-AS2 were transduced into NB4 cells to inhibit its expression. Four stable cell lines of NB4 were established: one with each targeting shRNA and one with the scramble control. These cell lines exhibited vigorous growth and microscopic appearance identical to the parental NB4 cells (data not shown). The efficacy of HOXA-AS2 knockdown by the shRNAs was analyzed by qRT-PCR. All three shRNA constructs caused a reduction of HOXA-AS2 RNA expression when compared with scramble (Fig. 3A). shRNA constructs A and C exhibited more efficient knockdown (clone A 76%, clone C 73%) and were therefore used in all other experiments. To determine the effect of HOXA-AS2 on proliferation and apoptosis, NB4 transductant clones were incubated with ATRA (1μM) and the number of viable cells determined at days 1, 2, 3, 4 and 5. The HOXA-AS2 knockdown cells treated with ATRA showed progressive relative decreases in cell number (Fig. 3B) and viability (Fig. 3C) compared to scramble-expressing cells; whereas HOX3AS knockdown did not affect proliferation or viability of uninduced NB4 cells. The scramble control showed a smaller, non-specific effect on proliferation and viability.
Figure 3.
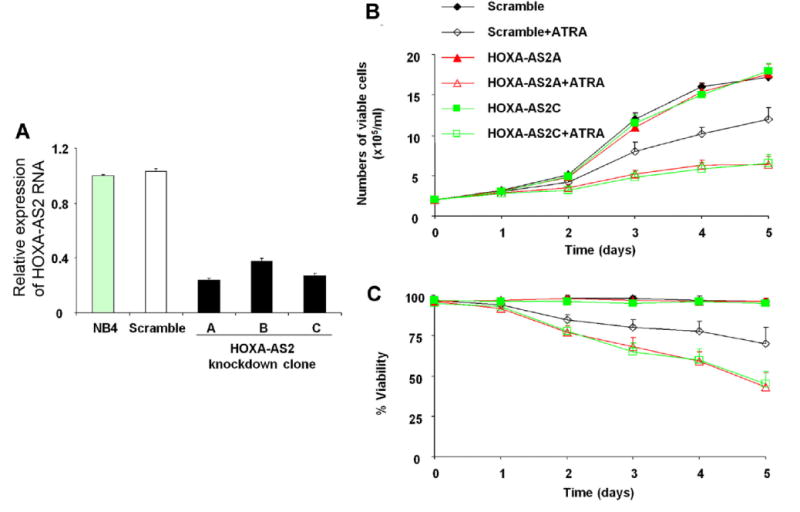
Effect of HOXA-AS2 knockdown on NB4 cell proliferation. (A) HOXA-AS2 gene expression, measured by qRT-PCR, in NB4 cells transfected with three HOXA-AS2 shRNAs. (B,C) NB4 cells transfected with the indicated constructs, with or without ATRA induction, as represented by the colors and symbols in the insert, were seeded at 2 × 105/mL and cell number (B) and viability (C) determined at the indicated numbers of days in culture. Points and error bars represent the mean ± SD of three independent experiments.
ATRA-induced apoptosis in knockdown cells
Since decreased cell counts and viability can be attributed to increased apoptosis, we investigated the effect of HOXA-AS2 on ATRA-induced apoptosis in NB4 cells. Cells were stained with FITC-labeled annexin V and analyzed by flow cytometry at 3 days after ATRA (1μM) treatment. The percentage of annexin V positive cells markedly increased in HOXA-AS2 knockdown cells compared to scramble (Fig. 4A,B), indicating enhanced apoptosis in HOXA-AS2 knockdown cells. Consistent with these results, colorimetrically-determined caspase-3 activity (Fig. 4C) and levels of cleaved caspase-3 on western blot (Fig. 4D) also increased in the knockdown clones. Thus, HOXA-AS2 plays a negative regulatory role in caspase-3-dependent apoptosis in ATRA-induced NB4 cells.
Figure 4.
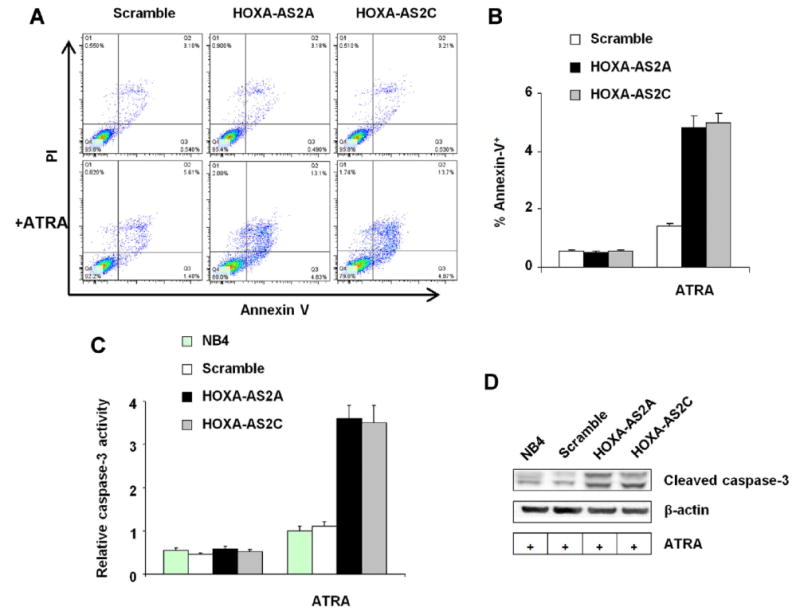
Effect of HOXA-AS2 knockdown on NB4 cell apoptosis. Annexin V staining of NB4 cells transfected with scramble shRNA or the indicated HOXA-AS2 targeting shRNAs, incubated with or without ATRA (1 μM for 3 days) as indicated. (A) Scatter plots; (B) Bar plot, with bars and error brackets representing mean ± SD of four independent experiments; p<0.001 for differences between scramble and knockdown clones A and C. Caspase-3 activity was measured (C) colorimetrically with substrate acetyl-Asp-Glu-Val-Asp-p-nitroanilide, bars and error brackets representing mean ± SD of four independent experiments, p<0.001 for differences between scramble and knockdown clones A and C; and (D) by western blot detection of cleaved caspase-3 protein; the blot is representative of three experiments.
Extrinsic and intrinsic pathways in apoptosis
In TRAIL-mediated apoptosis (Bodmer et al., 2000), death-inducing signaling complex activity is known to mediate the processing of caspase-8. In addition to direct activation of executor caspases, caspase-8 activation of the initiator caspase-9 also triggers activation of executor caspases (Adams and Cory, 2007;Garrido et al., 2006). To determine the involvement of caspases-8 and -9 in the effects of HOXA-AS2 knockdown on NB4 cell apoptosis, we measured intracellular caspase-8 and -9 activity in native NB4 cells, HOXA-AS2 knockdown clones, and scramble controls, with or without 4 days of treatment with ATRA (1 μM). Both caspase activities were increased in the ATRA-induced HOXA-AS2 knockdown cells, compared to controls (Fig. 5A,B). Consistent with these results, western blot analysis confirmed that cleaved products of caspase-8 and -9 in HOXA-AS2 knockdown cells were elevated over native NB4 and scramble controls (Fig. 5C). Western blot analysis also showed that HOXA-AS2 knockdown cells contain increased amounts of BAX (Fig. 5C), a proapoptotic protein of the BCL2 protein family (Gavathiotis et al., 2010). Thus, ATRA induces apoptosis in HOXA-AS2 knockdown cells through activation of both caspase-8 and caspase-9 pathways.
Figure 5.
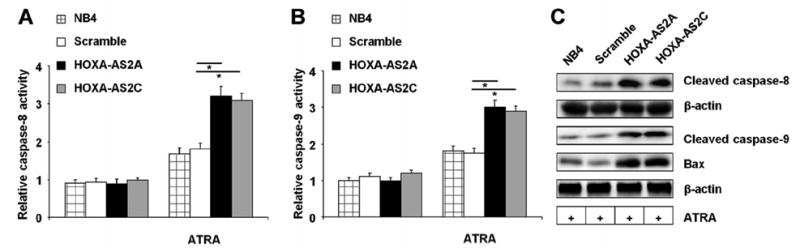
Effect of HOXA-AS2 on ATRA-induced extrinsic apoptosis pathways. (A) Caspase-8 and (B) caspase-9 activity, measured respectively as acetyl-Ile–Glu–Thr–Asp–p-nitroanilide-cleaving and acetyl-Leu-Glu-His-Asp-pnitroanilide-cleaving activity in NB4 cells with or without treatment with ATRA (1 μM for 4 days). Bars and error brackets represent mean ± SD of four independent experiments. Horizontal bars with asterisks indicate comparisons that reached statistical significance at p<0.05. (C) Western blot of cleaved caspases-8 and -9, Bax, and β-actin in the indicated cell lysates; the blot is representative of three experiments.
TRAIL levels in HOXA-AS2 knockdown cells
ATRA-induced cell death in APL and NB4 cells is linked to paracrine production of TRAIL (Altucci et al., 2001;Johnstone et al., 2008), a member of the tumor necrosis factor (TNF) superfamily that induces apoptosis via the extrinsic death receptor pathway and activation of caspases -3, -8, and -9. We therefore assessed the role of HOXA-AS2 on TRAIL induction in ATRA-treated NB4 cells. TRAIL protein (Fig. 6A) and mRNA (Fig. 6B) expression was markedly increased in HOXA-AS2 knockdown cells after 3 day of ATRA (1μM) treatment. These results indicate that HOXA-AS2 negatively regulates ATRA-induced TRAIL production. The elevation of caspase-8 and -9 activities in HAXA-AS2 knockdown cells occurred, at least in part, in response to the paracrine effects of TRAIL, as the addition of TRAIL-neutralizing antibody partially suppressed the levels of cleaved caspases-8 and -9 in ATRA-treated knockdown cells (Fig.6C).
Figure 6.
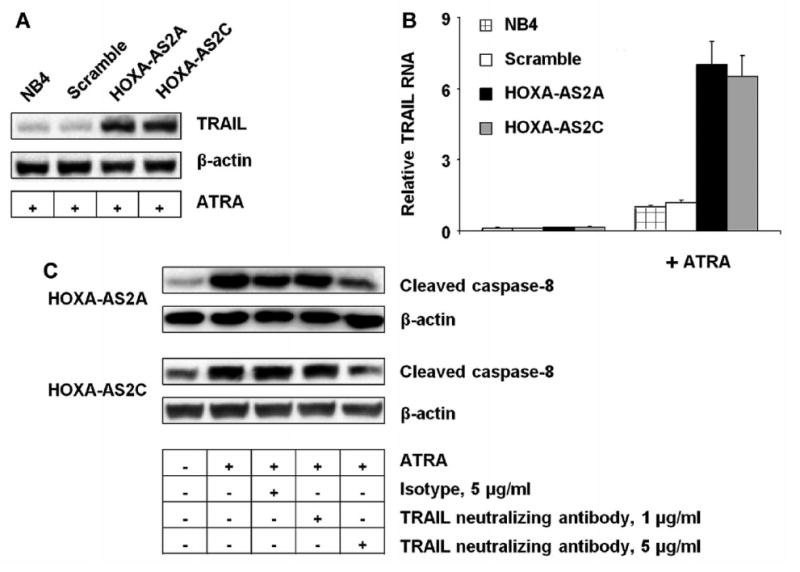
Effect of HOXA-AS2 on TRAIL expression and function. NB4 cells transfected with the indicated knockdown constructs were cultured with or without ATRA (1 μM for 3 days). (A) Western blot of TRAIL and β-actin in the indicated cell lysates; blot representative of three experiments. (B) TRAIL transcripts measured by qRT-PCR. Bars and error brackets represent mean ± SD of three independent experiments; p<0.01 for difference between scramble and knockdown clone A, and p<0.01 for difference between scramble and knockdown clone C. (C) Western blot of cleaved caspases-8 and 9, and β-actin, in NB4 cell lysates with or without ATRA (1 μM for 3 days) and with anti-TRAIL or isotype control antibodies as indicated; the blot is representative of three experiments.
HOXA-AS2 is not a target of TRAIL
As shown in Fig. 1B, HOXA-AS2 expression was selectively increased in ATRA-sensitive NB4 cells but not in the TRAIL-resistant K562 cell line (Nimmanapalli et al., 2001), raising the possibility that ATRA-mediated induction of HOXA-AS2 itself may be secondary to a paracrine function of TRAIL. We therefore added recombinant human TRAIL to NB4 cell cultures and evaluated cleaved caspase-8 level and HOXA-AS2 expression. TRAIL exposure did not result in up-regulation of HOXA-AS2 RNA expression (Fig. 7A), indicating that its induction is not TRAIL-mediated. Exogenous TRAIL was active in these cells, as evident in the dose-dependent increases in cleaved caspase-8 (Fig. 7B), as in previous studies (Altucci et al., 2001;Sakoe et al., 2010).
Figure 7.
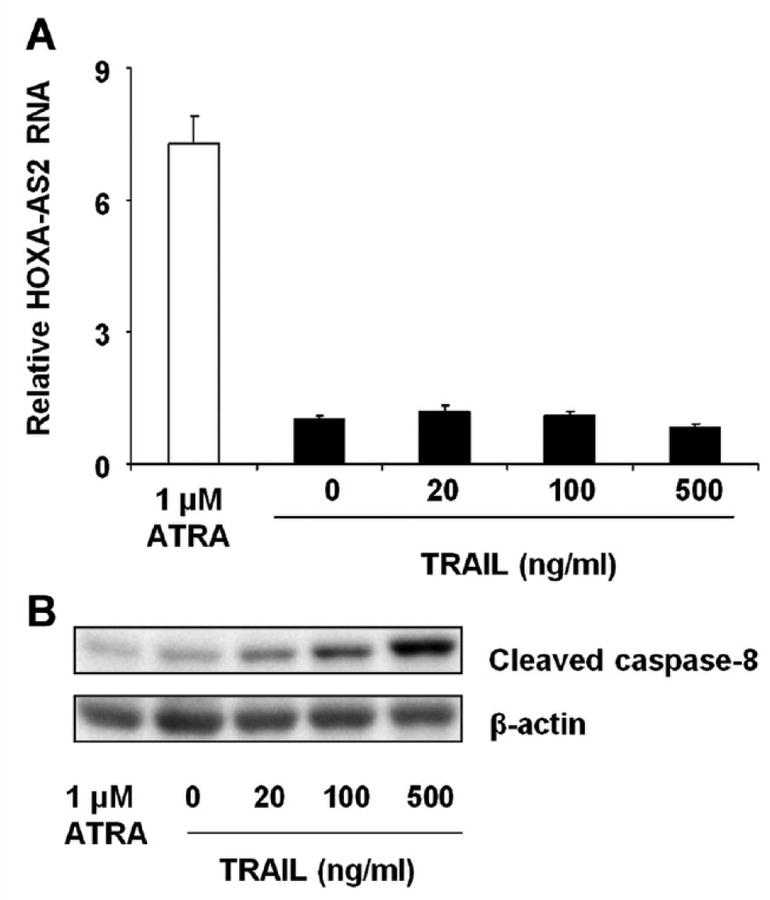
Effect of TRAIL on HOXA-AS2 expression. NB4 cells were incubated with ATRA (1 μM) or with the indicated concentrations of recombinant human TRAIL for 2 days. (A) HOXA-AS2 expression was analyzed by qRT-PCR. Bars and error brackets represent mean ± SD of three independent experiments. (B) Western blot analysis of cleaved caspase-8 and β-actin protein; the blot is representative of three experiments.
Discussion
Myeloid cell development critically relies on control mechanisms that maintain the balance between proliferation and apoptosis, and defective regulation of apoptosis during hematopoiesis may promote leukemogenesis or neutropenia. (de Thé and Chen, 2010;Grenda et al., 2007) In the current studies, we have shown that lincRNA HOXA-AS2 is expressed in both human peripheral blood PMN and in the human promyelocytic leukemia cell line NB4. In PMN, its expression shows rapid up-regulation by the inflammatory cytokine IFN-γ and a later response to TNFα, with a synergistic response to the combined cytokines. HOXA-AS2 is dramatically upregulated during ATRA-induced granulocytic differentiation of NB4 cells.
HOXA-AS2 knockdowns by shRNAs demonstrate that the lincRNA negatively regulates ATRA-induced NB4 cell apoptosis, with a mechanism that involves the key pro-apoptotic mediator, TRAIL. HOXA-AS2 appears to repress TRAIL mRNA and protein expression, as knockdown of HOXA-AS2 by transduced shRNAs result in increased TRAIL levels. Thus, HOXA-AS2-mediated regulation could contribute to the fine-tuning of apoptosis during induced myeloid differentiation. The anti-apoptotic effect of HOXA-AS2 reveals new layer of modulation of the tightly controlled cell death program that is required for the proper generation of mature myeloid cells. The lincRNA may also be involved in development of other tissues, as it is similarly up-regulated by ATRA in human embryonic stem cells (Colleoni et al., 2011;EMBL-EBI, 2012).
The precise events downstream of ATRA that result in HOXA-AS2 induction are not known. Up-regulation of HOXA-AS2 by ATRA may reflect either the engagement of regulatory networks following the formation of ATRA·PML-RARα complexes or regulation by other early biochemical cellular events induced by ATRA. HOXA-AS2 may contribute to a graded response that optimizes TRAIL output, in concert with other known mediators of ATRA-induced TRAIL-mediated apoptosis. ATRA induces interferon regulatory factor (IRF)-1-dependent TRAIL expression and subsequently triggers apoptosis in leukemia cells through a paracrine action of TRAIL (Clarke et al., 2004;Altucci et al., 2001). ATRA-induced STAT1 and p48/IRF-9 play a key role in the interferon response and induce the synthesis and the secretion of interferons (Gianni et al., 2000;Matikainen et al., 1997). FOXO3A also plays an important role in ATRA-induced TRAIL-mediated apoptosis in NB4 cells (Sakoe et al., 2010). More studies are required to determine whether any of these pathways mediate TRAIL repression by HOXA-AS2.
HOXA-AS2 may also directly affect transcription of the TRAIL gene, distantly located at chromosome 3q26. The archetypical HOX region lincRNA, HOTAIR, regulates target genes in trans: HOTAIR generated from the HOXC locus on chromosome 12 represses transcription across 40-kb of the HOXD locus on chromosome 2, by recruitment of polycomb repressive complex 2 (Rinn et al., 2007).
Emerging evidence suggests that lincRNAs are an important component of tumor biology. Inappropriate expression of lincRNAs in HOX loci has been implicated in the development of both hematopoietic malignancies and solid tumors (Gibb et al., 2011;Prensner and Chinnaiyan, 2011). HOTAIR is upregulated in breast and hepatocellular carcinomas and on pancreatic cancer (Gibb et al., 2011;Kim et al., 2012). Overexpression of HOTAIR in breast cancer is an independent predictor of overall survival and the progression of free survival (Gupta et al., 2010). Moreover, HOXA genes particularly HOXA9 are broadly known to be important for many malignancies, including acute myeloid leukemias (Thorsteinsdottir et al., 2001).
In conclusion, HOXA-AS2 functions as an apoptosis repressor in ATRA-treated NB4 cells, through mechanisms mediated in part by TRAIL. As targeted therapy of APL by ATRA and by arsenic compounds depends upon induction of apoptosis, further investigation of HOXA-AS2 in TRAIL-mediated apoptosis could provide insight into further therapeutic target for APL and possibly other myeloid malignancies. The lincRNA could also be important in the mediation of inflammatory disorders, where apoptosis plays a major role in the resolution of inflammation and prevention of tissue damage.
Supplementary Material
Acknowledgments
Current grant sponsors: NIH R01DK054369 (PEN), Fundação de Amparo à Pesquisa do Estado de São Paulo (ACN).
References
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H. Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL. Nat Med. 2001;7:680–686. doi: 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- Bertani S, Sauer S, Bolotin E, Sauer F. The noncoding RNA Mistral activates Hoxa6 and Hoxa7 expression and stem cell differentiation by recruiting MLL1 to chromatin. Mol Cell. 2011;43:1040–1046. doi: 10.1016/j.molcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N, Jimenez-Lara AM, Voltz E, Gronemeyer H. Tumor suppressor IRF-1 mediates retinoid and interferon anticancer signaling to death ligand TRAIL. EMBO J. 2004;23:3051–3060. doi: 10.1038/sj.emboj.7600302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni S, Galli C, Gaspar JA, Meganathan K, Jagtap S, Hescheler J, Sachinidis A, Lazzari G. Development of a neural teratogenicity test based on human embryonic stem cells: response to retinoic acid exposure. Toxicol Sci. 2011;124:370–377. doi: 10.1093/toxsci/kfr245. [DOI] [PubMed] [Google Scholar]
- de Thé H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigo R. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- Duprez E, Benoit G, Flexor M, Lillehaug JR, Lanotte M. A mutated PML/RARA found in the retinoid maturation resistant NB4 subclone, NB4-R2, blocks RARA and wild-type PML/RARA transcriptional activities. Leukemia. 2000;14:255–261. doi: 10.1038/sj.leu.2401683. [DOI] [PubMed] [Google Scholar]
- Eklund E. The role of Hox proteins in leukemogenesis: insights into key regulatory events in hematopoiesis. Crit Rev Oncog. 2011;16:65–76. doi: 10.1615/critrevoncog.v16.i1-2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMBL-EBI. E-MEXP-3577. [Accessed 10-11-2012]; http://www.ebi.ac.uk/gxa/experiment/E-MEXP-3577/ENSG00000253552?ef.
- Ensembl. HOXA-AS2. [Accessed 10-12-2012]; http://useast.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000253552;r=7:27147396-27173921;redirect=mirror;source=www.ensembl.org.
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni M, Ponzanelli I, Mologni L, Reichert U, Rambaldi A, Terao M, Garattini E. Retinoid-dependent growth inhibition, differentiation and apoptosis in acute promyelocytic leukemia cells. Expression and activation of caspases. Cell Death Differ. 2000;7:447–460. doi: 10.1038/sj.cdd.4400673. [DOI] [PubMed] [Google Scholar]
- Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenda DS, Murakami M, Ghatak J, Xia J, Boxer LA, Dale D, Dinauer MC, Link DC. Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis. Blood. 2007;110:4179–4187. doi: 10.1182/blood-2006-11-057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade D, Mistry AR, Solomon E, Guidez F. Acute promyelocytic leukemia: a paradigm for differentiation therapy. Cancer Treat Res. 2010;145:219–235. doi: 10.1007/978-0-387-69259-3_13. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Yuan B, Flygare J, Lodish HF. Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation. Genes Dev. 2011;25:2573–2578. doi: 10.1101/gad.178780.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idres N, Benoit G, Flexor MA, Lanotte M, Chabot GG. Granulocytic differentiation of human NB4 promyelocytic leukemia cells induced by all-trans retinoic acid metabolites. Cancer Res. 2001;61:700–705. [PubMed] [Google Scholar]
- Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanotte M, Martn-Thouvenin V, Najman S, Ballerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15:17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080. [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- Mainguy G, Koster J, Woltering J, Jansen H, Durston A. Extensive polycistronism and antisense transcription in the mammalian hox clusters. PLoS ONE. 2007;2:e356. doi: 10.1371/journal.pone.0000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen S, Ronni T, Lehtonen A, Sareneva T, Melen K, Nordling S, Levy DE, Julkunen I. Retinoic acid induces signal transducer and activator of transcription (STAT) 1, STAT2, and p48 expression in myeloid leukemia cells and enhances their responsiveness to interferons. Cell Growth Differ. 1997;8:687–698. [PubMed] [Google Scholar]
- NCBI UniGene. HOXA cluster antisense RNA 2 (HOXA-AS2) [Accessed 10-12-2012]; http://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=714774.
- Nimmanapalli R, Porosnicu M, Nguyen D, Worthington E, O'Bryan E, Perkins C, Bhalla K. Cotreatment with STI-571 enhances tumor necrosis factor alpha-related apoptosis-inducing ligand (TRAIL or apo-2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin Cancer Res. 2001;7:350–357. [PubMed] [Google Scholar]
- Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Wang JK, Liu H, Montgomery K, van de Rijn M, Chang HY. A systems biology approach to anatomic diversity of skin. J Invest Dermatol. 2008;128:776–782. doi: 10.1038/sj.jid.5700986. [DOI] [PubMed] [Google Scholar]
- Sakoe Y, Sakoe K, Kirito K, Ozawa K, Komatsu N. FOXO3A as a key molecule for all-trans retinoic acid-induced granulocytic differentiation and apoptosis in acute promyelocytic leukemia. Blood. 2010;115:3787–3795. doi: 10.1182/blood-2009-05-222976. [DOI] [PubMed] [Google Scholar]
- Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Kroon E, Jerome L, Blasi F, Sauvageau G. Defining roles for HOX and MEIS1 genes in induction of acute myeloid leukemia. Mol Cell Biol. 2001;21:224–34. doi: 10.1128/MCB.21.1.224-234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgner H, Knowles DG, Johnson R, Davis CA, Chakrabortty S, Djebali S, Curado J, Snyder M, Gingeras TR, Guigo R. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 2012;22:1616–1625. doi: 10.1101/gr.134445.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, Wysocka J, Lei M, Dekker J, Helms JA, Chang HY. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Cook D, Lawrence M. ggbio: an R package for extending the grammar of graphics for genomic data. Genome Biol. 2012;13:R77. doi: 10.1186/gb-2012-13-8-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lian Z, Padden C, Gerstein MB, Rozowsky J, Snyder M, Gingeras TR, Kapranov P, Weissman SM, Newburger PE. A myelopoiesis-associated regulatory intergenic non-coding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–2534. doi: 10.1182/blood-2008-06-162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


