Abstract
Objectives
Non-speech psychophysical tests of spectral resolution, such as the spectral-ripple discrimination task, have been shown to correlate with speech recognition performance in cochlear implant (CI) users (Henry et al., 2005; Won et al. 2007, 2011; Drennan et al. 2008; Anderson et al. 2011). However, these tests are best suited for use in the research laboratory setting and are impractical for clinical use. A test of spectral resolution that is quicker and could more easily be implemented in the clinical setting has been developed. The objectives of this study were 1) To determine if this new clinical ripple test would yield individual results equivalent to the longer, adaptive version of the ripple discrimination test; 2) To evaluate test-retest reliability for the clinical ripple measure; and 3) To examine the relationship between clinical ripple performance and monosyllabic word recognition in quiet for a group of CI listeners.
Design
Twenty-eight CI recipients participated in the study. Each subject was tested on both the adaptive and the clinical versions of spectral ripple discrimination, as well as CNC word recognition in quiet. The adaptive version of spectral ripple employed a 2-up, 1-down procedure for determining spectral ripple discrimination threshold. The clinical ripple test used a method of constant stimuli, with trials for each of 12 fixed ripple densities occurring six times in random order. Results from the clinical ripple test (proportion correct) were then compared to ripple discrimination thresholds (in ripples per octave) from the adaptive test.
Results
The clinical ripple test showed strong concurrent validity, evidenced by a good correlation between clinical ripple and adaptive ripple results (r=0.79), as well as a correlation with word recognition (r = 0.7). Excellent test-retest reliability was also demonstrated with a high test-retest correlation (r = 0.9).
Conclusions
The clinical ripple test is a reliable non-linguistic measure of spectral resolution, optimized for use with cochlear implant users in a clinical setting. The test might be useful as a diagnostic tool or as a possible surrogate outcome measure for evaluating treatment effects in hearing.
INTRODUCTION
Performance with cochlear implants (CIs) has traditionally been assessed using standard speech recognition tests. While these tasks require the ability to discriminate numerous spectral and temporal cues, they are also subject to cognitive and linguistic factors that are particularly susceptible to learning. Speech tests provide a good index of functional hearing ability with a CI over time; however, they might not be sensitive to more subtle differences in the ability to perceive spectral or temporal acoustic information when using different processing schemes or sound processor programs (Donaldson et al. 2011; Drennan et al. 2010).
Supin et al. (1994) argued a single-point measure of spectral resolution could be beneficial clinically. They developed an adaptive discrimination task which involves discriminating between a broadband noise containing alternating spectral peaks and valleys and another noise in which the frequency positions of the peaks and valleys have been reversed. Such an approach could rapidly evaluate spectral resolution ability, a fundamental tenet of hearing ability in the tonotopically organized auditory system. Henry et al.(2003; 2005) built upon this concept with hearing impaired and cochlear implant listeners. Their studies demonstrated a significant correlation between speech and spectral ripple discrimination across normal hearing, hearing impaired and cochlear implant listeners. The adaptive spectral-ripple discrimination task has been shown to reflect spectral resolution abilities (Jones et al. 2013; Won, Jones et al. 2011). Spectral ripple discrimination has also been shown to be sensitive to differences in signal processing (Berenstein et al. 2008; Drennan et al. 2010) and has been shown to correlate with speech understanding in cochlear implant users (Anderson et al. 2011; Henry and Turner 2003; Henry et al. 2005; Won et al. 2010; Won et al. 2007). A similar task, spectral ripple detection (Bernstein et al. 1987; Eddins et al. 2007), has also been shown to correlate well with speech understanding in cochlear implant users (Litvak et al. 2007; Saoji et al. 2009; Spahr et al. 2011). Anderson et al. (2012) has compared the two methods in CI listeners, and provides some insight into some potential mechanistic differences between the tasks; however, it is clear that both measures provide a measure closely related to spectral resolution (Jones et al. 2013; Litvak et al. 2007; Won, Jones et al. 2011) and correlate significantly with individual speech understanding differences in cochlear implant users.
The spectral ripple discrimination test was developed for laboratory research and would be impractical for use in a typical clinical setting because the test is relatively time-consuming, requiring approximately 25 minutes of testing time in its current form. A more efficient procedure for measuring spectral ripple resolution could hold potential utility in the clinic. Spectral ripple discrimination also has the advantage of providing an acute measure of hearing ability which is less susceptible to acclimatization (Drennan et al. 2011; Drennan et al. 2010). Acclimatization to speech in cochlear implant users creates a challenge in evaluating cochlear implant outcome, because it requires retesting after several months of experience. A rapid, single-point measure of spectral resolution might also prove useful in evaluating the spectral resolution afforded by different clinical maps, or in evaluating preoperative hearing ability which could have clinical implications in treatment decisions (Moon et al. 2013; Noble et al. 2013; Shim et al. submitted; Zhang et al. 2013).
A clinical measure of spectral ripple resolution that requires about 8 minutes to administer, including 6 minutes of test time plus a few minutes of instruction time, is reported here. Modeled after the Test of Basic Auditory Capabilities (Surprenant et al. 2001; Watson, Jensen et al. 1982; Watson, Johnson et al. 1982), the test uses a method of constant stimuli, yielding psychometric functions that describe performance across a wide range of spectral ripple densities. The effective area under the psychometric curve is estimated for each listener by calculating the proportion of correct responses across all tested ripple densities.
The current validation study compared performance by 28 CI recipients on the clinical ripple test to their performance on the original, adaptive spectral-ripple procedure (Henry et al. 2005; Won et al. 2007). The objectives were: 1) To determine if the clinical ripple test would yield individual results equivalent to the longer, adaptive version of the ripple discrimination test; 2) To evaluate test-retest reliability for the clinical ripple measure; and 3) To examine the relationship between clinical ripple performance and monosyllabic word recognition in quiet for the same CI listeners.
METHODS AND MATERIALS
Subjects
Twenty-eight CI users participated in this study, including 10 Advanced Bionics, 1 Med El, and 17 Nucleus recipients. Subjects ranged in age from 23 to 82 years, with length of CI use ranging from 0.5 to 18 years. Table I displays general subject characteristics. This study was approved by the University of Washington Institutional Review Board.
Table I.
Individual subject and device characteristics.
| Subj Code | Sex | Age | Yrs Hrg Loss | Ear | Yrs CI Use | Device | Strategy | Reported Etiology |
|---|---|---|---|---|---|---|---|---|
| S1 | F | 67 | <1 | L | 11 | Nucleus 24 Contour | ACE | Unk, Poss autoimmune |
| S3 | F | 66 | 5 | L | 3 | Nucleus CI512 | ACE | Unk, Poss genetic |
| S4 | M | 68 | 1 | L | 9 | Nucleus 24 Contour | ACE | Long-term infection |
| S12 | M | 55 | unk | L | 5 | Med El Pulsar | FSP | Genetic |
| S16 | M | 56 | 17 | L | 7 | Nucleus 24 | CCIS | “Nerve damage” |
| S48 | F | 71 | 10 | R | 5 | AB HiRes 90K | HIRes-P w/F120 | Familial |
| S52 | M | 81 | <1 | L | 4 | ABHiRes 90k | HiRes-S | Noise |
| S71 | F | 72 | 13 | R | 3 | AB HiRes 90K | HIRes w/F120 | Autoimmune |
| S79 | F | 66 | 10 | R | 12 | Nucleus 24 | ACE | Unk, Poss genetic |
| S80 | M | 60 | 2 | L | 1 | AB HiRes 90K | HIRes-P w/F120 | Otosclerosis |
| S84 | M | 47 | unk | L | 1 | AB HiRes 90K | HIRes-P w/F120 | Congenital; Menière’s contralat ear |
| S87 | M | 72 | 5 | R | 2 | Nucleus Hybrid | Unk | Unk, Poss genetic |
| S89 | F | 63 | 2 | R | 1 | Nucleus CI512 | ACE | Noise, Genetic, Poss measles |
| S91 | F | 23 | unk | R | 7.5 | Nucleus Freedom | ACE | EVA |
| S93 | M | 27 | 1 | L | 18 | Nucleus N22 | SPEAK | Unk |
| S94 | M | 82 | 10 | R | 3 | Nucleus Freedom | ACE | Unk |
| S95 | M | 69 | 35 | R | 12 | Clarion II | CIS | Unk, poss genetic |
| S97 | F | 58 | 6 | R | 0.5 | Nucleus 5 | ACE | Unk |
| S98 | F | 60 | 6 | R | 11 | Nucleus Freedom | ACE | Genetic |
| S99 | M | 77 | 8 | R | 2 | AB HiRes 90K | HiRes-P w/F120 | Noise |
| S102 | F | 75 | 0 | L | 5 | Nucleus Freedom | ACE | genetics, head trauma |
| S105 | F | 61 | 37 | R | 14 | Nucleus 24 | ACE | Genetic |
| S106 | M | 65 | <1 | L | 3 | Nucleus Freedom | ACE | Unk |
| S107 | M | 61 | <1 | R | 10 | AB Harmony | Unk | Unk |
| S108 | F | 41 | 15 | L | 11 | AB Clarion C-I | SAS | Unk |
| S109 | M | 79 | 3 to 5 | R | 2 | Nucleus 5 | ACE | Unk, Poss Noise |
| S0110 | F | 48 | 7 | R | 1 | AB HiRes 90K | Hi Res w/F120 | Genetic |
| S0111 | F | 41 | 29 | L | <1 | Nucleus Freedom | ACE | Autoimmune |
Gender; age at time of testing; duration of bilateral, severe-to-profound hearing loss prior to implantation; ear tested; duration of implant use prior to testing; implanted device; sound processing strategy in use; etiology of deafness. “Unk” is short for unknown, “Poss” is short for possibly.
Stimuli
Spectral ripple stimuli for the longer adaptive ripple task were identical to those used by Won, et al (2007). Rippled noise was generated by summing 200 random-phase pure tones, then shaping the noise with a full-wave rectified, sinusoidal spectral envelope to produce spectral ripples, or regular variations in amplitude on a log-frequency axis. The bandwidth of the stimuli was 100-5000 Hz. After constructing the ripples, stimuli were filtered to match the long-term speech spectrum (Byrne et al. 1994). Ripple densities were produced for 14 log-spaced values (0.125, 0.176, 0.25, 0.354, 0.5, 0.707, 1.0, 1.414, 2.0, 2.828, 4.0, 5.657, 8.0, and 11.314 ripples per octave, or rpo). A 30-dB modulation depth (peak-to-valley) was used. The stimuli were 500 ms in total duration, which included 150 ms onset/offset ramps and a 200 ms steady-state portion, and were presented in the sound field at 61 dBA, with a random level rove of ± 4 dB to greatly reduce the potential for the use of overall level cues.
Spectral ripple stimuli for the clinical ripple task were similar to those described above, with three main differences. First, denser noise was produced by summing 2555 random-phase pure tones, in order to minimize potential variations in the ripple spectrum at high ripple densities. Second, the phase of the spectral ripple envelope for the clinical ripple task was randomized to prevent listeners from focusing on level changes at the stimulus edges. The phase of the standard stimuli within a trial was determined by the equation θ = x*(2π) radians, where x is a random number between 0 and 1; the phase of the corresponding inverted stimuli = θstand+(π/2) radians. Third, ripple densities included 12, rather than 14, log-spaced values (0.125, 0.176, 0.25, 0.354, 0.5, 0.707, 1.0, 1.414, 2.0, 2.828, 4.0, and 5.657 rpo), eliminating the highest ripple densities in order to streamline the procedure.
Procedure
Stimuli for non-speech and speech tests were presented in the sound field using custom MATLAB programs via a Macintosh G5 computer and Crown D45 amplifier, within a sound-treated booth (IAC). A single B&W DM303 speaker (B&W Loudspeakers of America, North Reading, MA) was located one meter from the listener’s seated position. The B&W DM303 speaker is a studio monitor with amplitude and phase responses exceeding ANSI standards for speech audiometry. Subjects used their own speech processors with their typical use settings. A three-interval, three-alternative forced-choice (3I-3AFC) task was employed. On each trial, two standard and one inverted stimulus were presented, and the task was to select the interval that sounded different from the other two. No correct-response feedback or training trials were provided.
The Adaptive ripple task employed a 2-up, 1-down procedure, converging on 70.7% correct, to determine the ripple discrimination threshold -- the highest ripple density (in rpo) at which listeners were able to discriminate an inverted signal from two standard stimuli, identical to the inverted one except that the positions of spectral peaks and valleys were reversed (see Figure 1 for a diagram of the ripple stimuli spectra). Six test runs were completed for each subject, and the average of the six individual thresholds was determined.
Figure 1.
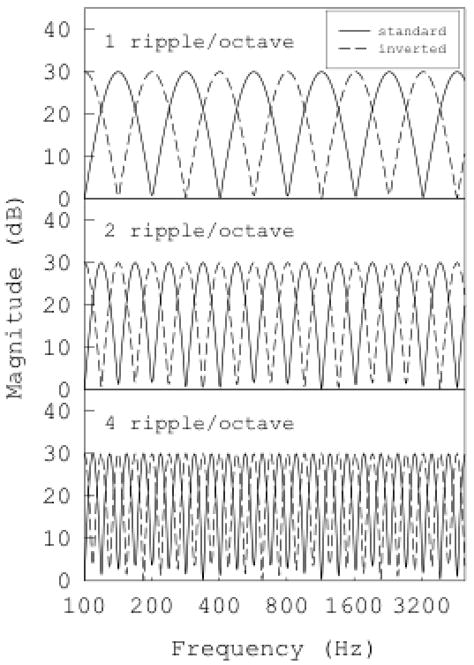
Amplitude spectra (magnitude, in dB, as a function of frequency) of spectral-ripple stimuli used in the spectral-ripple discrimination tasks. Standard and inverted ripples for ripple densities of 1, 2, and 4 ripples/octave are displayed.
In the Clinical ripple task, a method of constant stimuli procedure was used, with each ripple frequency represented on six trials that occurred in random order. Proportion correct (p[c]) for each of the ripple densities was calculated, and psychometric functions were produced. The p(c) across all ripple densities for each subject was calculated, representing a measure of the area under the psychometric function. Two separate experimental repetitions were completed on different dates, usually at least one week apart. One subject, S4, completed both experimental repetitions on the same day, separated by several hours, because the distance the subject lived from the laboratory precluded a separate second visit. Another subject, S98, withdrew from the study after completing just the first experimental administration of clinical ripple.
Word recognition
Subjects were assessed on monosyllabic word recognition using Consonant-Nucleus-Consonant (CNC) word lists (Peterson et al. 1962), spoken by one male talker and presented in quiet in the sound field from the same single speaker as the spectral ripple stimuli. The words were presented at 62 dBA. Subjects used their own speech processors at typical use settings; programs optimized for noise reduction were not used. Subjects verbally repeated each word presented; their responses were recorded by one of the experimenters, sitting outside of the booth and listening via an intercom. Results were scored in terms of percent words and percent phonemes correct; these scores were later transformed to rationalized arcsine units (RAU) for analysis.
RESULTS
1. Clinical ripple vs. Adaptive ripple
Adaptive ripple discrimination results are reported as ripple discrimination thresholds, in rpo. The average ripple discrimination threshold across subjects was 2.60 rpo, with a range of 0.63 to 7.03 rpo. This represents somewhat better performance than previous studies; Anderson et al. (2011) reported average ripple discrimination performance of 1.68 rpo, with a range of 0.41 to 4.27, and Won et al.(Won et al. 2007) reported an average of 1.73 rpo, with a range of 0.6 to 4.87 rpo.
Clinical ripple results were quantified for each subject as average proportion correct performance, p(c), across all ripple densities. The clinical ripple p(c) across all ripple densities and subjects was 0.78 (i.e., 78% correct), with a range of 0.57 to 0.96 and a normal distribution.
Figure 2 displays adaptive ripple discrimination thresholds as a function of clinical ripple scores for all subjects. Panel a includes clinical ripple scores for the first test repetition only; panel b shows the average of two test repetitions. Linear regression analysis of the data revealed significant correlations between the adaptive and clinical ripple tests: r=0.79, p<0.001 for the first repetition only, and r=0.82, p<0.001 for the average of two clinical ripple repetitions. Since the adaptive ripple data did not meet the statistical criterion for normal distribution (Shapiro-Wilk statistic=0.90, p=0.01), a nonparametric procedure, the Spearman’s rank order correlation coefficient, was also calculated. The results did not change; the Spearman’s rho revealed a statistically significant relationship between adaptive and clinical ripple tests: ρ=0.817, p<0.001 for the first repetition only, and ρ=0.882, p<0.001 for the average of two clinical ripple repetitions.
Figure 2.
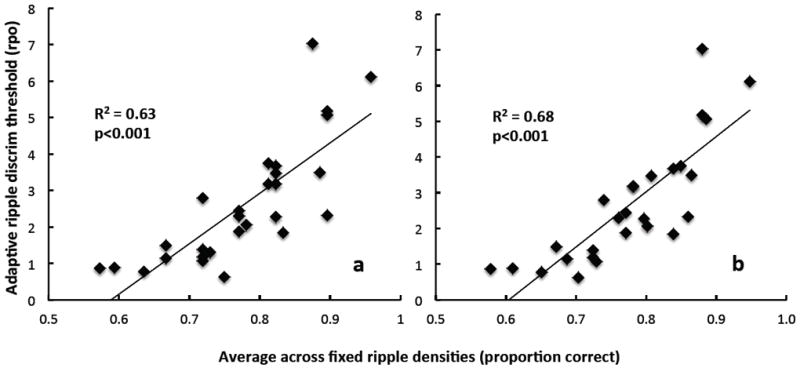
Adaptive ripple discrimination thresholds (rpo) as a function of clinical ripple performance (average proportion correct across all ripple densities). Panel a: first repetition of clinical ripple test. Panel b: average of two repetitions of clinical ripple test.
2. Test-retest reliability
The test-retest reliability of the clinical ripple test, evaluated by comparing performance obtained at two different test sessions, was high (r = 0.90, p < 0.0001). A comparison of the two repeated measures is displayed in Figure 3 with p(c) for the first repetition represented on the abscissa, and for the second repetition on the ordinate. Data for 27 subjects are included and are plotted against a diagonal line projecting perfect correspondence between the two measures.
Figure 3.
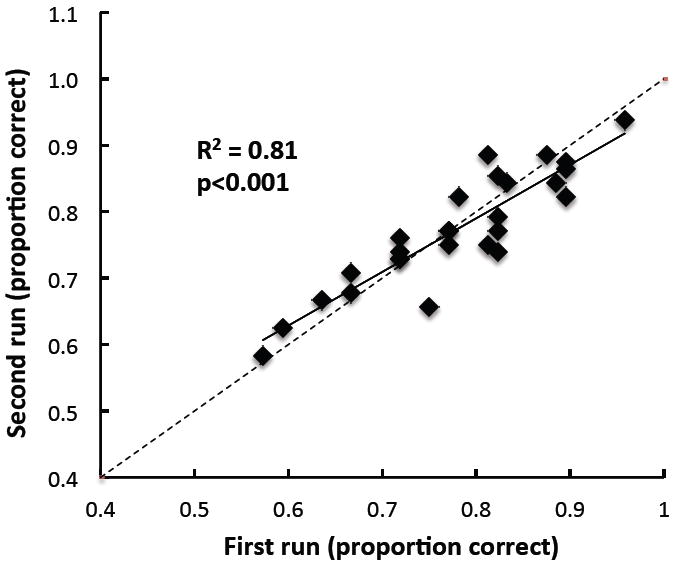
Performance on second repetition of clinical ripple test as a function of performance on first repetition, for 27 subjects. The diagonal line represents perfect correspondence.
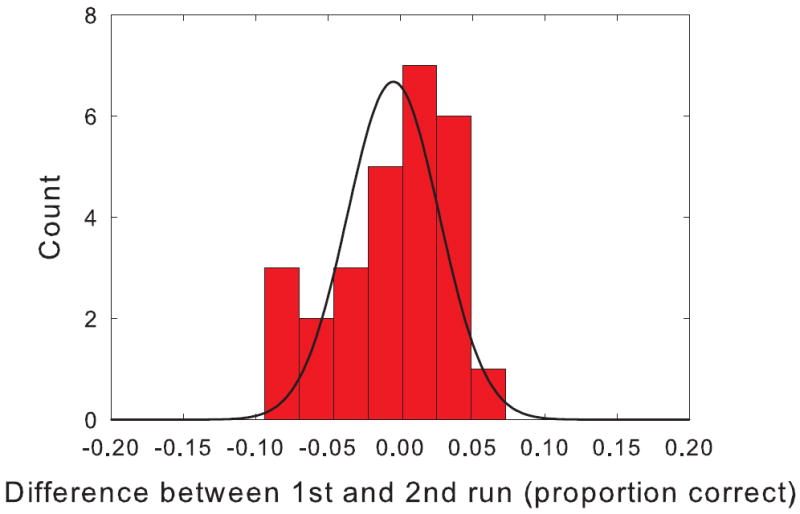
The average change in performance from the first repetition of the clinical ripple test to the second was -0.58% which was not statistically different from 0. The standard deviation of the test-retest difference scores was 4.2%. Figure 4 shows the distribution of difference scores with a best fit normal curve. The distribution was not significantly different from normal. The 95% confidence interval for a single run in a single patient is about two times the standard deviation or 8.4%. Based on these data, there is less than a 1 in 20 probability that a difference of more than 8.4% would occur by chance. Thus, if a single patient were tested in two conditions and the difference in the clinical spectral ripple discrimination score was greater than 8.4%, the difference could be considered to be statistically significant.
Figure 4.
Histogram showing the differences scores between the 1st and 2nd run of the clinical spectral ripple discrimination test. A normal curve is shown fit to the data.
3. Relationship between clinical ripple and word recognition
Performance on CNC word recognition was relatively good within this group of CI listeners and well distributed. Average percent correct was 68.85, with a range of 12% to 100%. For comparison, the group mean for CNC monosyllabic word recognition for 115 unilateral CI users reported by Gifford et al.(2008) was 55.7%. Drennan et al. (2010) reported mean performance of approximately 70% for 10 Advanced Bionics implant users. The average score converted to RAU units was 69.66. Average percent phonemes correct was 82.67.
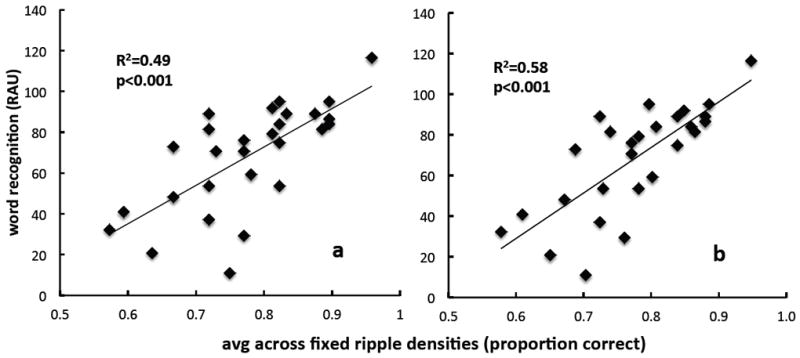
Linear regression analysis, comparing clinical ripple results with CNC word recognition scores, indicated a significant correlation: r=0.70, p<0.001 for the first experimental repetition only (Figure 5, panel a), and r=0.76, p<0.001 for the average of two repetitions (panel b). This corresponds to 49% and 58%, respectively, of the variance in word recognition accounted for using the clinical ripple test.
Figure 5.
Word recognition (rau scores) as a function of clinical ripple performance (proportion correct) for all subjects. Panel a: first repetition of clinical ripple test. Panel b: average of two repetitions of clinical ripple test.
The corresponding linear relationship between adaptive ripple thresholds and word recognition was similar: r = 0.66, corresponding to 44% of variance accounted for. However, when a logarithmic function (best fit, in a least-squares sense) was used, the proportion of word recognition variance accounted for by adaptive ripple thresholds rose to 54% (r = 0.73) (Figure 6). It appears that there is a level of adaptive spectral ripple performance, ~3 rpo, beyond which it provides little additional benefit to CNC performance.
Figure 6.
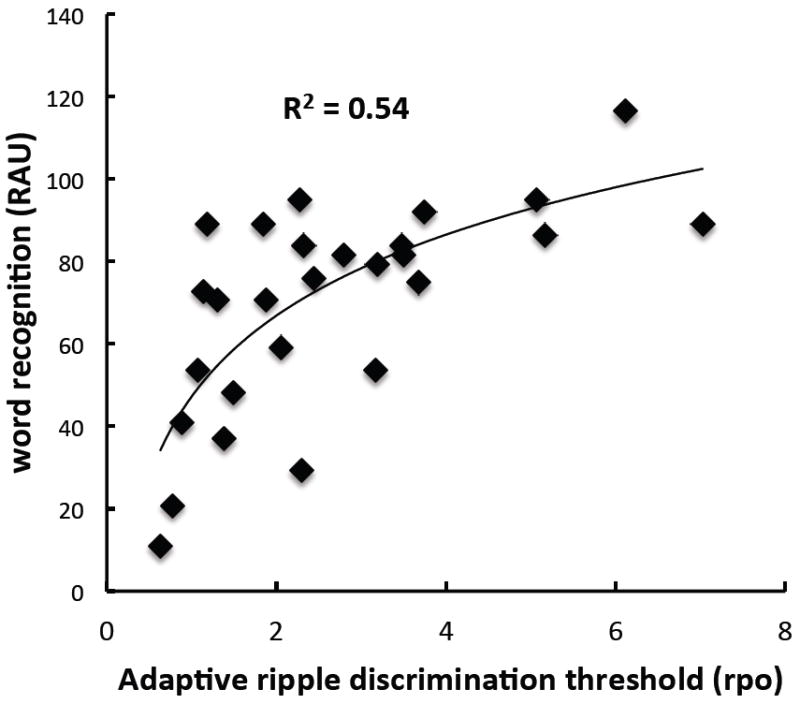
Word recognition (rau scores) as a function of adaptive spectral-ripple discrimination thresholds (rpo). A logarithmic fit is displayed.
DISCUSSION
The work demonstrates that the clinical spectral ripple discrimination test is viable for clinical use because it can be implemented reliably and rapidly. Furthermore, the study demonstrates that the clinical spectral ripple discrimination test correlates well with the more time-consuming adaptive spectral ripple discrimination procedure, which has been shown to correlate well with speech and music perception measures (Henry et al. 2005; Won et al. 2010; Won et al. 2007). This measure has also been proven to reflect spectral resolution abilities in cochlear implant users (Jones et al. 2013; Won, Jones et al. 2011). Most importantly, the clinical ripple discrimination test scores correlate significantly with open-set word recognition in quiet. Demonstration of the correlation of the measure with a clinically important measure is the first and primary step in establishing spectral ripple discrimination as a viable surrogate outcome measure for evaluating hearing ability.
Surrogate measures are used to evaluate clinical outcome when comparing treatment approaches and are intended to reflect the most important clinical endpoint, namely how well a patient feels, functions and survives (Fleming et al. 2012). A good, validated surrogate clearly demonstrates the clinical efficacy of a treatment. Speech recognition is regarded as a good surrogate for hearing loss treatments. In this study, a correlation was observed between CNC word recognition and spectral ripple discrimination in cochlear implant users; however, “A correlate does not a surrogate make” (Fleming et al. 1996). While it might be reasonably expected that an improvement in spectral resolution would reflect improvement in true clinical outcome, spectral ripple discrimination has only been established as a correlate with another established surrogate, speech perception. Further work must be done to determine whether treatment-based improvements in spectral ripple discrimination coincide with improved speech recognition or with improved clinical outcome.
As a correlate, a rapid spectral resolution test could be used clinically as a diagnostic tool. It would not be required necessarily that the measure be a fully validated surrogate for evaluating treatment effects if the test is used for diagnostic purposes. For example, such a tool could be used preoperatively, to help determine cochlear implant candidacy (Moon et al. 2013; Shim et al. submitted). A similar spectral resolution test, spectral ripple detection, has been shown to predict which ear is most likely to benefit from amplification (Zhang et al. 2013). Thus, such a test might be used to help determine which ear to implant. Furthermore, the clinical spectral ripple discrimination test could be most valuable when speech testing is not possible. The test has the advantage of being non-linguistic, so could be useful for subjects who speak any language, or it could be used in children (Jung et al. 2012), or, with modified techniques, for young children, such as observer-based approaches (Dasika et al. 2009) or electrophysiological approaches (Won, Clinard et al. 2011). Given the extended pre-operative assessment commonly required in children and its subjective nature in some cases, use of validated non-linguistic tests could potentially save significant time and improve objectivity.
While the current results show a correlation with CNC word recognition (r = 0.7), the power of the clinical ripple test to predict speech perception might be limited in the mid range of 70-75% correct on the ripple test (see Figure 5). This mid-range variance might be partially the result of random outliers i.e., a sampling effect, or it might reflect that some factor other than spectral resolution is contributing to speech understanding ability in quiet. Temporal resolution is a plausible candidate. For example, Won, Drennan et al.(2011) showed that both adaptive temporal modulation detection and adaptive spectral-ripple discrimination thresholds correlate with CNC word recognition scores in quiet, but that the two tests did not correlate with each other. Using a two-parameter model, they showed that combined, the tests correlate more highly with CNC word scores than the best correlate alone. A clinical assessment of temporal modulation ability used in combination with the spectral ripple test, or a non-linguistic test that combines both spectral and temporal abilities, might reduce the midrange variance.
It is important to note that while the current results show a significant correlation between scores from the clinical ripple test and CNC word scores, they do not actually evaluate how well clinical ripple performance predicts CNC word performance, because this is a single random sample. The results generate a plausible hypothesis that this clinical spectral ripple discrimination task could predict speech performance well, but in order to evaluate the clinical spectral ripple discrimination task as a predictor, the current results should be used as a model to predict performance in another cohort.
In summary, a clinical measure of spectral ripple discrimination has been developed, and the results of the present study suggest that it is a reliable and valid measure, giving results consistent with those obtained using the lengthier adaptive version of the test. The test has potential for estimating spectral resolution in an efficient manner in clinical settings.
Acknowledgments
Sources of Funding: This research was supported by NIH grants R01-DC010148 and P30-DC04661, and an educational grant from Advanced Bionics. JTR has been a paid consultant for Cochlear Ltd and Advanced Bionics Corporation.
Footnotes
Conflicts of Interest For the remaining authors, none were declared.
Contributor Information
Ward. R. Drennan, VM Bloedel Hearing Research Center, Department of Otolaryngology, University of Washington
Elizabeth S. Anderson, CCC-A, Envoy Medical Corp., St. Paul, MN
Jong Ho Won, Department of Audiology and Speech Pathology, University of Tennessee
Jay T. Rubinstein, VM Bloedel Hearing Research Center, Department of Otolaryngology, University of Washington
References
- Anderson ES, Nelson DA, Kreft H, et al. Comparing spatial tuning curves, spectral ripple resolution, and speech perception in cochlear implant users. J Acoust Soc Am. 2011;130:364–375. doi: 10.1121/1.3589255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, Oxenham AJ, Nelson PB, et al. Assessing the role of spectral and intensity cures in spectral ripple detection and discrimination in cochlear-implant users. J Acoust Soc Am. 2012;132 doi: 10.1121/1.4763999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenstein CK, Mens LHM, Mulder JJS, et al. Current steering and current focusing in cochlear implants: comparison of monopolar, tripolar and virtual channel electrode configurations. Ear Hear. 2008;29:250–260. doi: 10.1097/aud.0b013e3181645336. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Green DM. Detection of simple and complex changes of spectral shape. J Acoust Soc Am. 1987;82 doi: 10.1121/1.395147. [DOI] [PubMed] [Google Scholar]
- Byrne D, Dillon H, Tran K, et al. An international comparison of long-term average speech spectra. J Acoust Soc Am. 1994;96:2108–2120. [Google Scholar]
- Dasika VK, Werner LA, Norton SJ, et al. Measuring sound detection and reaction time in infant and toddler cochlear implant recipients using an observer-based procedure: A first report. Ear Hear. 2009;30:250–261. doi: 10.1097/AUD.0b013e3181986dfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Dawson PK, Borden LZ. Within-subjects comparison of the HiRes and Fidelity120 speech processing strategies: speech perception and its relation to place-pitch sensitivity. Ear and hearing. 2011;32:238–250. doi: 10.1097/AUD.0b013e3181fb8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, Won JH, Jameyson EM, et al. Stability of clinically-meaningful, non-linguistic measures of hearing performance with a cochlear implant. 2011 Conference on Implantable Auditory Prostheses; Pacific Grove, CA. 2011. p. 297. [Google Scholar]
- Drennan WR, Won JH, Nie K, et al. Sensitivity of psychophysical measures to signal processor modifications in cochlear implant users. Hear Res. 2010;262:1–8. doi: 10.1016/j.heares.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins DA, Bero EM. Spectral modulation detection as a function of modulation frequency, carrier bandwidth, and carrier frequency region. J Acoust Soc Am. 2007;121:363–372. doi: 10.1121/1.2382347. [DOI] [PubMed] [Google Scholar]
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: Are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Statistics in Medicine. 2012;31:2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: Consideration for cochlear implant programs. Audiol Neurotol. 2008;13:193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Jones GL, Won JH, Drennan WR, et al. Relationship between channel interaction and spectral-ripple discrimination in cochlear implant users. J Acoust Soc Am. 2013;133:425–433. doi: 10.1121/1.4768881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Won JH, Drennan WR, et al. Psychoacoustic performance and music and speech perception in prelingually deafened children with cochlear implants. Audiol Neurootol. 2012;17:189–197. doi: 10.1159/000336407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Saoji AA, et al. Relationship between percpetion of spectral ripple and speech recognition in cochlear implant and vocoder listeners. J Acoust Soc Am. 2007;122:982–991. doi: 10.1121/1.2749413. [DOI] [PubMed] [Google Scholar]
- Moon IJ, Shim HJ, Won JH, et al. Use of psychoacoustic measures to evaluate cochlear implant candidacy. 2013 Conference on Implantable Auditory Prostheses; Lake Tahoe, CA. 2013. p. 220. [Google Scholar]
- Noble JH, Labadie RF, Gifford RH, et al. Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Trans Neural Syst Rehabil Eng. 2013 doi: 10.1109/TNSRE.2013.2253333. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Saoji AA, Litvak L, Spahr AJ, et al. Spectral modulation detection and vowel and consonant identifications in cochelar implant listeners (L) J Acoust Soc Am. 2009;126:955–958. doi: 10.1121/1.3179670. [DOI] [PubMed] [Google Scholar]
- Shim J, Won JH, Moon IJ, et al. Can unaided non-linguistic measures predict cochlear implant candidacy? Otol Neurotol. doi: 10.1097/MAO.0000000000000323. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr A, Saoji A, Litvak L, et al. Spectral cues for understanding speech in quiet and noise. Int J Audiol. 2011;12:S66–S69. doi: 10.1179/146701011X13001035753056. [DOI] [PubMed] [Google Scholar]
- Supin AY, Popov VV, Milekhina ON, et al. Frequency resolving power measured by rippled noise. Hear Res. 1994;78:31–40. doi: 10.1016/0378-5955(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Surprenant AM, Watson CS. Individual differences in the processing of speech and nonspeech sounds by normal-hearing listeners. J Acoust Soc Am. 2001;110:2085–2095. doi: 10.1121/1.1404973. [DOI] [PubMed] [Google Scholar]
- Watson CS, Jensen JK, Foyle DC, et al. Performance of 146 normal adult listeners on a battery of auditory discrimination tests. J Acoust Soc Am. 1982;71:S73. [Google Scholar]
- Watson CS, Johnson DM, Lehman JR, et al. An auditory discrimination test battery. J Acoust Soc Am. 1982;71:S73. [Google Scholar]
- Won JH, Clinard C, Kwon S, et al. Relationship between behavioral and physiological spectral-ripple discrimination. J Assoc Res Otolaryngol. 2011;12:375–393. doi: 10.1007/s10162-011-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Kang RS, et al. Psychoacoustic abilities associated with music perception in cochlear implant users. Ear Hear. 2010;31:796–805. doi: 10.1097/AUD.0b013e3181e8b7bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Nie K, et al. Acoustic temporal modulation and speech perception in cochlear implant users. J Acoust Soc Am. 2011;130:376–388. doi: 10.1121/1.3592521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolarygol. 2007;8:384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Jones GL, Drennan WR, et al. Evidence of across-channel processing for spectral-ripple discrimination in cochlear implant listeners. J Acoust Soc Am. 2011;130:2088–2097. doi: 10.1121/1.3624820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Spahr AJ, Dorman MF, et al. Relationship between auditory function of nonimplanted ears and bimodal benefit. Ear Hear. 2013;34:133–141. doi: 10.1097/AUD.0b013e31826709af. [DOI] [PMC free article] [PubMed] [Google Scholar]


