Abstract
Elevated levels of combustion-derived particulate matter (CDPM) are a risk factor for the development of lung diseases such as asthma. Studies have shown that CDPM exacerbates asthma, inducing acute lung dysfunction and inflammation; however, the impact of CDPM exposure on early immunological responses to allergens remains unclear. To determine the effects of early-life CDPM exposure on allergic asthma development in infants, we exposed infant mice to CDPM and then induced a mouse model of asthma using house dust mite (HDM) allergen. Mice exposed to CDPM+HDM failed to develop a typical asthma phenotype including airway hyperresponsiveness, Th2-inflammation, Muc5ac expression, eosinophilia, and HDM-specific Ig compared to HDM-exposed mice. Although HDM-specific IgE was attenuated, total IgE was two-fold higher in CDPM+HDM mice compared to HDM-mice. We further demonstrate that CDPM exposure during early life induced an immunosuppressive environment in the lung, concurrent with increases in tolerogenic dendritic cells and Tregs, resulting in suppression of Th2 responses. Despite having early immunosuppression, these mice develop severe allergic inflammation when challenged with allergen as adults. These findings demonstrate a mechanism whereby CDPM exposure modulates adaptive immunity, inducing specific-antigen tolerance while amplifying total IgE, and leading to a predisposition to develop asthma upon rechallenge later in life.
Keywords: particulate matter, immunosuppression, neonatal
Introduction
Exposure to elevated levels of ambient particulate matter (PM) during early life has long been associated with development of acute/chronic pulmonary morbidities (e.g., asthma) later in life.1 Though epidemiology suggests young children are most affected,2, 3 relatively little data, mechanistic or otherwise, exist detailing the impacts of PM exposure on this immunologically immature population.4 Exacerbation of pulmonary dysfunction has been demonstrated in allergen-sensitized adult mice exposed to PM during allergen challenge.5, 6 While clinically relevant, these studies focus heavily on the acute effects of PM exposure during the “challenge” phase of asthma which bypasses possible PM effects during earlier sensitization. In addition, adult mice with mature immune systems respond very differently than infants, therefore failing to inform us about the role of early-life exposure in the development of the airway disease itself.
Our previous work with combustion-derived particulate matter (CDPM) has shown that acute early-life exposure induces oxidative stress and inflammation in the lung.7 These effects are accompanied by significant pulmonary remodeling including airway smooth muscle thickening brought upon by CDPM exposure-induced epithelial-to-mesenchymal transitions (EMT).8 Bronchiolar epithelial disruption caused by CDPM exposure imparts a potentially important change in “programming” of subsequent adaptive immune responses primarily through actions of dendritic cells (DCs), the professional antigen-presenting cell. When E-cadherin-mediated cell:cell junctions are disrupted, DCs undergo a phenotypic shift toward the promotion of tolerance.9
In the present study, we sought to determine the role of early life exposure to PM on the development of allergic asthma (i.e., PM exposure prior-to and during allergen sensitization and challenge). The question arose due to the lack of data on the impacts of PM exposure in infants and the lack of studies examining the effects of allergen exposure during PM exposure in infants, an equally clinically relevant scenario. We found that exposure to CDPM during early life creates an immunosuppressive environment in the lung which impairs T effector cell production in vivo. Combining CDPM exposure with exposure to house dust mite allergen (HDM) in a mouse model of allergic asthma10, we showed that infant mice exposed to CDPM and HDM fail to develop certain hallmarks of asthma while maintaining others. Interestingly, this immunosuppression is lost over time; mice that exhibited dampened Th2 effector responses early in life developed an exacerbated asthma phenotype when rechallenged with allergen as adults. Taken together, our data suggest a mechanism whereby CDPM exposure during infancy alters pulmonary immune responses and modulates long-term pulmonary inflammatory disease development.
Results
Exposure to CDPM modulates early-life allergic airway disease in mice
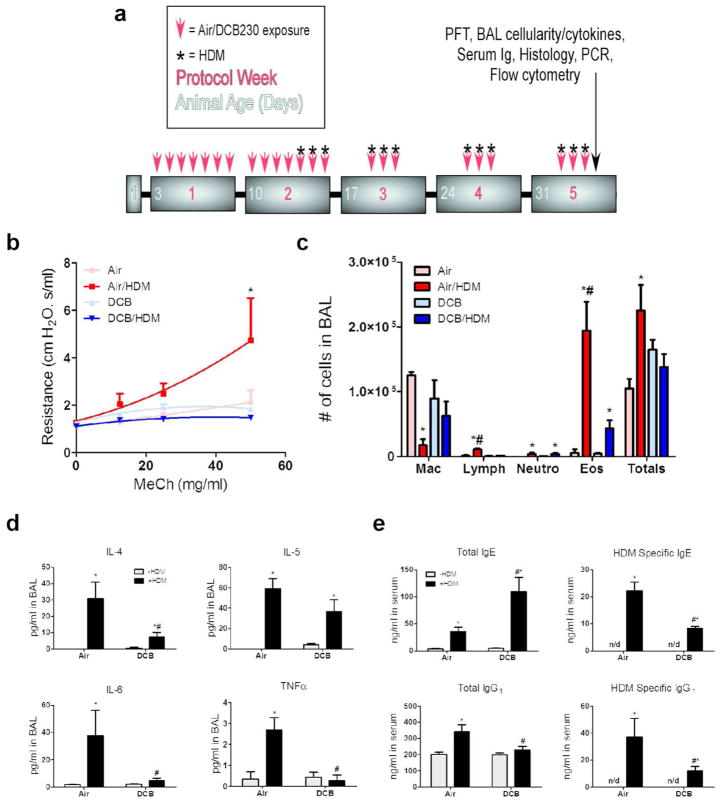
To investigate the impact of CDPM exposure in an early-life model of allergic airways disease, we exposed mouse pups beginning at 3d of age to CDPM (DCB) before and during the establishment of an HDM-model of asthma (Fig. 1a). Twenty four hours following the final exposure day, pulmonary function was analyzed by flexiVent (Fig. 1b). Interestingly, AHR, a physiological hallmark of asthma, was only observed in the Air/HDM mice and not DCB/HDM mice. Following functional tests, bronchoalveolar lavage (BAL) fluid was collected and analyzed for cellularity (Fig. 1c). A significant increase in total cell number occurred only in Air/HDM mice. Furthermore, a large increase in eosinophils, commonly seen in asthmatics, was observed only in the Air/HDM mice but was noticeably lower in the DCB/HDM mice. Lymphocyte numbers were also increased in the Air/HDM group, while neutrophils were equally increased in both Air/HDM and DCB/HDM mice. Cell-free BAL fluid was analyzed for cytokine content using multiplex (Fig. 1d). As expected, Th2 cytokines (IL-4, IL-5) were significantly elevated in Air/HDM and DCB/HDM mice compared to respective controls, however, IL-4 was approximately 3-fold lower in DCB/HDM mice compared to Air/HDM mice. Other proinflammatory cytokines (IL-6, TNF-α) were elevated in only Air/HDM mice. Serum from these animals was analyzed for immunoglobulin production (Fig. 1e). Surprisingly, total IgE was statistically increased in both Air/HDM and DCB/HDM mice compared to controls and levels were approximately 3-fold higher in DCB/HDM mice compared to Air/HDM mice. Conversely, HDM-specific IgE levels were significantly lower in DCB/HDM mice compared to Air/HDM mice. Total IgG1 was elevated only in Air/HDM mice and once again HDM-specific IgG1 levels were reduced in DCB/HDM mice compared to Air/HDM mice.
Figure 1.
Exposure to combustion-derived particulate matter (CDPM) modulates early-life allergic airway disease in mice. (a) Schematic of early-life HDM ± CDPM asthma model in mice. All analyses were done 24 hr following final protocol day. (b) Airway hyperresponsiveness, determined by airway resistance in response to increasing doses of inhaled methacholine. Airway resistance values were determined by forced oscillation technique using snapshot perturbation. (c) Total number of cells and differential cell counts for macrophages (Mac), lymphocytes (Lymph), neutrophils (Neutro), and eosinophils (Eos) recovered in BAL. (d) Cytokine protein levels in recovered BAL determined by multiplex assay. (e) Serum antibody concentrations determined by ELISA. Data plotted as means ± SEM. *p<0.05 vs. −HDM within exposure group, #p<0.05 vs. +HDM in opposite exposure group; Two-way ANOVA with Bonferroni post-tests.
CDPM exposure decreases Th2 and Treg inflammation in early-life asthma model
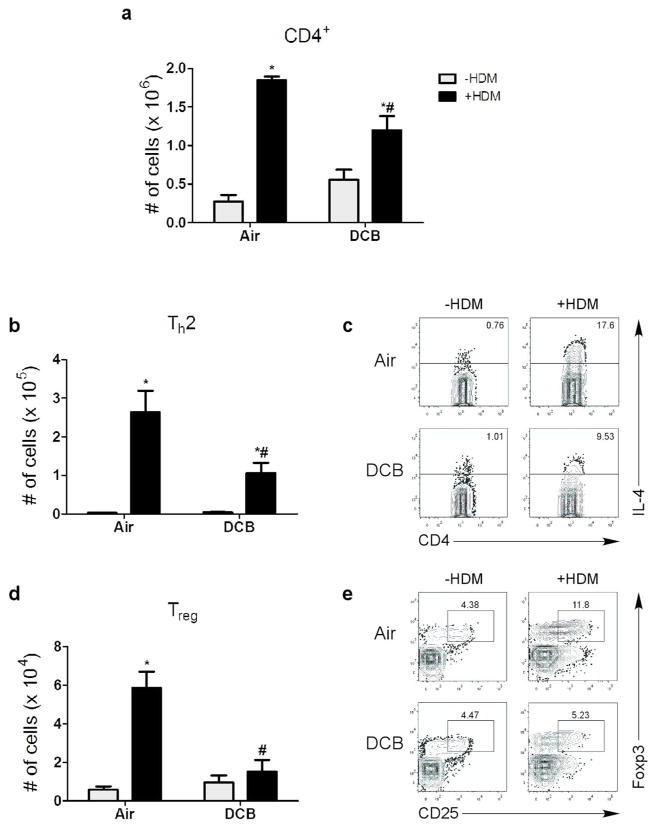
IL-4 producing Th2 lymphocytes are regularly associated with allergic asthma, and relative level of Th2 inflammation is positively correlated with asthma symptoms.11 To study the effects of CDPM exposure on Th2 inflammation in an asthma model, levels of Th2 lymphocytes (CD4+ IL-4+) were assessed in mice (Fig. 2). Total CD4+ lymphocytes were elevated in all mice given HDM relative to controls, but a statistical decrease was observed in DCB/HDM mice compared to Air/HDM (Fig. 2a). Expectedly, levels of Th2 lymphocytes were elevated in all HDM groups (Fig. 2b, c). However, Th2 levels were significantly lower in DCB/HDM mice compared to Air/HDM mice. Due to the previously observed inflammatory suppression in CDPM-exposed mice, we assessed Treg (CD25+ Foxp3+) levels. We observed an increase in Treg cell numbers in the lungs of Air/HDM mice compared to Air mice (Fig. 2d, e). Conversely, Treg levels were significantly decreased in mice exposed to DCB/HDM. Neither Th2 nor Treg cell numbers were different between Air and DCB groups.
Figure 2.
CDPM exposure decreases Th2 and Treg inflammation in early-life asthma model. (a) Total numbers of CD4+ lymphocytes in the lungs measured by flow cytometry. (b, c) Th2 lymphocyte (CD4+ IL-4+) levels in the lungs, shown as (b) total numbers and (c) representative flow dot plots. (d, e) Treg (CD25+ Foxp3+) numbers in the lungs, shown as (d) total and (e) representative dot plots. Data plotted as means ± SEM. *p<0.05 vs. −HDM within exposure group, #p<0.05 vs. +HDM in opposite exposure group; Two-way ANOVA with Bonferroni post-tests.
CDPM exposure modulates mucus-associated gene expression but not airway mucus production in early-life asthma model
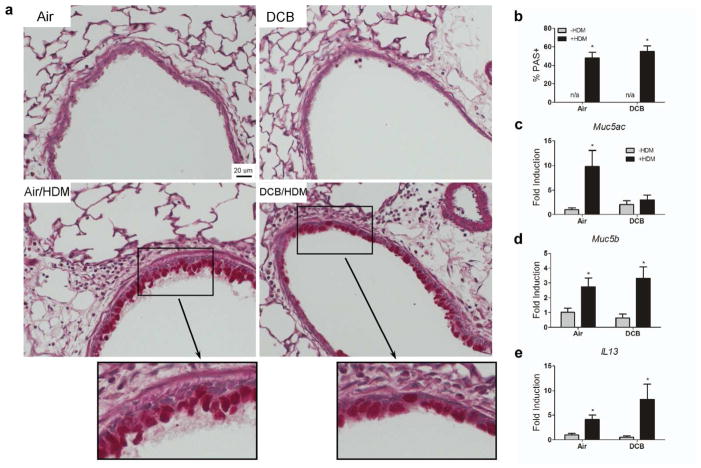
Increased airway mucus production via goblet cell-hyperplasia is commonly seen in allergic airway diseases (i.e., asthma). To determine the impact of CDPM exposure on HDM-induced mucus in the airways, we quantified mucus content in the airways using Periodic Acid-Schiff (PAS) staining on lung sections and mucus gene expression in the lungs (Fig. 3). Percent mucus positive cells (Fig. 3a, inset) were found to be significantly elevated in all mice receiving HDM, with no statistical difference in DCB/HDM and Air/HDM mice (Fig. 3b). Mucus-associated gene expression was investigated by RT-PCR on whole lung tissue (Fig. 3c–e). Expression of Muc5b and IL13 was elevated in both groups receiving HDM relative to controls. Interestingly, Muc5ac expression was only elevated in Air/HDM mice relative to controls.
Figure 3.
CDPM exposure modulates mucus-associated gene expression but not airway mucus production in early-life asthma model. (a) Representative micrographs of histological sections of mouse lungs stained for mucus (PAS, bright pink exemplified in inset). (b) Percent mucus-positive cells per total epithelial cells in airways. (c–e) Gene expression (Muc5ac, Muc5b, IL13) in whole lung tissue. Data plotted as means ± SEM. *p<0.05 vs. −HDM within exposure group, Two-way ANOVA with Bonferroni post-tests.
Acute CDPM exposure causes an immunosuppressive environment in the lung
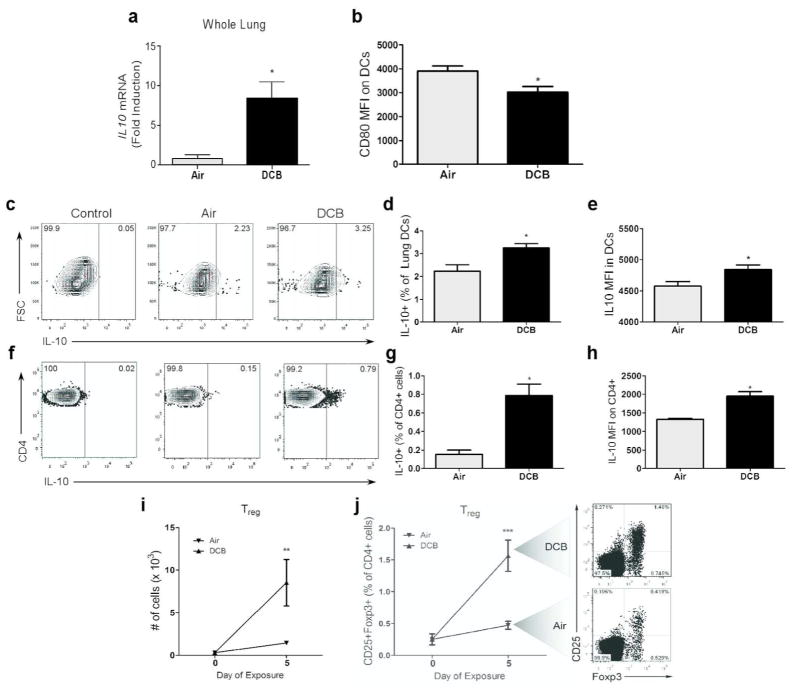
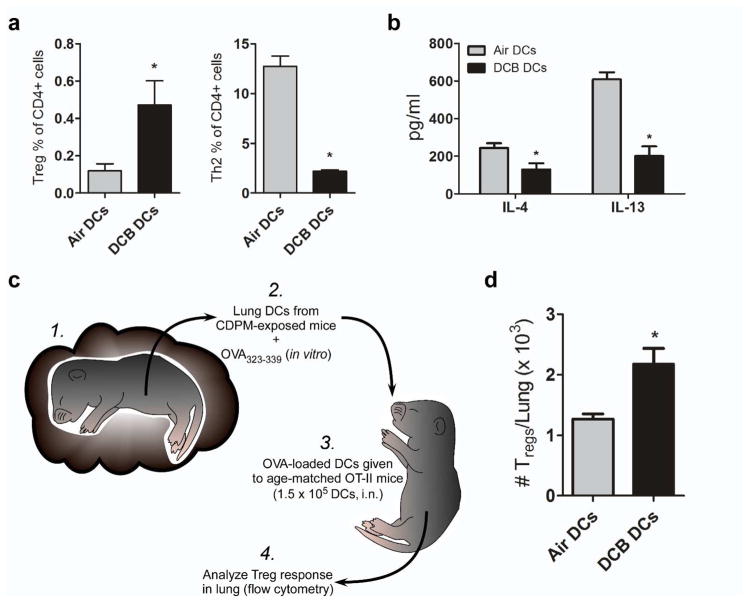
Immunosuppressive effects of CDPM were investigated by measuring levels/sources of IL-10 in the lung (Fig. 4a–h). After acute (5 consecutive days) exposure to DCB, expression of IL10 in whole lung tissue was elevated compared to air controls (Fig. 4a). Lung DCs (CD11c+ F4/80−), which are crucial determinants in adaptive immune responses, were less activated following exposure as evidenced by decreased co-stimulatory molecule (CD80) expression (Fig. 4b). Using IL-10-GFP reporter mice to discern major sources of IL-10, an increased number of IL-10-producing DCs were observed in the lungs of DCB-exposed mice (Fig. 4c, d). In addition to increased number, these DCs produced higher levels of IL-10 compared to IL-10+ DCs from control mice as evidenced by increased MFI (Fig. 4e). Further investigation of DC subsets (mDC, MHCIIhi CD11b+; interstitial DC, MHCIIhi CD11b−) showed interstitial DCs as the main source of DC-derived IL-10 (Supplementary Fig. S1). Increased numbers of IL-10+ CD4 lymphocytes were also observed in DCB-exposed mice (Fig. 4f, g). These IL-10+ cells also expressed higher levels of IL-10 compared to controls (Fig. 4h). No difference in IL-10 production was observed in other cell types investigated (data not shown). The ability of CDPM exposure to elicit Treg responses in the lung was investigated by flow cytometry (Fig. 4i, j). Following an acute exposure to DCB, pulmonary Treg (CD3+ CD4+ CD25+ Foxp3+) numbers were increased compared to control mice.
Figure 4.
Acute CDPM exposure causes an immunosuppressive environment in the lung. (a) IL10 expression in whole lung tissue measured by RT-PCR. (b) Mean fluorescence intensity (MFI) of stimulation marker CD80 on lung DCs (CD11c+ F4/80−). (c–e) IL-10 expression in DCs from IL-10-GFP reporter mice expressed as (d) a percentage (IL-10+) of total lung DCs and (e) MFI as determined by flow cytometry. (f–h) IL-10 expression in lung CD4+ T cells (CD3+ CD4+) of IL-10-GFP reporter mice expressed as (g) a percentage (IL-10+) of total lung CD4+ T cells and (h) MFI. (i, j) Increase in regulatory T cell (Treg; CD3+ CD4+ CD25+ Foxp3+) numbers in the lungs of OT-II mice during acute exposure expressed as (i) total number of cells and as (j) a percentage of all CD4+ T cells. Data plotted as means ± SEM. *p<0.05, t-test.
CDPM exposure dampens Th2 inflammation in favor of a regulatory response in vitro and in an adoptive transfer model
The function of phenotypically immunosuppressive DCs resulting from CDPM exposure was investigated using an in vitro T cell activation assay. When co-cultured with antigen-specific naïve CD4+ cells from age-matched OT-II mice, lung DCs isolated from DCB-exposed mice promote significantly increased Treg and significantly decreased Th2 effector polarization compared to lung DCs isolated from control mice (Fig. 5a). Supernatant Th2 cytokine (IL-4, IL-13) concentrations were also significantly decreased in co-cultures with DCB-exposed DCs compared to control DCs (Fig. 5b). In order to see if DCB-exposed DCs were capable of driving Treg-biased differentiation in vivo, we isolated pulmonary DCs from Air or DCB exposed mice, loaded them with OVA323-339 peptide in vitro, and then adoptively transferred those DCs into age-matched OT-II mice (Fig. 5c). Similarly to effector responses observed in vitro, the DCs from DCB-exposed mice generated a significantly higher Treg response in the lungs of recipient mice (Fig. 5d). Negligible Th2 responses were observed in all groups (data not shown).
Figure 5.
CDPM exposure dampens Th2 inflammation in favor of a T regulatory response in vitro and in an adoptive transfer model. In an in vitro T cell activation assay using DCs isolated from Air/DCB mice lungs to present OVA323-339 antigen to OT-II naïve splenic CD4+ cells (cultured 10:1, respectively), (a) T cell polarization and (b) cytokine concentration in culture supernatant were determined by flow cytometry and multiplex, respectively. (c) Schematic of adoptive transfer experiment: DCs were isolated from lungs of CDPM-exposed and unexposed mice and cultured 2 hr with OVA323-339. Age-matched OT-II mice were then given OVA323-339-loaded DCs (1.5 × 105 DCs/pup) and analyzed for Treg numbers in lungs. (d) Total number of Tregs/lung of adoptive transfer recipient mice. Data plotted as means ± SEM. *p<0.05, t-test.
Acute CDPM exposure dampens lung T effector response in antigen-challenged OT-II mice
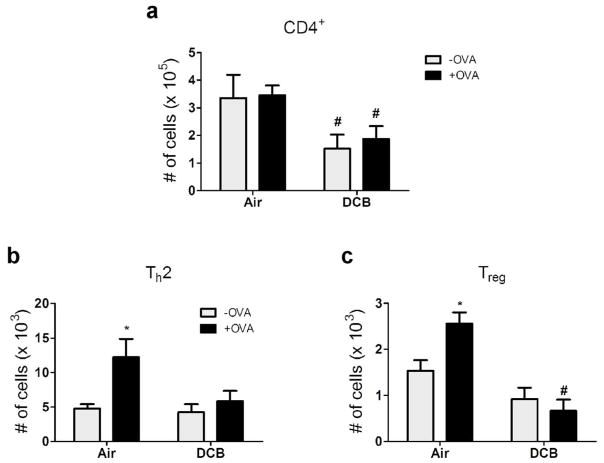
In order to model CDPM-induced immunosuppression in response to antigen, OT-II mice were exposed to DCB for one week starting at three days of age and administered OVA323-339 (2 μg) i.n. on exposure days 5 and 7. Four days later, T effector responses were measured in the lung by flow cytometry (Fig. 6). Suppression of T effector cell numbers was noted in DCB exposed mice compared to controls. Specifically, decreased levels of total CD4+ cells were observed (Fig. 6a) and allergen failed to elicit an increase in Th2 cell numbers (Fig. 6b) in DCB exposed mice. Surprisingly, Treg levels were also decreased in lungs from allergen challenged, DCB-exposed mice compared to controls (Fig. 6c). No significant differences were observed in other T effector populations analyzed (Th1 and Th17; data not shown).
Figure 6.
Acute CDPM exposure dampens lung T effector response in antigen-challenged OT-II mice. (a–c) T effector profiles of mice exposed to air/DCB and challenged intranasally with OVA323-339. Levels of (a) total CD4+, (b) Th2 (IL-4+), and (c) Treg cells in the lungs determined by flow cytometry. Data plotted as means ± SEM. *p<0.05 vs. −OVA within exposure group; #p<0.05 vs. +OVA in opposite exposure group, Two-way ANOVA with Bonferroni post-tests.
Mice exposed to CDPM and sensitized to OVA as neonates, then rechallenged with OVA as adults display an exacerbated asthma phenotype
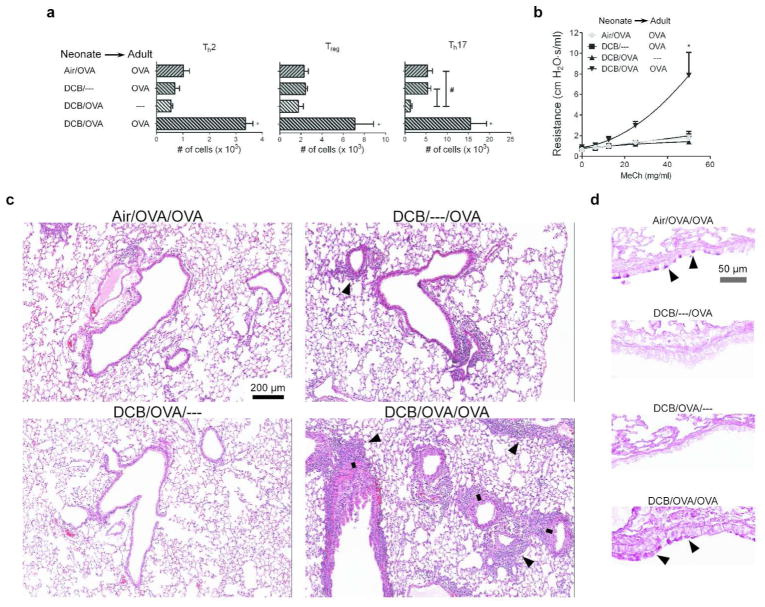
To examine the effect of early-life CDPM exposure during antigen sensitization on responses to antigen later in life (i.e., as an adult), OT-II neonates (groups as in Fig. 6) were allowed to mature until 8 weeks of age and then rechallenged with OVA323-339. In contrast to previously-observed immunosuppression in the lungs of CDPM-exposed mice, allergic responses in mice exposed to CDPM during initial OVA sensitization as neonates were significantly elevated above all other groups (Fig. 7a). Specifically, increased levels of Th2 and Th17 cells were observed in addition to increased levels of Tregs. This group also displayed AHR (b) and severe peribronchial inflammation with an apparent increase in peribronchial smooth muscle mass (c). Levels of mucus were comparable in exposed and unexposed groups that were rechallenged with OVA (d).
Figure 7.
Mice exposed to CDPM and sensitized to OVA as neonates, then rechallenged with OVA as adults display an exacerbated asthma phenotype. (a) CD4+ T cell levels (Th2, Treg, Th17; respectively) in the lungs as determined by flow cytometry. (b) Airway hyperresponsiveness, determined by airway resistance in response to increasing doses of inhaled methacholine. (c–d) Representative micrographs of histological sections of mouse lungs stained with hematoxylin and eosin (c) or PAS (d); black arrows denote areas of significant peribronchiolar inflammation (c) or mucus (d); black line denotes smooth muscle mass surrounding bronchiole. Data plotted as means ± SEM. *p<0.05 vs. all other groups; #p<0.05 vs. indicated group. One-way ANOVA (a) or Two-way ANOVA (b) with Bonferroni post-tests.
Discussion
Positive correlations are observed between elevated PM levels and adverse pulmonary effects including developmental/functional deficits that present during childhood and last into adulthood.12–15 The increasing rate of childhood asthma over recent decades, particularly in urban areas,16 indicates a larger influential role of environmental factors like PM. Indeed, elevated PM is a factor in childhood asthma onset,17 with the highest risk associated with traffic-related CDPM exposure during an early age.18 The fact that early insults on the lung associate with such dire, persistent consequences is not surprising, given the immaturity of the immune system during the first three years of life. During infancy, humans display a natural tendency toward tolerance, which is believed to be extended from antimaternal immunity in utero.19 This is also observed (and has been studied) in neonatal mice.20–22 Despite infants and young children representing a population highly affected by both PM and asthma, there is very little data concerning the relationship of the two in the early stages of asthma development.4
In this study, we demonstrated a temporary immunosuppressive environment in the lung in response to early-life CDPM exposure. The major determinants of adaptive immune responses, DCs, were less activated and produced more IL-10 after CDPM exposure. Within this same acute exposure time period, we observed increases in CD4+ T cells producing IL-10, Tregs, and IL10 mRNA in the whole lung. In an isolated in vitro system, DCs from the lungs of CDPM-exposed mice promoted less Th2 differentiation and effector function including lower expression of IL-4 and IL-13 in favor of a higher Treg differentiation of naïve T cells. When DCs from CDPM exposed mice were adoptively transferred into naïve OT-II mice, an increase in Tregs was observed in the lungs of recipient mice. A measureable Th2 response was not observed in any group. This was not unexpected, given the weak antigen challenge with respect to the OT-II mice being challenged with OVA. When directly allergen challenged during early-life, CDPM-exposed mice were unable to elicit Th2 responses while non-exposed mice had significantly increased Th2 cell numbers. The increased numbers of Tregs in the lungs of OT-II mice exposed to CDPM (Fig. 4) followed by subsequent decrease of Tregs after allergen challenge (Fig. 6) suggests a transient Treg response to CDPM that is suppressive to all effector T proliferation to allergen, including Tregs themselves. This immunosuppression could have beneficial effects with respect to early asthma symptoms, but could lead to exacerbated allergic responses upon allergen rechallenge when suppression wanes. Indeed, our data demonstrated an exacerbation of allergic responses in mice exposed to CDPM and sensitized to OVA as infants and then rechallenged with OVA as adults. This was evidenced by AHR and increased Th2 cell numbers in lungs of adult mice exposed to CDPM during OVA sensitization as neonates (Fig. 7). Interestingly, we also observed significant Th17 cell numbers in this same group of mice even in the absence of measurable Th17 responses to OVA as neonates. A Th17-mediated response has been previously demonstrated in OVA-challenged adult OT-II mice,23 and our previous CDPM-exposure study in adult mice also shows a dominant Th17 component.5 Therefore, it is of little surprise that combining CDPM exposure with adult OVA challenge yielded such large Th17 responses. Despite having significant pulmonary inflammation, minimal amounts of mucus were observed in these mice; we believe this to be a consequence of the use of OVA peptide as allergen and/or the use of OT-II mice. We believe that the exacerbated Th2 and Th17 responses and pathological changes induced by early-life exposure to CDPM may be responsible for the increases in asthma severity.
In an early-life HDM model of asthma, exposure to CDPM abolished AHR. This functional effect was accompanied by significant decreases in BAL total inflammation and eosinophils and Th2 cytokines (i.e., IL-6, TNFα/IL-4, respectively). A trending, but non-significant decrease in IL-5 was observed in BAL fluid of CDPM-exposed mice. Additionally, the chemokine CXCL10, which is linked to AHR in human asthma,24 was elevated in BAL fluid of Air/HDM mice but not CDPM-exposed HDM mice (Supplementary Fig. S1) and may partially explain the absence of AHR in the latter group. Furthermore, we observed decreased Th2 cell numbers in the lungs of CDPM-exposed HDM mice, which agrees with our other antigen challenge data (Fig. 6). Morphometric analysis of airway mucus revealed no quantifiable difference in the amount of mucus producing cells and RT-PCR revealed no quantifiable difference in the expression of mucus-associated genes Muc5b and IL13 in the lungs of CDPM exposed and non-exposed HDM mice. However, Muc5ac expression was decreased in the lungs of CDPM-exposed mice given HDM vs. non-exposed HDM mice.
Serum antibody responses were of note, particularly due to the contrast that the data have to the rest of the suppressive effects of CDPM on HDM asthma. Whereas CDPM-exposed HDM mice appeared to be protected from functional/inflammatory effects of HDM, this group had approximately double the concentration of total IgE in serum. Interestingly, however, the serum concentrations of both HDM-specific IgE and IgG1 were approximately two-fold lower in CDPM-exposed HDM mice vs. non-exposed HDM mice. These data agree with CDPM-mediated immunosuppression to HDM, but also indicate a more complex adaptive immune response. The antigen specificity of the IgE that account for the increase in CDPM-exposed HDM mice is unclear, but it is possible that oxidative damage to airway cells that we have previously observed7, 8, 25, 26 is releasing oxidized self-antigen able to initiate adaptive responses. In any case, elevated total IgE is strongly associated with development of hypersensitivity,1 and, though our data suggest CDPM-mediated protection from the development of specific allergy (i.e., HDM asthma) during CDPM exposure, we clearly observed heightened asthma-like responses if mice were rechallenged with allergen as adults. It is possible that the elevated total IgE observed early plays a role in the heightened susceptibility to allergens later in life.
Our previous work focusing on the early-life effects of CDPM exposure has shown the significant disruption airway epithelium resulting in EMT and increased smooth muscle mass.8 There are some in vitro and intestinal in vivo data suggesting that loss of E-cadherin interactions between DCs results in a tolerogenic DC phenotype via β-catenin transcriptional signaling.9, 27 In this study, we show that the DC subset responsible for IL-10 production following CDPM exposure is interstitial DCs (intraepithelial resident cDCs) which rely on E-cadherin interactions to reside between airway epithelial cells. Therefore, it is possible that loss of E-cadherin interactions between epithelial cells and interstitial DCs as a result of CDPM exposure is involved in inducing tolerance here also. In agreement with this theory, we have observed β-catenin-responsive transcription events such as increases in Snai1 in CDPM-exposed mice.8 The impact of CDPM exposure on regulation of β-catenin itself is currently being investigated by our laboratory. The suppressive effects of IL-10-producing, tolerogenic DCs have been demonstrated to increase the presence/activity of Tregs and abrogate AHR, airway eosinophilia, Th2 cytokines, and circulating HDM-specific IgE/IgG1 in an HDM asthma model.28 The actions of local Foxp3+ Tregs are vital in maintaining tolerance,29 and the significant increase that we observed in these cells in CDPM-exposed mice in vivo coupled with the lack of Th2 differentiation/elevated Treg numbers perpetuated from the CDPM-exposed mice DCs in vitro suggests that tolerance to HDM in this respective group may also be derived from CDPM-induced tolerogenic DCs/Tregs. Although beyond the scope of this manuscript, it is possible that following epithelial repair, E-cadherin interactions between DCs and epithelial cells release the DC from its tolerogenic state allowing “normal” responses to allergens/pathogens. Since adaptive T effector responses were developed to allergen during the initial CDPM exposure and allergen challenge (evidenced by HDM-specific IgE), this suggests that upon re-exposure, persisting T and B cells are able to mount a memory response to allergen. This response is unhampered by tolerogenic DCs and Tregs, allowing for an exacerbated asthma phenotype as our data show.
The inflammation-attenuating effect of PM on allergic asthma models has been previously demonstrated. When OVA-sensitized mice are co-exposed to nanoparticles or mild cigarette smoke (which contains high amounts of CDPM) along with OVA, significant decreases in pulmonary inflammation (e.g., total cells, eosinophilia, BAL IL-4/IL-5) are observed along with abolishment of AHR.30, 31 Additionally, Muc5ac expression is also decreased which agree with our data. To our knowledge, no data concerning PM and Muc5b expression exist, making it difficult to explain why we observed a difference in expression of Muc5b vs. Muc5ac or why mucus cell frequency remained unchanged by CDPM. There is evidence that Muc5b is prominent in developing lungs while Muc5ac is not.32 Furthermore, Muc5ac is stabilized by TNFα, which was decreased in CDPM mice, while Mub5b is unaffected.33 Differential regulation of these mucus genes is further evidence of age-dependent responses and enforces the necessity of age-appropriate studies.
In contrast to our data, IgE levels are not affected by co-exposure in aforementioned studies.30, 31 It is likely that the differences in OVA vs. HDM co-exposure asthma protocols are responsible; OVA co-exposure protocols require prior sensitization to OVA (i.e., intraperitoneal injections of OVA complexed to adjuvant) followed by challenge with OVA during the PM exposure whereas in our HDM model mice were exposed first to CDPM and exposure to CDPM continued throughout the HDM exposure. Finally, HDM is a more physiologically relevant allergen and elicits asthma in the absence of a Th2-driving adjuvant in mice. The implications of PM exposure-induced immunosuppression in the lung reach beyond allergic sensitization, and we are currently exploring this effect in the context of other disease. For example, an early immunosuppressive environment could be detrimental during contact with actual pathogen (e.g., influenza virus, K. pneumonia, etc.) due to the inability to mount an appropriate immune response (manuscript in preparation). In conclusion, we were able to demonstrate the immunosuppressive effects of CDPM exposure, due in part to promotion of tolerogenic DCs and Treg differentiation. In the presence of this suppressive environment, antigen-specific responses were attenuated in the lung leading to lessened overall inflammation and AHR. Despite the reduced antigen-specific effects observed, total IgE was more highly elevated in CDPM-exposed HDM mice indicating a susceptibility to other hypersensitivity diseases. These findings illustrate a mechanism for the development of asthma and other allergic disease in the context of high PM exposure during early-life. Knowledge gained from these types of age-appropriate studies is crucial to our understanding of origins of complex diseases such as asthma, and highlights the importance of preventing exposure to CDPM during early-life.
Methods
Combustion-generated particulate matter and exposures
Combustion-generated particulate matter, DCB, was generated and characterized as previously described.7 Immediately before use, vacuum-sealed vials were opened and 5 mg DCB were suspended in 25 ml sterile saline containing 0.02% Tween-80 using sonication to monodisperse particles, giving a mean aerodynamic diameter of 0.2 Qm (Supplemental Methods). For in vivo inhalation exposures, pups were placed in a small chamber with nebulized particles for 30 min/day (beginning at three days of age and continuing for experiment-specific amount of time), at an average airborne PM concentration of 200 μg/m3 which is equivalent to that of an above average polluted urban area. Exposure conditions are based on modeling calculations (MPPD v2.0; Supplemental Methods) to produce equivalent particle deposition per alveolus to that of a human infant exposed over 24 h.
Animals
C57BL/6J, OT-II (C57BL/6-Tg(TcraTcrb)425Cbn/J), and IL-10-GFP (B6.129S6-Il10tm1Flv/J) reporter mice were purchased from Jackson Labs (Bar Harbor, ME), kept in ventilated cages in a specific pathogen-free environment, and time-mated to obtain experimental age-appropriate pups. IL-10-GFP mice pups were genotyped by PCR, and heterozygotes (+/−) were used for studies. All animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the LSUHSC/UTHSC Institutional Animal Care and Use Committee.
Neonatal allergic asthma model
A neonatal mouse model of HDM-induced airway allergy was adapted from published methods.10 Starting at three days of age, mice were exposed to DCB230 every day for two weeks (graphic in Fig. 1). HDM extract (Greer Labs, Lenoir, NC) was administered to isofluorane-anesthetized mice intranasally starting on the last three days of the two week exposure, then for three consecutive days/week for three consecutive weeks (10 μg/day during administration blocks 1 & 2; 15 μg/day during administration blocks 3 & 4). In DCB230-exposed groups, mice were only exposed on the same day and 2 h before HDM administration. All analyses/tissues were taken 24 h following the final protocol day.
Pulmonary mechanics
For invasive measurement of pulmonary function, mice were anesthetized and evaluated for AHR using flexiVent forced oscillation maneuvers (Scireq, Montreal, Canada). Lung resistance to airflow was measured in response to increasing doses of (0, 12.5, 25, 50 mg/ml) methacholine.
BAL cellularity and cytokines
BAL was collected in 1 ml PBS containing 2% BSA. Total cell counts were obtained, and cells were spun onto glass slides (one slide/animal sample) and stained with Hema-3 kit (Fisher Scientific, Hampton, NH). Cells were differentiated based on morphology/stain and a total of 200 cells/slide were counted. Cytokine levels were measured in cell-free BAL using a multiplex assay (Millipore, Billerica, MA). The following cytokines were assayed: GM-CSF, IFN-γ, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, KC, TNF-α, VEGF, and IP-10. Raw data were plotted against a standard curve using a 5-parameter logistic regression to interpolate unknowns. Data presented exclude numbers beyond the sensitivity of the assay.
Circulating immunoglobulin
Serum was isolated from whole blood after 30 min of clotting followed by centrifugation. Samples were flash frozen in liquid nitrogen and stored at −80°C until further analysis. Levels of total IgE and IgG1 were measured by ELISAs according to manufacturer’s protocol (eBioscience, San Diego, CA). To assess HDM-specific IgE and IgG1 levels in serum, ELISA plates were coated with 50 μg/ml HDM overnight at 4°C, incubated with diluted serum (1:10 IgE; 1:10,000 IgG1) and isotype-specific HRP-linked detection antibody (Southern Biotech, Birmingham, AL).
Lung histopathology
Lungs were excised, inflated at a constant fluid pressure of 25 cm with Zinc-formalin, and fixed overnight. Fixed lungs were dehydrated, paraffin-embedded, and sectioned at 4 μm onto slides. Mucus positive cells were quantified from sections stained with periodic acid-Schiff (PAS) and recorded as a percentage of PAS+ cells per total number of epithelial cells in randomly selected bronchi (ImageJ) by an unbiased observer. All images were taken on a Zeiss microscope using AxioVision software.
Quantitative Real-Time PCR
Total RNA was isolated from 40 mg lung tissue using RNAspin Mini Isolation Kit (GE, Pittsburgh, PA) and converted to cDNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). qPCR was performed on a Roche LightCycler 480 using SYBR Green I Master (Roche, Indianapolis, IN) and gene-specific, intron-spanning primers for Muc5b (Forward primer, 5′-CATGGATGGCTGCTTCTGT-3′; Reverse primer, 5′-TAA ATTCGGCCACCGTGT-3′), Muc5ac (Forward primer, 5′-TCCCATTCTGGGTCCTAGC-3′; Reverse primer, 5′-GATTTCAGAGAGTCCTGTTGAGC-3′), IL13 (Forward primer, 5′-CCTCTGACCCTTAAGGAGCTTAT-3′; Reverse primer, 5′-CGTTGCACAGGGGAGTCT-3′), IL10 (Forward primer, 5′-CAGAGCCACATGCTCCTAGA-3′; Reverse primer, 5′-GTCCAGCTGGTCCTTTGTTT-3′), and HPRT (Forward primer, 5′-TCCTCCTCAGACCGCTTTT-3′; Reverse primer, 5′-CCTGGTTCATCATCGCTAATC-3′). Single-amplicon quality was verified by melting curve. Relative expression was calculated using ΔΔCt method normalized to reference gene HPRT.
Flow cytometry
Mice were euthanized and exsanguinated to prevent the flow of blood into the lungs and blood was removed by retrograde perfusion with PBS. The lungs were excised, gently mechanically dissociated (Octodissociator; Miltenyi, Germany), and digested for 30 min at 37°C in HBSS supplemented with 1 mg/ml collagenase I (Invitrogen, Grand Island, NY) and 150 ng/ml DNase I (Sigma-Aldrich, St. Louis, MO). After incubation, lung pieces were vigorously mechanically dissociated (Octodissociator) and mashed through a 40 μm cell strainer (BD Biosciences, San Jose, CA) followed by treatment with RBC lysis buffer (eBioscience) to get RBC-depleted, single-cell lung suspensions. For intracellular cytokine staining, cells were stimulated for 5 h at 37°C in RPMI 1640 media (5% heat-inactivated FBS) with 5 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of protein transport inhibitor brefeldin A (GolgiPlug; BD Biosciences). The following antibodies were used for T cells: eFluor450-CD3 (17A2), PerCP-CD4 (RM4-5), PE-IFNγ (XMG1.2), PE-Cy7-IL-4 (BVD6-24G2), FITC-IL-17 (eBio17B7), PE-CD25 (PC61.5), and FITC-Foxp3 (FJK-16s) and for DCs/Macs: FITC-CD11c (N418), PE-CD11b (M1/70), eFluor450-F4/80 (BM8), APC-Cy7-MHCII (M5/114.15.2), APC-IL-10 (JES5-16E3), and PerCP-Cy5.5-CD80 (16-10A1). A fixable live/dead marker was used to exclude dead cells (eFluor506/780). Cells were analyzed using flow cytometry (FACSCanto II; BD Biosciences), and all flow data were analyzed using FlowJo software v7.6.5 (Tree Star, Inc., Ashland, OR).
In vitro T cell activation
C57BL/6 neonatal mice were exposed to DCB230 or control air starting at three days of age as described above for five consecutive days. After exposure on the fifth day, lungs were harvested, pooled according to exposure group, digested into single-cell suspensions, and DCs were positively selected using CD11c+ selection kit (StemCell; Vancouver, Canada) followed by brief plating to select for non-adherence (to exclude macrophages). Concurrently, naïve OVA323-339-specific CD4+ T cells were positively selected using CD4+ selection kit (StemCell) from single-cell spleen suspensions of age-matched OT-II neonates. DCs (5 × 103) from air/DCB230-exposed neonate lungs were cultured with naïve OVA323-339-specific CD4+ T cells (5 × 104) in the presence of 100 ng/ml OVA323-339 in 200 μl total of RPMI 1640 (10% heat-inactivated FBS) for 72 h at 37°C in a round-bottom 96-well plate. After 72 h, cytokine expression was measured from cell-free media supernatant using method described above. Cells were washed with PBS and either stained for Treg markers or stimulated in order to stain intracellularly for T cells subsets. Stained cells were analyzed by flow cytometry.
Adoptive transfer
C57BL/6 neonatal mice were exposed to DCB230 or control air starting at three days of age as described above for five consecutive days. After exposure on the fifth day, lungs were harvested, pooled according to exposure group, digested into single-cell suspensions, and DCs were positively selected using CD11c+ selection kit (StemCell). After selection, DCs from both exposure groups were separately incubated at 37°C for 2 h in RPMI 1640 with 10 μg/ml OVA323-339. Following incubation, cells were washed with PBS and adoptively transferred intranasally to age-matched OT-II neonates (1.5 × 105 OVA323-339-loaded DCs/pup). After seven days, pups were challenged intranasally with 2 μg OVA323-339 to boost T cell response, and then lungs were harvested 72 h later to analyze Treg levels using flow cytometry.
In vivo T effector response
OT-II neonates were exposed to DCB230 or control air starting at three days of age as described above for seven consecutive days. On exposure days 5 and 7, mice were challenged intranasally with 2 μg OVA323-339 and lung T effector populations were assessed using flow cytometry six days after the final exposure/challenge. To measure responses in adult mice, a similar challenge/response time frame was used; mice were challenged intranasally with OVA323-339 twice (48 hr apart) and had lung T effector populations assessed using flow cytometry.
Statistical Analysis
All data were analyzed using GraphPad software. Two-way ANOVA with Bonferroni post-tests or Student’s t-test was used to determine differences between all groups. All results are expressed as mean ± SEM, representative of at least 3 independent experiments. Values of p≤0.05 were considered statistically significant. Statistical comparisons in Bonferroni post-tests were made within exposure groups (*p; Air vs. Air/HDM; DCB vs. DCB/HDM; Air vs. Air/OVA; DCB vs. DCB/OVA) or within allergen groups (#p; Air vs. DCB; Air/HDM vs. DCB/HDM; Air/OVA vs. DCB/OVA).
Supplementary Material
Acknowledgments
This work was supported by NIEHS grants (R01ES015050 and P42ES013648) to SAC. In addition, JS was supported by a training grant from the Louisiana Board of Regents (LEQSF (2009-14)-GF-08) and PT and GL were supported by a training grant from NIAAA (AA007577). We thank Drs. Owen Price and Bahman Asgharian for lending their expertise with MPPD. We thank Dr. Christopher Waters for providing access to his flexiVent at UTHSC. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the State of Louisiana.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Supplementary material is linked to the online version of this paper at http://www.nature.com/mi
References
- 1.Sly PD. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol. 2011;11(1):24–28. doi: 10.1097/ACI.0b013e328342309d. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- 3.Nicolai T. Air pollution and respiratory disease in children: what is the clinically relevant impact? Pediatr Pulmonol Suppl. 1999;18:9–13. [PubMed] [Google Scholar]
- 4.Saravia J, Lee GI, Lomnicki S, Dellinger B, Cormier SA. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: a review. J Biochem Mol Toxicol. 2013;27(1):56–68. doi: 10.1002/jbt.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang P, Thevenot P, Saravia J, Ahlert T, Cormier SA. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am J Respir Cell Mol Biol. 2011;45 (5):977–983. doi: 10.1165/rcmb.2011-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, et al. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir Res. 2011;12:19. doi: 10.1186/1465-9921-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakrishna S, Saravia J, Thevenot P, Ahlert T, Lominiki S, Dellinger B, et al. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Particle and Fibre Toxicology. 2011;8:11. doi: 10.1186/1743-8977-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thevenot PT, Saravia J, Jin N, Giaimo JD, Chustz RE, Mahne S, et al. Radical-Containing Ultrafine Particulate Matter Initiates Epithelial-to-Mesenchymal Transitions in Airway Epithelial Cells. Am J Respir Cell Mol Biol. 2013;48(2):188–197. doi: 10.1165/rcmb.2012-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saglani S, Mathie SA, Gregory LG, Bell MJ, Bush A, Lloyd CM. Pathophysiological features of asthma develop in parallel in house dust mite-exposed neonatal mice. Am J Respir Cell Mol Biol. 2009;41(3):281–289. doi: 10.1165/rcmb.2008-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, et al. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61(3):202–208. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He QQ, Wong TW, Du L, Jiang ZQ, Gao Y, Qiu H, et al. Effects of ambient air pollution on lung function growth in Chinese schoolchildren. Respir Med. 2010;104(10):1512–1520. doi: 10.1016/j.rmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Dales R, Chen L, Frescura AM, Liu L, Villeneuve PJ. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: a panel study of schoolchildren with asthma. Eur Respir J. 2009;34(2):316–323. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- 14.Mauad T, Rivero DH, de Oliveira RC, Lichtenfels AJ, Guimaraes ET, de Andre PA, et al. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178(7):721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D, Wallis C, Wexler AS, Schelegle ES, Van Winkle LS, Plopper CG, et al. Small particles disrupt postnatal airway development. J Appl Physiol. 2010;109(4):1115–1124. doi: 10.1152/japplphysiol.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Midodzi WK, Rowe BH, Majaesic CM, Saunders LD, Senthilselvan A. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: results from the Canadian Early Childhood Development cohort study. J Asthma. 2010;47(1):7–13. doi: 10.3109/02770900903380996. [DOI] [PubMed] [Google Scholar]
- 17.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118(7):1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118(2):284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. NKG2D Blockade Inhibits Poly(I:C)-Triggered Fetal Loss in Wild Type but Not in IL-10−/− Mice. J Immunol. 2013;190(7):3639–3647. doi: 10.4049/jimmunol.1203488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18(10):1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Wang S, Curtiss R., 3rd Evaluation of Regulated Delayed Attenuation Strategies for Salmonella Typhi Vaccine Vectors in Neonatal and Infant Mice. Clin Vaccine Immunol. 2013 doi: 10.1128/CVI.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109(9):3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171(10):1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 25.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol. 2009;6:11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahmy B, Ding L, You D, Lomnicki S, Dellinger B, Cormier SA. In vitro and in vivo assessment of pulmonary risk associated with exposure to combustion generated fine particles. Environ Toxicol Pharmacol. 2010;29(2):173–182. doi: 10.1016/j.etap.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu M, Dawicki W, Zhang X, Huang H, Nayyar A, Gordon JR. Therapeutic induction of tolerance by IL-10-differentiated dendritic cells in a mouse model of house dust mite-asthma. Allergy. 2011;66(5):612–620. doi: 10.1111/j.1398-9995.2010.02526.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SM, Batzer G, Ng N, Lam D, Pattar SS, Patel ND, et al. Regulatory T cells contribute to allergen tolerance induced by daily airway immunostimulant exposures. Am J Respir Cell Mol Biol. 2011;44(3):341–349. doi: 10.1165/rcmb.2010-0001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang S, Park JW, Cha HR, Jung SY, Lee JE, Jung SS, et al. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int J Nanomedicine. 2012;7:1329–1343. doi: 10.2147/IJN.S27159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hizume DC, Toledo AC, Moriya HT, Saraiva-Romanholo BM, Almeida FM, Arantes-Costa FM, et al. Cigarette smoke dissociates inflammation and lung remodeling in OVA-sensitized and challenged mice. Respir Physiol Neurobiol. 2012;181(2):167–176. doi: 10.1016/j.resp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Roy MG, Rahmani M, Hernandez JR, Alexander SN, Ehre C, Ho SB, et al. Mucin production during prenatal and postnatal murine lung development. Am J Respir Cell Mol Biol. 2011;44(6):755–760. doi: 10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borchers MT, Carty MP, Leikauf GD. Regulation of human airway mucins by acrolein and inflammatory mediators. Am J Physiol. 1999;276(4 Pt 1):L549–555. doi: 10.1152/ajplung.1999.276.4.L549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.