Abstract
It is now widely accepted that some forms of necrosis are controlled by a dedicated signaling pathway triggered by various cell surface and intracellular receptors. This regulated form of necrosis is mediated by the kinase activity of receptor-interacting protein kinase 1 (RIP1/RIPK1) and/or RIP3/RIPK3. A number of studies using the RIP1 kinase inhibitor Necrostatin-1 (Nec-1) and its derivatives, or RIP3-deficient mice demonstrated that RIP1 and RIP3 are involved in various infectious and sterile inflammatory diseases. As a consequence, these specific phenotypes were construed to depend on necrosis. However, emerging evidence indicates that the RIP1 kinase activity and RIP3 can also control apoptosis and inflammatory cytokine production independent of necrosis. Therefore, we may need to re-interpret conclusions drawn based on loss of RIP1 or RIP3 functions in in vivo models. We propose that studies of RIP1 and RIP3 in different inflammatory responses need to consider cell death-dependent and independent mechanisms of the RIP kinases.
Keywords: RIP3/RIPK3, RIP1/RIPK1, Programmed Necrosis, Necroptosis, necrostatin-1, necrosome, apoptosis, NF-κB, Inflammasome, Inflammation, TNF, caspase 8, Fadd, MLKL, Pgam5
1. Introduction
Necrosis is cell death defined by characteristic morphologies including swelling of cytoplasm and organelles, and disruption of plasma membrane integrity. Historically, necrosis is often associated with cell damage caused by exposure to physical stress or extreme extracellular conditions such as severe temperature, osmotic change, strong acidity, and depletion of oxygen and nutrients. These observations led to the assumption that necrosis is passive and unregulated cell death. The discovery of caspase-dependent apoptosis further strengthened the notion that necrosis is unregulated. However, a number of studies in the last decade demonstrated that physiological and pathological necrosis could be elicited in a regulated manner [1]. This type of regulated necrosis is now called “programmed necrosis” or “necroptosis” to distinguish it from passive necrosis [2]. Death ligands in the tumor necrosis factor (TNF) superfamily are prototypical inducers of programmed necrosis. Because of the importance of TNF in many inflammatory diseases, necrosis signaling pathway downstream of TNF receptor 1 (TNFR1) has been most intensively studied. Death receptor-mediated necrosis is controlled by the kinase activity of receptor-interacting protein kinase 1 (RIP1/RIPK1) [3] and RIP3/RIPK3 [4, 5]. Unlike RIP1−/− mice, which die in the early postnatal period [6], RIP3−/− mice are viable and have been used to understand the patho-physiological functions of RIP3. The pro-necrotic function of RIP1 has been examined in various disease models using necrostatins, a series of chemical inhibitors against RIP1 kinase activity [7]. Collectively, studies using these biological and chemical reagents revealed that intact RIP1 kinase activity and RIP3 are essential in immune responses against virus and bacterial infections [4, 8, 9], sepsis [10, 11], pancreatitis [5, 12], liver diseases [13–17], retinitis [18–20], atherosclerosis [21], and ischemia-reperfusion injury in brain, myocardium, and kidney [22–27]. These results suggest RIP1 and RIP3 as possible therapeutic targets in various inflammatory diseases.
Although apoptosis is pervasive during development and in normal tissue turnover, apoptotic cells are rapidly cleared by phagocytes and therefore are difficult to detect in vivo. The rapid clearance of apoptotic cells prior to membrane rupture prevents debilitating auto-inflammaotry reactions [28, 29]. In contrast, cells dying by necrosis elicit inflammatory immune responses through damaged plasma membrane and release of intracellular immunogenic proteins, nucleotides, and metabolites [30]. These endogenous “danger-associated molecular patterns” (DAMPs) or alarmins are functionally analogous to pathogen-associated molecular patterns (PAMPs) and are sensed by specific pattern recognition receptors such as toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), nucleotide-binding, oligomerization domain (NOD)-like receptors (NLRs), and C-type lectin receptors (CLR) expressed on the surface of immune effector cells [31, 32]. Although it is widely believed that RIP1 and RIP3 promote inflammatory responses in various diseases through the release of alarmins [33], several recent reports show that RIP3 can also promote inflammation independent of necrosis. Similarly, in addition to necrosis, RIP1 kinase activity has been implicated in apoptosis and cytokine production. Here, we discuss recent findings that contribute to the emerging paradigm that RIP1 and RIP3 can synergize with each other to promote inflammation through necrotic and non-necrotic signaling.
2. Molecular mechanism of necrosis induced by TNF, RIP1 and RIP3
RIP1 and RIP3 share a conserved kinase domain in their amino termini. In addition, they contain a unique protein-protein interaction motif called the “RIP homotypic interaction motif” (RHIM) that is not present in other RIP family kinases [34]. The core sequences of the RHIM I/VQI/VGXXN are made of hydrophobic residues that are predicted to form β-strands. Recent study demonstrated that the RHIM mediates assembly of a RIP1-RIP3 complex termed the necrosome [35]. Surprisingly, the RIP1-RIP3 necrosome is not a simple heterodimer. Rather, they form an amyloid-like filamentous complex [36]. Since Nec-1 blocked TNF-induced formation of the necrosome, RIP1 kinase activity and perhaps RIP1 autophosphorylation might be crucial events preceding necrosome formation [4]. Because alanine substitution of individual serine residues had little effects on RIP1 kinase activity and TNF-induced necrosis [37], we propose that multiple phosphorylation events are required to synergize amyloid conversion and activation of the RIP1-RIP3 necrosome. Interestingly, alanine substitution of a highly conserved serine residue on RIP1, Ser89, augmented RIP1 kinase activity and TNF-induced necrosis [37]. By contrast, phospho-mimetic mutant of Ser89 reduced RIP1 kinase activity. These results suggest the possibility that RIP1 kinase activity and necrosis can be regulated by inhibitory phosphorylation at Ser89. Thus, the balance between positive and negative phosphorylation events on RIP1 may impinge on the outcome of necrosis signaling.
Like RIP1, a number of serine and threonine residues in RIP3 have been identified as phospho-acceptor sites by mass spectrometry [38, 39]. An inactive RIP3 failed to undergo necrosis-specific phosphorylation and to recruit its downstream substrate mixed lineage kinase domain-like (MLKL) [38–40]. This suggests that some of these residues are the targets of RIP3 autophosphorylation. Phosphorylation of human RIP3 at Ser227 (Ser232 in mouse RIP3) was postulated to be essential for the recruitment of MLKL. Surprisingly, we found that phospho-mimetic mutant of Ser232 also abolished RIP3 kinase activity and necrosis induction, suggesting that the negative charge from Ser232 phosphorylation alone is not sufficient to mediate MLKL recruitment and necrosis signaling [37]. Ser199 in human RIP3 is another putative phosphorylation site that is highly conserved among different species [5]. Alanine substitution of Ser199 or the corresponding Ser204 in mouse RIP3 compromised RIP3 kinase activity and necrosis induction [37]. Strikingly, phospho-mimetic mutations at this site led to TNF-induced necrosis that is RIP1-independent. Collectively, these results show that TNF stimulates RIP1 kinase activity to drive formation of the RIP1-RIP3 complex, which acts as a scaffold for RIP3 kinase activation. However, full activation of RIP1 and RIP3 likely requires conversion of the complex into amyloid-like fibrils and further phosphorylation events beyond Ser199/Ser204.
Although RIP1 and RIP3 phosphorylation is critical for recruitment of MLKL, MLKL itself does not exhibit any kinase activity [41]. Instead, MLKL was reported to facilitate recruitment of another RIP3 substrate, the mitochondrial fission factor phosphoglycerate mutase family member 5 (PGAM5) [42]. The importance of PGAM5 in necrosis has recently been challenged [41]. In support of the lack of mitochondria involvement in necrosis, a recent report shows that MLKL forms trimers and is redistributed to the plasma membrane to facilitate calcium influx and necrosis in response to TNF-induced necrosis [43]. Blockade of the non-voltage-sensitive channel transient receptor potential cation channel subfamily M member 7 (TRPM7) inhibited calcium influx and necrosis (Fig. 1). Interestingly, reactive oxygen species (ROS) scavenger and TRPM7 inhibitor synergized with each other to inhibit TNF-induced necrosis. This suggests that ROS and calcium flux may be parallel effector mechanisms that execute necrosis. The discovery of calcium flux in necrosis may also explain why inhibition ROS did not always block necrosis efficiently in some cell types.
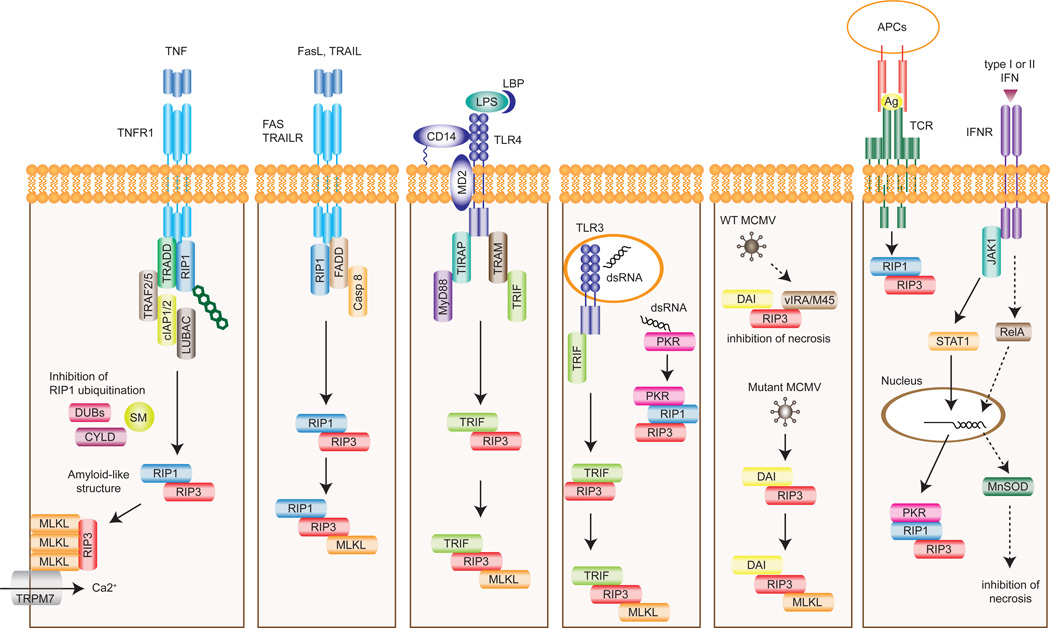
Figure 1. Receptor-specific activation of RIP1 and/or RIP3-dependent necrosis.
Different upstream stimuli can cause necrosis through pro-necrotic RHIM-containing proteins such as RIP1, RIP3, TRIF, and DAI. TNF induces the recruitment of RIP1 to TNFR1 complex where RIP1 is ubiquitinated. Inhibition of RIP1 ubiquitination by deubiquitinating enzymes (DUBs) such as CYLD or SMAC mimetic (SM) leads to formation of the necrosome. RIP3 activates MLKL by phosphorylation. Trimeric MLKL localizes to the plasma membrane to promote Ca2+ influx via TRPM7. Fas ligand and TRAIL also induce necrosis through RIP1- and RIP3-dependent manner. Stimulation of TLR4 or TLR3 by LPS or dsRNA respectively leads to the interaction between RIP3 and TRIF. dsRNA also stimulates PKR and subsequently induces necrosis through RIP1 and RIP3. Wild type (WT) MCMV encodes vIRA/M45 that blocks necrosis by sequestering RIP3 from DAI. In the absence of a functional RHIM in vIRA/M45, MCMV infection promotes RIP3-DAI binding and necrosis. T cell receptor (TCR) stimulation also induces RIP1/RIP3-dependent necrosis, albeit the mechanism remains unknown. Type I or II IFNs can promote or inhibit necrosis. When caspases are inhibited, PKR facilitates assembly of the PKR/RIP1/RIP3 complex. On the other hand, PKR also stimulates NF-κB-dependent expression of MnSOD, which neutralizes ROS to inhibit necrosis. TRAILR, TRAIL receptor; APC, antigen presenting cell; Ag, antigen; IFNR, IFN receptor.
3. RIP1/RIP3-dependent necrosis mediated by other RHIM-containing proteins
Besides RIP1 and RIP3, the RHIM is found in several molecules with crucial functions in innate immunity: Toll/IL-1 receptor (TIR) domain-containing adapter inducing interferon (IFN)-β (TRIF), DNA-dependent activator of IFN-regulatory factors (DAI), and the viral inhibitor of RIP activation (vIRA/M45). TLR4 on the cell surface detects lipopolysaccharide (LPS) found in most Gram-negative bacteria in cooperation with LPS binding protein (LBP) [44], CD14 [45], and MD-2 [46]. Activation of TLR4 leads to two distinct signaling pathways: the first one is mediated by TIR domain-containing adaptor protein (TIRAP) and myeloid differentiation primary response 88 (MyD88), and a second pathway that is mediated by TRIF-related adaptor molecule (TRAM) and TRIF [47]. TRIF is a TLR3/TLR4 adaptor that mediates induction of pro-inflammatory cytokines and type I interferons (IFNs) through the transcription factors NF-κB and interferon regulatory factor 3 (IRF3) [48, 49]. Unlike TLR4 and other TLRs, TLR3 signals exclusively through TRIF and is localized in the endosome to detect double stranded RNA (dsRNA) from viruses [50] and endogenous RNA from damaged and necrotic cells [51]. Similar to TNF-induced necrosis, TLR3- and TLR4-induced necrosis also requires caspase inhibition, intact RIP3 activity, RHIM-dependent binding between TRIF and RIP3, and MLKL (Fig. 1) [52, 53]. Surprisingly, RIP1 was required for TLR3- and TLR4-mediated necrosis in macrophages, but not in fibroblasts or endothelial cells [53]. The molecular basis for the differential requirement of RIP1 for TLR3/4-induced necrosis in different cell types is unknown at present.
DAI (aka DLM-1/ZBP1) is a cytosolic DNA sensor that can activate type I IFN responses [54]. Infection of mutant murine cytomegalovirus (MCMV) lacking a functional vIRA/M45 led to RHIM-mediated interaction between DAI and RIP3 and necrosis of the infected cells [9, 55], indicating that vIRA/M45 prevents necrosis by blocking DAI-RIP3 interaction through its RHIM. Although DAI-RIP3 mediated necrosis requires MLKL, RIP1 is dispensable (Fig. 1) [9, 53, 55]. Thus, RIP3 partners with distinct RHIM-containing adaptors to signal for necrosis under different conditions. Whether RIP3-TRIF, RIP3-DAI or vIRA-RIP3 complexes adopt similar β-amyloid-like scaffold as the RIP1/RIP3 necrosome will require further investigation.
Recently, dsRNA-dependent protein kinase (PKR) was reported to be a novel pro-necrotic partner of RIP1 and RIP3 [56]. Type I or II IFNs induced PKR activation through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway. Activated PKR interacted with RIP1 and subsequently initiate RIP1/RIP3-dependent necrosis in the absence of FADD or caspase-8. However, since PKR does not contain a RHIM, it is unclear how it engages the RIP kinases. It is also noteworthy that IFNs has been reported to counteract pro-necrotic signaling through NF-κB-dependent induction of manganese superoxide dismutase (MnSOD), which limits cellular damage caused by excessive ROS [57]. Thus, whether IFNs induce necrosis is tightly controlled by NF-κB-dependent survival signals and caspase-8 activity (Fig. 1).
4. Non-necrotic function of RIP1 in apoptosis and inflammatory cytokine production
4.1. RIP1 regulates NF-κB activation
Although RIP1 was originally identified as a death domain (DD)-containing adaptor for Fas that mediates apoptosis [58], its primary function is to promote pro-survival through activation of NF-κB downstream of TNFR-1, TLR3, TLR4 and RIG-I. In addition to the RHIM, RIP1 binds to DD-containing receptors and adaptors such as TNFRSF1A-associated via death domain (TRADD) through homotypic DD-DD interaction [58, 59]. TNF stimulates the recruitment of TRADD, RIP1, and TNF receptor-associated factor 2 (TRAF2), TRAF5, cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2, and linear ubiquitin chain assembly complex (LUBAC) to TNFR1 [60]. RIP1 recruited to this membrane-associated complex, often referred to as Complex I [61], is heavily ubiquitinated. This polyubiquitin scaffold facilitates recruitment of NF-κB essential modulator (NEMO) and TAK1 binding protein 2 or 3 (TAB2/3) [62, 63], leading to activation of inhibitor of kappa B (IκB) kinase beta (IKKβ) and transforming growth factor beta (TGFβ)-activated kinase 1 (TAK1), NF-κB nuclear translocation, inflammatory gene expression, and cell survival (Fig. 2). In support of the role of RIP1 ubiquitination in NF-κB activation, cells lacking RIP1 or those expressing non-ubiquitinated mutant of RIP1 (K377R) were defective in TNF-induced NF-κB activation [63, 64].
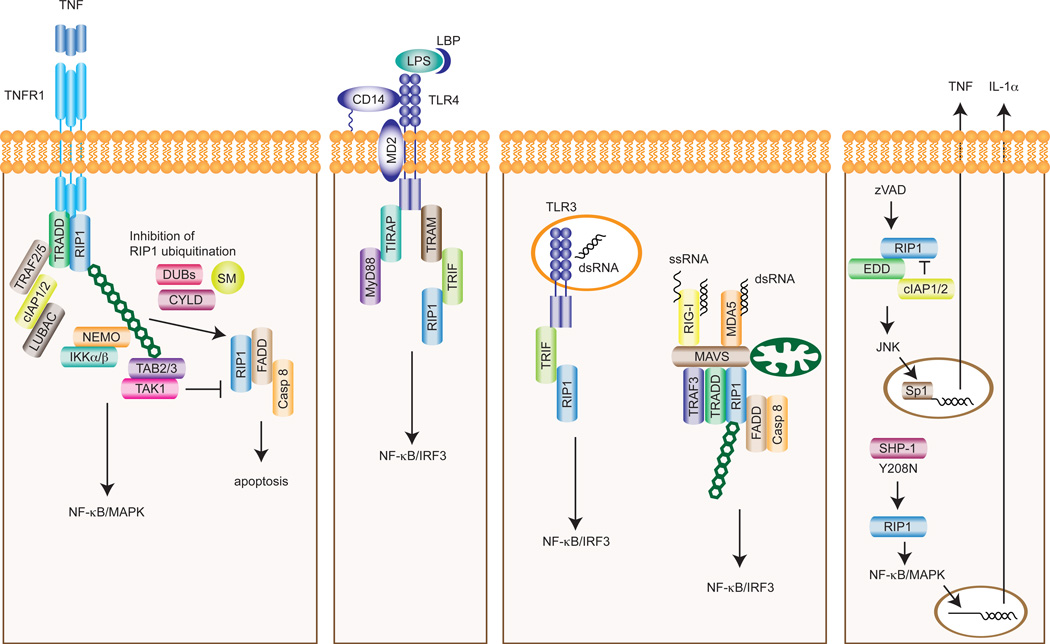
Figure 2. RIP1-dependent regulation of cytokine production and apoptosis.
Downstream of TNFR1, ubiquitination of RIP1 and other adaptors acts as a scaffold to recruit the IKKα/β/NEMO and TAB2/3/TAK1 complexes to activate NF-κB and MAPK pathways. When RIP1 ubiquitination or TAK1 is inhibited, RIP1 kinase activity promotes apoptosis. In addition, RIP1 is involved in TRIF-mediated activation of NF-κB and IRF3 pathways downstream of TLR4 and TLR3. The recognition of viral RNA by RIG-I or MDA5 causes the formation of MAVS signaling complex on mitochondria. RIP1 is similarly ubiquitinated to activate NF-κB and IRF3 within this complex. Treatment with the pan-caspase inhibitor zVAD induces the interaction between RIP1 and the E3 ubiquitin ligase component n-recognin 5 (EBR5, also known as EDD), leading to JNK-dependent TNF secretion in certain cell types. RIP1 contributes to the destructive inflammatory disease in SHP-1 mutant mice through promoting IL-1α expression. SM, SMAC mimetic.
4.2. RIP1 regulates TLR or RLR-induced anti-viral responses
Besides TNFR-1, RIP1 is also involved in signaling pathways induced by pattern recognition receptors (Fig. 2). RIP1−/− cells exhibited defective TLR3-induced NF-κB activation and type I IFN production. Surprisingly, this effect of RIP1 is also mediated by RHIM-dependent interaction with TRIF [65–67]. A similar role for RIP1 in TLR4-induced, TRIF-dependent NF-κB activation has also been reported [66]. When RLRs such as RIG-I [68] and melanoma differentiation-associated gene 5 (MDA5) [69] sense viral RNAs in the cytoplasm, they recruit the mitochondrial adaptor protein MAVS (also called IPS-1, Cardif, VISA) through the caspase recruitment domains (CARDs) to assemble a signaling complex containing TRADD, Fas-associated via death domain (FADD), caspase-8/10, and RIP1 [70–72]. The regulation of RIP1 activity downstream of RIG-I is similar to that in TNFR1 signaling. For example, RIP1 ubiquitination at Lys377 promotes IRF3 activation [73], while caspase-8 cleavage of RIP1 at Asp324 inhibits RIG-I signaling [73]. These observations underscore the parallel nature of cell death and innate immune signaling pathways.
4.3. RIP1 promotes apoptosis in the absence of cIAPs or TAK1
Death receptor induced apoptosis is optimally induced when NF-κB activity is inhibited. Under such conditions, RIP1 is dispensable for death receptor-mediated apoptosis [6, 64]. However, when cIAPs are depleted in response to IAP antagonists or SMAC mimetics [74, 75], RIP1 becomes indispensable for caspase-dependent apoptosis. SMAC mimetics sensitize cells to apoptosis through NIK, which drives non-canonical NF-κB activation, autocrine TNF production [75] and RIP1-dependent apoptosis [76–78]. Importantly, the kinase activity of RIP1 is required for apoptosis under these conditions. Smac mimetics also sensitize cells to apoptosis induced by TLR3 [79]. Since cIAPs are E3 ligases that ubiquitinate RIP1, RIP1 polyubiquitination is an important checkpoint that dictates whether cells stay alive or undergo death (Fig. 2). In agreement with this notion, deubiquitination of RIP1 by the tumor suppressor cylindromatosis (CYLD) facilitates apoptosis and necrosis [80–82]. In addition to cIAPs, RIP1 also contributes to TNF-induced apoptosis in the absence of TAK1. In TAK1−/− cells, TNF-induced caspase cleavage, RIP1-FADD interaction, and apoptosis were efficiently blocked by Nec-1 [83–85]. In contrast to cIAPs depletion, TAK1 inhibition did not affect RIP1 ubiquitination within Complex I, indicating that TAK1 inhibits TNF-induced apoptosis downstream of RIP1 ubiquitination (Fig. 2) [85]. Finally, RIP1 was recently reported to be involved in eleostearic acid-induced atypical apoptosis by promoting reactive oxygen species (ROS) production [86].
5. RIP1 kinase activity in cytokine expression
RIP1 kinase activity is not essential for TNF-induced NF-κB activation or mitogen-activated protein kinase (MAPK) activation, since neither necrostatins nor kinase inactive RIP1 had any effects on NF-κB and MAPK activities [7, 87]. On face value, these results would suggest that RIP1 kinase activity is not required for inflammatory cytokine expression. However, in L929 cells, pan-caspase inhibitors stimulated RIP1 kinase-dependent and c-Jun N-terminal kinase (JNK)-mediated transcription of TNF (Fig. 2) [88]. RIP1 kinase activity also underlies the IL-1α mediated auto-inflammatory disease in mice carrying loss of function mutation in Src homology region 2 (SH2) domain-containing phosphatase-1 (SHP-1) (Fig. 2) [89]. Hence, RIP1 kinase activity can promote apoptosis, necrosis and inflammatory cytokine expression in a context-dependent manner. Hence, results obtained using necrostatins should be carefully interpreted.
6. Non-necrotic functions of RIP3 in apoptosis and inflammasome activation
RIP3 was originally identified as a mediator of apoptosis and NF-κB activation [90, 91]. However, apoptosis and TNF- or TLR-induced NF-κB activation were normal in RIP3−/− thymocytes, MEFs and macrophages [92]. In contrast to these results, a recent paper showed that in the absence of cIAPs or TAK1, RIP3 cooperate with RIP1 to promote TNFR1-mediated apoptosis [85]. Hence, RIP3 may facilitate apoptosis under certain conditions.
The pro-inflammatory cytokine IL-1β is a key player in the pathogenesis of many acute and chronic inflammatory diseases. Pro-IL-1β requires cleavage by caspase-1 within the inflammasome to generate the biologically active mature IL-1β. The inflammasome is a macro-molecular complex composed of a sensor molecule such as NLR family, pyrin domain containing 3 (NLRP3), the adaptor apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1 [93]. A recent report shows that in LPS-primed bone marrow (BM)-derived macrophages (BMDMs) and BM-derived dendritic cells (BMDCs), depletion of cellular IAP proteins with Smac mimetics was sufficient to activate the NLRP3 inflammasome in a RIP3-dependent manner (Fig. 3) [94]. In agreement with results obtained using SMAC mimetics, macrophages lacking cIAP-1, cIAP-2 and XIAP readily produce mature IL-1β in response to LPS stimulation alone. In addition to caspase-1, caspase-8-mediated IL-1β processing was dependent on RIP3 under these conditions. RIP3 was also responsible for the inflammasomeindependent, caspase-8-mediated IL-1β processing in BMDCs costimulated with LPS and pro-apoptotic chemotherapeutic agents such as doxorubicin or staurosporine (Fig. 3) [95]. In contrast to these results, we were unable to detect mature IL-1β expression in SMAC mimetics and LPS-primed macrophages (unpublished observation). Because most SMAC mimetics do not affect XIAP expression, the discrepant results might be explained by whether XIAP expression is also affected in response to SMAC mimetics.
Figure 3. RIP3-dependent inflammasome activation.
When cIAP1/2 and XIAP are depleted by SMAC mimetics or genetic inactivation, LPS induces RIP3-dependent inflammasome and caspase-8 activation in BMDCs and BMDMs. Inflammasome-independent caspase-8 activation is induced by LPS in the presence of pro-apoptotic chemotherapeutic agents such as doxorubicin (Dox) or staurosporine (STS). In caspase-8−/− DCs, LPS-induced inflammasome activation is greatly increased in a RIP1- and RIP3-dependent manner.
Moreover, RIP3 was reported to promote NLRP3 inflammasome activation in caspase-8−/− DCs. NLRP3 activation and IL-1β secretion were substantially augmented in LPS-primed caspase-8−/− DCs, which was dependent on the RIP1 kinase activity and RIP3 (Fig. 3). This led to significantly elevated level of mature IL-1β and hypersensitivity to LPS-induced septic shock in mice with DC-specific deletion of caspase-8 [96]. These effects of RIP3 on inflammasome activation and IL-1β secretion were reported to be independent of necrosis induction. In contrast, DC-specific FADD−/− mice developed spontaneous systemic inflammation that was attributed to commensal microbiota driven inflammation and excessive RIP3-dependent necrosis [97]. Based on available genetics evidence, it is unlikely that these disparate results were due to distinct functions of FADD and caspase-8 in cell death and inflammation. Further analyses will be required to reconcile these findings. Nonetheless, these results highlight the importance of considering necrosis-dependent and independent effects of RIP3 in different inflammatory diseases. They also illustrate the cell type-specific roles of RIP3 in controlling cell death and inflammatory signaling.
7. Concluding remarks
Emerging evidence indicates that the RIP kinases can promote apoptosis, necrosis and inflammasome activation. Several questions arise from these recent observations. For example, necrosis is regulated by ubiquitination, phosphorylation, and caspase cleavage of RIP1 and RIP3. Do similar molecular mechanisms regulate RIP1 and RIP3 functions in apoptosis and inflammasome activation? Specifically, do RIP1 and RIP3 form amyloid-like structures beyond necrosis? What are the downstream effectors that mediate apoptosis and inflammasome signaling by RIP1 and RIP3? One recent study suggests that MLKL and PGAM5 are common downstream effectors of RIP3 in inflammasome signaling. Quantitative tools that positively distinguish RIP1 and RIP3 activities during apoptosis, necrosis and inflammasome activation will help to decipher these puzzles. In addition, it will be important to distinguish the contribution of these signaling pathways in RIP kinase-dependent inflammatory diseases. To this end, novel animal models that allow tracking of RIP1 and RIP3 functions in distinct cells and tissues will be useful. Such knowledge will facilitate examination of RIP1 and RIP3 as potential therapeutic targets in inflammatory diseases.
Acknowledgement
This work is supported by NIH grant AI083497 to F.C. K.M. is supported by a fellowship from the Japan Society for the Promotion of Science.
Biographies

Kenta Moriwaki is a senior fellow at the University of Massachusetts Medical School. He earned his PhD in cancer biology at Osaka University. His research interest is in the area of how cell death processes regulate inflammation and cancer growth.

Francis Ka-Ming Chan is an Associate Professor in the Department of Pathology at the University of Massachusetts Medical School. He received his PhD at the University of California, Berkeley, where he developed an interest in how cell death processes modulate immune cell development and functions. He did his postdoctoral work at the National Institutes of Health in Bethesda, MD, USA. His current work focuses on the molecular regulation of programmed necrosis or necroptosis and how this form of non-apoptotic cell death regulates inflammatory diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan FK. Fueling the flames: Mammalian programmed necrosis in inflammatory diseases. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 4.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 7.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, et al. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 13.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013 doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Dai W, Lin C, Wang F, He L, Shen M, et al. Protective Effects of Necrostatin-1 against Concanavalin A-Induced Acute Hepatic Injury in Mice. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/706156. 706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4:776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami Y, Matsumoto H, Roh M, Suzuki J, Hisatomi T, Ikeda Y, et al. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc Natl Acad Sci U S A. 2012;109:14598–14603. doi: 10.1073/pnas.1206937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Li S, Gordon WC, He J, Liou GI, Hill JM, et al. Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J Neurosci. 2013;33:17458–17468. doi: 10.1523/JNEUROSCI.1380-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 23.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 24.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 26.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 27.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 30.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 33.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 35.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McQuade T, Cho Y, Chan FK. Positive and Negative Phosphorylation Regulates RIP1 and RIP3-Induced Programmed Necrosis. Biochem J. 2013 doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 46.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 49.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 50.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 51.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 52.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like Receptor 3-mediated Necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 55.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thapa RJ, Basagoudanavar SH, Nogusa S, Irrinki K, Mallilankaraman K, Slifker MJ, et al. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol. 2011;31:2934–2946. doi: 10.1128/MCB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 59.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 60.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev. 2011;244:9–28. doi: 10.1111/j.1600-065X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 61.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 62.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. Embo J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 65.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 66.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 67.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 68.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 69.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 71.Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 74.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 75.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 76.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 79.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujikura D, Ito M, Chiba S, Harada T, Perez F, Reed JC, et al. CLIPR-59 regulates TNF-alpha-induced apoptosis by controlling ubiquitination of RIP1. Cell Death Dis. 2012;3:e264. doi: 10.1038/cddis.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arslan SC, Scheidereit C. The prevalence of TNFalpha-induced necrosis over apoptosis is determined by TAK1-RIP1 interplay. PLoS One. 2011;6:e26069. doi: 10.1371/journal.pone.0026069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33:582–595. doi: 10.1128/MCB.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20:1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obitsu S, Sakata K, Teshima R, Kondo K. Eleostearic acid induces RIP1-mediated atypical apoptosis in a kinase-independent manner via ERK phosphorylation, ROS generation and mitochondrial dysfunction. Cell Death Dis. 2013;4:e674. doi: 10.1038/cddis.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 88.Christofferson DE, Li Y, Hitomi J, Zhou W, Upperman C, Zhu H, et al. A novel role for RIP1 kinase in mediating TNFalpha production. Cell Death Dis. 2012;3:e320. doi: 10.1038/cddis.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, et al. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9:539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 91.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 92.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic Chemotherapeutic Drugs Induce Noncanonical Processing and Release of IL-1beta via Caspase-8 in Dendritic Cells. J Immunol. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Young JA, He TH, Reizis B, Winoto A. Commensal microbiota are required for systemic inflammation triggered by necrotic dendritic cells. Cell Rep. 2013;3:1932–1944. doi: 10.1016/j.celrep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]