Abstract
BLyS family members govern selection and survival of cells in the preimmune B cell compartment, and emerging evidence suggests similar roles in antigen-experienced B cell pools. We review the features of this family, with particular emphasis on recent findings of how BLyS influences affinity maturation in germinal centers, which lie at the intersection of the pre-immune and antigen-experienced B cell compartments. We propose a model whereby tolerogenic selection at the transitional stage and affinity maturation in the germinal center employ the same BLyS driven mechanism.
Keywords: BLyS, BAFF, TACI, germinal center
1. Introduction to the BLyS family of cytokines and receptors
B cells are the effectors of humoral immunity. Quiescent, pre-immune B cells are generated throughout life from hematopoietic stem cells and, when activated by antigen exposure, expand and further differentiate into antibody-forming plasma cells (PCs) or memory B cells (Bmem) that mediate long-term immunity. Members of the B Lymphocyte Stimulator (BLyS, a.k.a. B cell activating factor of the TNF family, BAFF) family of ligands and receptors play unique, lineage-specific roles in B cell development, selection, persistence, and function. Much research and speculation to date has focused on how members of this family govern the size and composition of pre-immune B cell pools. However, more recent evidence reveals roles for this molecular family in dictating the differentiation, selection and persistence of activated and antibody secreting effector cells of the B lineage. Accordingly, we herein briefly overview the basic features of BLyS family members and their roles in pre-immune B cell selection and homeostasis. We then provide a more forward thinking and detailed consideration of recently appreciated influences on other B lineage subsets, with emphasis on the selective processes acting on germinal center (GC) B cells.
1.1 BLyS family ligands: BLyS (BAFF) and APRIL
The BLyS family is a subset of the tumor necrosis factor (TNF) superfamily, and includes two ligands (reviewed in detail in (1, 2)). A Proliferation Inducing Ligand (APRIL, TNFSF13a) was the first to be described, and is also termed TALL-2 (TNF- and ApoL-related Leukocyte- expressed Ligand 2) or TRDL-1 (TNF-related Death Ligand-1a). Subsequently, several laboratories simultaneously reported B lymphocyte stimulator (BLyS; TNFSF13b), which also appears in the literature as BAFF (B cell Activating Factor of the TNF superfamily), THANK (a TNF Homologue that activates Apoptosis, Nuclear factor-kappaB, and c-Jun NH2-terminal Kinase), TALL-1 (TNF- and ApoL-related Leukocyte-expressed Ligand 1) and zTNF4. While these two ligands share only ~25% identity with the conserved carboxy-terminal regions of other TNF family members, they share 33% amino acid and 48% DNA homology with each other. Moreover, amino acid sequence homologies for each cytokine between mammals ranges from 80%-97%.
Both APRIL and BLyS are type II transmembrane proteins that are cleaved into soluble forms by protein convertases ((3, 4); reviewed in (2)). Their active forms are composed of homotrimers and, while heterotrimers have been demonstrated, the biological relevance of such hybrid molecules is not yet understood. Nevertheless, heterotrimers are active in vitro and are elevated in the serum of some autoimmune patients (for example, (5)). BLyS can also assemble into 60mers, which exhibit distinct binding and signaling characteristics (6). Finally, membrane-bound forms of BLyS have been observed which might reflect incomplete cleavage of the membrane form or the expression of an alternative splice form known as delta BAFF, which lacks the stalk region and consequently cannot be cleaved (7). Unlike BLyS, APRIL is cleaved in the Golgi prior to secretion, precluding expression as a membrane-bound form (4). Nonetheless, it can bind heparan sulfated proteoglycans (HSPG) via its amino terminus, allowing oligomerization and presentation on cell surfaces (8).
BLyS and APRIL are produced by cells of non-hematopoietic as well as hematopoietic origin (reviewed in (9, 10)). Radioresistant stromal cells maintain systemic BLyS levels, with apparently minimal contribution from cells of hematopoietic origin (11). Similar assessments have not yet been made for APRIL. Tumor cell lines derived from non-hematopoietic tissues as well as astrocytes are enriched for APRIL production (12, 13), but the extent of the contribution of these sources to overall APRIL production has not yet been determined. Among hematopoietic cells, myeloid cells/cell lines such as monocytes, eosinophils, osteoclasts, and neutrophils produce both cytokines, albeit with a generally greater propensity to produce APRIL than BLyS (10). Macrophages and dendritic cells express membrane-bound BLyS, and expression levels can be augmented or depressed by cytokines such as IFNγ or IL-4, respectively (3, 14). Further, compared to macrophages and B-1 B cells, resting splenic B-2 cells in mice express neither BLyS nor APRIL message (15). However, TLR agonists or surrogate BCR cross linking in vitro may induce transcripts for both cytokines (15, 16). Similarly, quiescent T cells express no BLyS or APRIL, although expression can be induced by TCR-driven activation in some circumstances (4, 15, 17). Curiously, in autoimmune-prone mice, depletion of CD4 T cells significantly reduces circulating levels of BLyS. Whether this is due to the absence of T cell-derived cytokines (such as IFNγ) that augment BLyS secretion by myeloid cells, or to a significant contribution of BLyS from excessive activated CD4 T cells themselves, is not yet known. Among activated CD4 T effectors, antigen-specific follicular helper T cells (TFH) are enriched for BLyS mRNA expression and express BLyS protein in the germinal center (18), as discussed further in section 3 below.
1.2. BLyS family Receptors: BR3/BAFFR, TACI, and BCMA
BLyS and APRIL can interact with three receptors, BR3 (BLyS Receptor 3, also termed BAFFR), TACI (Transmembrane Activator and Calcium modulator and cyclophilin ligand Interactor) or BCMA (B Cell Maturation Antigen). These interactions are extensively reviewed elsewhere, for both mice and humans ((1, 2, 10, 19), and briefly addressed here. These receptors possess characteristic canonical cysteine rich domains (CRDs) that are comprised of 6 cysteine residues, and transduce TNF Receptor Associated Factor (TRAF)-mediated signals. However, unlike other TNF receptors that express 3-6 CRDs, BR3 has only a partial CRD, BCMA has one CRD, and TACI has two. These atypical structures confer exquisite specificity for BLyS and APRIL, but not for other TNF ligands (20, 21). As noted above, APRIL can passively bind to proteoglycans, though whether such interaction induces downstream signaling events is not yet known (8, 22). Finally, TWE-PRIL, a fusion protein between the intracellular, transmembrane and stalk region of TWEAK (TNF Weak inducer of apoptosis) coupled to the extracellular receptor-binding part of APRIL, recognizes BCMA and TACI (19).
BLyS binds with much higher affinity to BR3 than to BCMA, whereas APRIL has a greater affinity for BCMA and little or no binding capacity for BR3 (23). Moreover, BLyS has a higher affinity for TACI compared to BCMA, and the converse is true for APRIL (21). Nonetheless, BLyS binds to BR3- or TACI-transfected cells with similar strength, and TACI has ~ 25 fold higher affinity for BLyS than for APRIL (24, 25). Therefore, it is conceivable that under steady state conditions, BLyS is largely bound to TACI. Indeed, reagents that detect pre-bound BLyS on circulating? B cells in mice have revealed that TACI is the key receptor involved in binding of BLyS to mature naïve B cells (18). Consistent with the inefficient binding of BLyS to TACI-deficient B cells, elevated levels of circulating BLyS are observed in TACI knockouts (data not shown). Additionally, BLyS 60mers bind to TACI with much higher affinity than BLyS trimers, and thus are readily detectable in the circulation of TACI-deficient mice (26).
1.3. BLyS family members govern B lineage homeostasis and selection
Homeostasis in the various functional B cell compartments is achieved by regulating generation rates, selection thresholds, and cellular lifespan. Steadily accumulating evidence over the last decade has revealed that BLyS family members play critical and non-redundant roles in all of these processes. A key feature of the BLyS family is that it includes multiple receptors and ligands with different binding preferences. We and others have posited that differential expression of the three BLyS family receptors affords coexisting, yet distinct and independently regulated, homeostatic niches for mature naïve, antibody-secreting, and memory B cell subsets (27). Consistent with this idea, the various pre-immune and antigen-experienced B cell subsets express different combinations of BLyS receptors at varying levels (reviewed in (28, 29)), and display differential reliance on the two cytokines (summarized in Table 1). The following sections expand on this theme, and review evidence supporting this idea for resting, activated, and antibody secreting B cell populations. Moreover, we propose a model based on recent findings that suggests transitional (TR) and germinal center (GC) B cell selection proceed via the same, BLyS-mediated, mechanism.
Table 1.
BLyS family receptor expression patterns and cytokine dependence for developing, pre-immune, and antigen-experienced B cell pools
| Differentiation Stage | Receptor Expression1 |
Cytokine Dependence2 |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| BR3 | TACI | BCMA | BLyS | APRIL | ||
|
Developing and
Pre-immune pools |
Bone marrow Pro-B and Pre-B | − | − | − | N | N |
| Bone marrow immature | + | ± | − | Y (?) | N | |
| Transitional | + | + | − | Y | N | |
| Follicular | + | + | − | Y | N | |
| Marginal zone | + | ++ | − | Y | N | |
|
Antigen-experienced
pools |
Germinal center | + | ↓ | − | Y | N |
| Short-lived plasma cell | ↓ | ↑ | − | N (?) | Y | |
| Long-lived plasma cell | ↓ | ↓ | ↑ | Y | Y | |
| Memory B cell | ↓ | + | ↑ | N (?) | N (?) | |
No expression (−), relative expression level (± or +), downregulation (↓), or upregulation (↑) compared to pre-immune pools
Y = yes, N = no evidence/not known
2. The BLyS-BR3 axis governs pre-immune B-2 cell selection and homeostasis
B cells are broadly divided broadly into two categories, B-2 and B-1 B cells. Cells of the B-2 lineage are continuously replenished from bone marrow (BM) precursors, exhibit extensive B cell antigen receptor (BCR) diversity, and comprise the bulk of recirculating B cells in the blood and associated secondary lymphoid organs. Following lineage specification from lymphoid progenitors, developing B-2 cells undergo RAG-mediated rearrangement of their immunoglobulin heavy and light chain genes during the pro- and pre-B cell stages, respectively, culminating in the expression of a functional B cell antigen receptor (BCR). The immature B-2 cells thus formed undergo negative and positive selection based on BCR specificity, and then exit the BM as transitional (TR) B cells. After further BCR-mediated selection, cells that successfully complete TR differentiation enter the mature, quiescent B-2 pools as either follicular (FO) or marginal zone (MZ) B cells. Negative selection during immature and TR differentiation purge many autoreactive BCR specificities, eliminating ~95% of all incipient B-2 cells. In contrast to these B-2 characteristics, cells of the B-1 lineage are derived largely from progenitors in the fetal liver and neonatal spleen, display a restricted BCR repertoire, and are generally confined to particular anatomic locales, including the peritoneal cavity and some mucosal sites. Emerging evidence that BLyS family members are involved in B-1 lineage homeostasis has recently been reviewed elsewhere (30), so we focus herein on roles for the BLyS family in the B-2 lineage.
2.1 BLyS receptor expression commences at the TR developmental stages
Within the B2 lineage, neither pro- nor pre-B cells express BLyS family receptors. The CD23+ subset of immature BM B cells express BR3 and TACI transcripts, but show minimal surface protein. As immature B cells emigrate from the BM and continue maturation in the periphery as TR B cells, surface expression of both BR3 and TACI increases (28). Early TR B cells (T1 subset) express surface BR3 but little TACI, while later TR subsets (T2, T3) maintain surface BR3 and up regulate TACI message and surface protein (29, 31, 32). These TR B cells in turn give rise to mature naïve B cells of the FO or MZ subsets, which express the highest levels of surface BR3 and TACI (33). Thus, as B cells progress through peripheral maturation stages, both BR3 and TACI are up regulated on the cell surface, and are stably maintained on mature quiescent B cells. BCMA transcript and surface protein are low to undetectable on all pre-immune human and murine B cell subsets (32, 34).
2.2 BLyS signaling through BR3 governs transitional differentiation and mature B cell survival
BLyS signaling via BR3 is indispensable for successful TR differentiation and for mature pre-immune B cell survival. Genetic deficiency in either BLyS or BR3 stalls B cell development at the late TR stages (reviewed in (1, 35)), and yields profound reductions in FO and MZ B cells. Similar effects are observed after treatment with BLyS neutralizing antibody or soluble decoy receptors (20, 21, 36, 37). Conversely, administration of exogenous BLyS or over-expression of BLyS in transgenic mice significantly increases FO and MZ B cell numbers (38, 39). In contrast, TACI or BCMA knockouts, as well as APRIL-deficient mice, have no defects in pre-immune B-2 B cell maturation (40-42).
BLyS also regulates an elastic threshold for BCR-mediated selection during TR development. B cells with relatively high BCR signal strengths are normally eliminated through negative selection at this TR checkpoint. However, elevated BLyS levels relax selective stringency, broadening the range of acceptable BCR signal strength and allowing more TR cells to survive, effectively increasing the production rate of mature FO and MZ B cells ((43); reviewed in (44, 45)). Likewise, reduced BLyS levels increase the stringency of TR selection, allowing fewer cells through than normal. Thus, BLyS levels dictate homeostatic “space” for pre-immune B-2 cells by governing both the proportion and quality of TR B cells that complete differentiation, as well as the lifespan of mature B cells themselves, thereby controlling the overall size and composition of FO and MZ B cell populations.
These observations also suggest that BCR and BR3 signals are integrated and non-redundant, since the absence of either signaling arm severely curtails peripheral B cell maturation and maintenance (reviewed in (46, 47)). The molecular mechanisms of interplay between BCR and BR3 signaling pathways appear complex (32, 48, 49), but likely involve cross-talk between downstream signaling and transcriptional regulatory systems including non-classical NF-κB, PI3-Kinase, and Syk (32, 49-52). More recent work indicates that the BCR/Igα function as adapter proteins in the BR3 signaling pathway, instead of delivering an independent survival signal (48). Regardless of the exact molecular mechanisms involved, the ability of BLyS to serve as a limited, and therefore competitive, resource in specificity-driven selection of the developing pre-immune B2 repertoire raises the question of whether it plays a similar role in other competitive selection events of this lineage, such as affinity maturation during the GC reaction (see below).
3. BLyS family members play multiple roles among antigen-experienced B cells
Upon antigen challenge and BCR cross-linking, mature pre-immune B cells may differentiate directly into plasma cells. Alternatively, they may become germinal center (GC) B cells that will alter their BCRs through somatic hypermutation (SHM) and undergo further specificity-based selection, subsequently giving rise to either Bmem or long-lived plasma cells (LLPC) (reviewed in (53, 54)). The propensity for adopting these alternate fates depends on the so-called second signals received following BCR ligation, that direct the ensuing expansion and differentiation. If the second signals do not include cognate CD4 T cell help (T cell independent or TI responses), then the predominant fate is direct differentiation to relatively short-lived plasma cells (SLPCs). In contrast, activated B cells that receive cognate CD4 T cell help (T cell dependent or TD responses) are directed to adopt GC B cell characteristics, undergo affinity maturation, and subsequently give rise to long lived subsets including LLPCs and Bmem cells.
Marked alterations in BLyS receptor expression occur after all types of antigen encounter and activation, but the exact expression patterns varying, depending on the fate adopted (summarized in Table 1). In the following sections, we first consider the changes observed in BLyS family receptor expression and ligand dependence as cells enter antibody secreting PC pools, then focus on the recently revealed role for BLyS family members in affinity maturation during the GC reaction.
3.1 TACI and BCMA may distinguish alternative routes to antibody-secreting effectors
Accumulating evidence indicates that local sources of APRIL are key in the establishment and maintenance of antibody-secreting B cells. Accordingly, these interactions appear to be mediated primarily through the ARIL-binding receptor partners, TACI, BCMA, and HSPGs. However, the distribution of these receptors differs among PC subsets and, while not absolute, these may reflect distinct receptor expression patterns among SLPC versus LLPC.
In general, TI antigens circumvent the need for cognate T help either by engaging innate receptors such as Toll-like receptors (TLRs) or through extensive BCR ligation. Such antigens trigger B cell proliferation and accumulation at extrafollicular sites within 4-5 days (55), and largely result in SLPCs that persist for days to weeks (56). TLR stimulation up regulates TACI on both FO and MZ B cells, and the SLPC generated during TI responses up-regulate TACI (16). Further, both the overall TI responses as well as class switching are blunted in TACI-deficient mice (41, 57, 58). Similarly, APRIL-deficient mice are also impaired in their ability to induce TI responses and in class switching to IgA (42, 56). Moreover, HSPG assist APRIL binding to TACI, thereby promoting IgA production (59). Extrafollicular PCs reside next to macrophages that are rich sources of APRIL (60). Together, these observations indicate an important role for APRIL interactions with TACI, and possible tripartite interactions between APRIL, TACI and HSPGs, in establishing or maintaining cells in the SLPC niche.
In contrast to the SLPC generated in TI responses, LLPC are mainly products of GC reactions (reviewed in (53)). However, our understanding of events that occur between GC resolution and LLPC establishment is limited. Nevertheless, BCMA clearly mediates survival of LLPC in mouse bone marrow, with both APRIL and BLyS likely required for establishment and maintenance of the LLPC niche (reviewed in (46, 61)). BCMA is the only BLyS family receptor expressed on most murine BM LLPC, though a small subset express TACI (Quinn et al., submitted). HSPG are expressed on plasma cells and indeed may assist APRIL binding to BCMA in the bone marrow, thereby promoting PC retention (8, 22, 62). Moreover, many BM resident and recirculating cell types express HSPG and/or produce APRIL, including osteoclasts, RANKL-stimulated macrophages and eosinophils (Quinn et al., submitted; (63-65). Taken together, these observations suggest that APRIL elaborated and sequestered by BM stromal cells creates a survival niche for BCMA-expressing BM plasma cells (61, 63, 66).
3.2 Memory B cells are largely BLyS independent
Memory B cells are also thought to arise mainly or solely from GC reactions, and although they do not secrete antibody, are mentioned here because of evidence from mouse models that establishment and maintenance of the memory B cell niche are independent of both BLyS and APRIL (37, 67). For example, in studies where the memory pool was allowed to establish for 6-8 weeks before ablating systemic BLyS, there was no impact on either memory B cell numbers or the ability to respond to secondary challenge. Further subsetting however revealed a small but consistent impact o on non-switched memory, suggesting some Bmem subsets may be BLyS sensitive (37). Whether these distinctions between switched and unswitched Bmem reflects differential BLyS receptor expression on these subsets of cells is not known.
3.3 Systemic and locally produced BLyS play distinct roles in GC maintenance and selection
Germinal centers are transient structures that form at the T-B interface of lymphoid follicles in the spleen and lymph nodes following antigen encounter. Somatic hypermutation in GC B cells results in activated B cell clones with novel BCR specificities that subsequently undergo both positive selection for higher affinity antigen-specific clones, as well as negative selection against incipient autoreactive somatic variants (reviewed in (53, 54)). The mechanisms mediating this specificity-driven selection remain unclear, but likely involve a combination of cellular interactions and competitive processes that, in concert, favor the survival of appropriate clonotypes. Substantial evidence indicates this process relies on interactions with accessory cells such as T follicular helper (TFH) cells (reviewed in (68)) and follicular dendritic cells (FDCs). TFH, a subset of activated helper T cells that are critical for GC responses, up regulate the chemokine receptor CXCR5 and migrate into the light zone of the germinal center (69). The requisite for cognate help in optimal evolution of the GC response as well as for normal GC selection has long been appreciated (68). Follicular dendritic cells (FDCs) display immune complexes on their surface and are thought to be a source of antigen for GCB cells; however, the role of immune complex presentation on FDCs is still controversial, since mice lacking the ability to secrete antibody show minimal impact on affinity maturation (70).
Several lines of evidence suggest roles for BLyS and BR3 in the GC reaction. First, mice that are BLyS-deficient or lack BR3 signaling capacity can form GCs, but are unable to maintain them (71, 72). Further, neutralization of BLyS alone or both BLyS and APRIL impairs GC maturation and function (72, 73). In contrast, APRIL-deficient mice have normal GC responses (42). Although these studies point to a role for BLyS-mediated signals in sustaining GCs, the B lymphopenia accompanying global systemic BLyS or BR3 deficiency thwarts straightforward interpretation. For example, GCs are constantly fed by naïve B cells (74), and antigen-presenting B cells are critical for complete TFH differentiation (75-78). Further, BLyS knockout mice also exhibit impaired FDC network maturation (72). These considerations raise the possibility that BLyS plays only an indirect role in the GC reaction, by maintaining sufficient naive B cell numbers for replenishment, antigen presentation, and organogenesis. Alternatively, BLyS might be directly involved in GC B cell selection, since BCR occupancy – and presumably signal strength - is linked to a survival advantage, analogous to TR selection. However, this raises the conundrum of how cells within the GC could compete for BLyS independently from naïve cells in the physically proximal and much larger FO and MZ pools -- unless the GC provides a unique local microenvironment that enables this.
Recent findings from our laboratory (18) have now separated the roles played by systemic BLyS versus locally produced BLyS in GC maintenance versus selection respectively. These studies in toto show that: (1) GC B cells downregulate TACI, resulting in their inability to sequester BLyS; (2) this in turn creates a BLyS-poor microenvironment within GCs; (3) TFH are a major source of BLyS within the GC; and (4) TFH-derived BLyS is required to promote the survival of high-affinity GC B cells. An intriguing aspect of these findings was that GCs are largely devoid of BLyS, despite a lack of physical barriers separating them from neighboring follicles where ample systemic BLyS is available. Moreover, this sequestration of systemic BLyS into the follicles and out of GCs correlated with complete loss of TACI on GC B cells; and TACI down regulation is driven by IL-21 in the context of BCR and CD40 signals (18).
This somewhat surprising set of observations has several implications. First, it indicates that even though naïve B cells rely on BR3 and not TACI for their BLyS-mediated survival signals, the TACI receptor is nonetheless critical for retention of systemic BLyS on FO and MZ B cell surfaces. Second, it suggests TACI down-regulation is a distinguishing feature of GC B cells, which may prove useful in identifying cells that have participated in GC reactions. Third, it implies that within the GC, BLyS availability is highly limited. Finally, since GC B cells maintain surface BR3 (18), this is the sole receptor through which BLyS can act.
Thus, the GC provides a unique microenvironment that is largely separated from any influence of systemic BLyS, and that contains activated B cells with a unique, BR3 only, receptor profile. A key observation suggesting how this might be leveraged to influence GC selection was revealed in histological and gene expression analyses: at the peak of the GC response, TFH cells are a local source of BLyS within the otherwise BLyS-poor GC. Moreover, when T cells are BLyS-deficient but systemic BLyS and B cell numbers are normal, GC size, structure and kinetics, as well as total serum antibody levels, are unimpaired, yet high-affinity antibody production is compromised. Together, these results indicate that while TFH-derived BLyS is dispensable for GC initiation and maintenance, it is critical to the persistence of high-affinity clones and efficient affinity maturation.
4. A unified model for TR and GC B cell selection
Because selection within the GC is based on BCR specificity, it has been called a “second window” of tolerogenic selection (79, 80) and termed “neoteny (81),” thus suggesting similarity to BCR-mediated selection during the TR stages of pre-immune B cell development. A long-standing caveat in this analogy has been the question of why GC B cells with increased affinity for the immunizing antigen – and therefore highest BCR occupation and signal strength – would undergo positive selection, contrasting TR selection where the strongest BCR signals yield death. This line of reasoning is further complicated by the simultaneous negative selection of GC B cells that have acquired self reactivity, inasmuch as these should also be experiencing high BCR occupation by the self antigen.
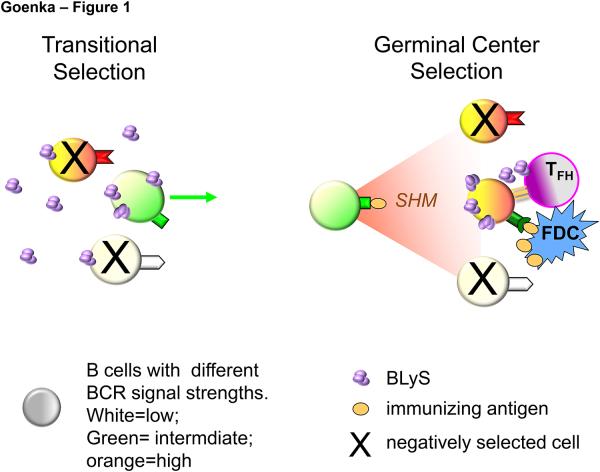
We suggest that the previously unappreciated sequestration of systemic BLyS out of GCs, combined with local production by TFH, reconciles these caveats; and we propose a unified model whereby TR and GC selection employ the same mechanism. This model is schematized in Figure 1. At the TR checkpoint (Fig. 1 left panel), selection based on BCR signal strength is modulated by systemic BLyS levels (purple trimers), such that cells with sub-threshold BCR signaling are better competitors for BLyS and survive; whereas cells with the highest BCR signal strength are negatively selected (large X) except when BLyS levels are abnormally high. Because systemic BLyS is uniformly available to all emerging TR B cells, an upper BCR signaling threshold for negative selection is thereby evenly applied across the population. A central feature of this model is that within the GC (Fig. 1 right panel), cells with strong BCR signal strength are, in fact, similarly disadvantaged, and must rely on BLyS-mediated rescue to survive. However, because systemic BLyS is meager within the GC and GC B cells are unable to retain surface BLyS, only cells engaged in sustained contact with TFH will acquire this locally produced BLyS. Accordingly, cells that acquire self reactivity will die because they no longer capture the immunizing antigen and are unable to present to and engage in sustained interactions with TFH. In contrast, cells that have mutated to higher affinity for the immunizing antigen will capture and present antigen efficiently, and can thus be rescued by TFH-produced BLyS, despite their high BCR signal strength. This model for GC selection is consistent with a requirement for competitive B cell antigen capture from FDC as a key event in the process, as well as with reports that TFH interactions are crucial for GC positive selection. Furthermore, it predicts that, as with TR selection, failures in BLyS family components – such as BLyS overproduction by TFH, inappropriate BR3 upregulation or TACI down regulation -- could thwart GC selection, resulting in poor protective humoral responses or autoimmunity.
Figure 1.
Systemic BLyS levels afford survival of only B cells with sub-threshold BCR signals at the transitional checkpoint (left), while TFH-produced BLyS selectively spares antigen-presenting GC B cells with very strong BCR signals (right).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mackay F, Schneider P. Cracking the BAFF code. Nature reviews Immunology. 2009 Jul;9(7):491–502. doi: 10.1038/nri2572. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- 2.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006 Oct;18(5):263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001 Jan 1;97(1):198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001 Oct;2(10):945–51. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon SR, Harder B, Lewis KB, Moore MD, Liu H, Bukowski TR, et al. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthritis Res Ther. 2010;12(2):R48. doi: 10.1186/ar2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Xu L, Opalka N, Kappler J, Shu HB, Zhang G. Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell. 2002 Feb 8;108(3):383–94. doi: 10.1016/s0092-8674(02)00631-1. [DOI] [PubMed] [Google Scholar]
- 7.Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, et al. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005 Jul 1;175(1):319–28. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 8.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005 May 2;201(9):1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003 Jun-Aug;14(3-4):311–24. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 11.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. The Journal of experimental medicine. 2003 Sep 15;198(6):937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thangarajh M, Masterman T, Hillert J, Moerk S, Jonsson R. A proliferation-inducing ligand (APRIL) is expressed by astrocytes and is increased in multiple sclerosis. Scand J Immunol. 2007 Jan;65(1):92–8. doi: 10.1111/j.1365-3083.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 13.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998 Sep 21;188(6):1185–90. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999 Jul 9;285(5425):260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 15.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007 Nov 1;179(9):5947–57. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 16.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007 Jun 15;178(12):7531–9. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 17.Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999 May;65(5):680–3. [PubMed] [Google Scholar]
- 18.Goenka R, Matthews AH, Zhang B, O’Neill PJ, Scholz JL, Migone TS, et al. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. Dec 23, [DOI] [PMC free article] [PubMed]
- 19.Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006 May 19;281(20):13964–71. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 20.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001 Oct 2;11(19):1547–52. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 21.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000 Jun 29;10(13):785–8. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 22.Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M, et al. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J. 2009 May;23(5):1584–95. doi: 10.1096/fj.08-124669. [DOI] [PubMed] [Google Scholar]
- 23.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005 Feb 15;44(6):1919–31. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000 Nov 10;275(45):35478–85. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001 Sep 14;293(5537):2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 26.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008 Feb 1;111(3):1004–12. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 27.Crowley JE, Treml LS, Stadanlick JE, Carpenter E, Cancro MP. Homeostatic niche specification among naive and activated B cells: a growing role for the BLyS family of receptors and ligands. Semin Immunol. 2005 Jun;17(3):193–9. doi: 10.1016/j.smim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Cancro MP. Peripheral B-cell maturation: the intersection of selection and homeostasis. Immunological reviews. 2004 Feb;197:89–101. doi: 10.1111/j.0105-2896.2004.0099.x. [Review] [DOI] [PubMed] [Google Scholar]
- 29.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009;53(1):1–16. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sindhava VJ, Scholz JL, Cancro MP. Roles for BLyS family members in meeting the distinct homeostatic demands of innate and adaptive B cells. Front Immunol. 4:37. doi: 10.3389/fimmu.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002 Jun 15;168(12):5993–6. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 32.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nature immunology. 2008 Dec;9(12):1379–87. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancro MP. The BLyS family of ligands and receptors: an archetype for niche-specific homeostatic regulation. Immunol Rev. 2004 Dec;202:237–49. doi: 10.1111/j.0105-2896.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 34.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007 Dec 1;179(11):7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 35.Crowley JE, Scholz JL, Quinn WJ, 3rd, Stadanlick JE, Treml JF, Treml LS, et al. Homeostatic control of B lymphocyte subsets. Immunol Res. 2008;42(1-3):75–83. doi: 10.1007/s12026-008-8036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vugmeyster Y, Seshasayee D, Chang W, Storn A, Howell K, Sa S, et al. A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am J Pathol. 2006 Feb;168(2):476–89. doi: 10.2353/ajpath.2006.050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15517–22. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3370–5. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999 Dec 6;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001 Dec 3;194(11):1691–7. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001 May;14(5):573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 42.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hondowicz BD, Alexander ST, Quinn WJ, 3rd, Pagan AJ, Metzgar MH, Cancro MP, et al. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. Int Immunol. 2007 Apr;19(4):465–75. doi: 10.1093/intimm/dxm011. [DOI] [PubMed] [Google Scholar]
- 44.Miller JP, Stadanlick JE, Cancro MP. Space, selection, and surveillance: setting boundaries with BLyS. J Immunol. 2006 Jun 1;176(11):6405–10. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- 45.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009 May;119(5):1066–73. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011 Nov;244(1):115–33. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009 Sep 15;183(6):3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 48.Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013 Mar 21;38(3):475–88. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997 Sep 19;90(6):1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 50.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002 Oct;17(4):515–24. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 51.Tucker E, O’Donnell K, Fuchsberger M, Hilton AA, Metcalf D, Greig K, et al. A novel mutation in the Nfkb2 gene generates an NF-kappa B2 “super repressor”. J Immunol. 2007 Dec 1;179(11):7514–22. doi: 10.4049/jimmunol.179.11.7514. [DOI] [PubMed] [Google Scholar]
- 52.Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998 Jan 19;187(2):147–59. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007 Jun;19(3):275–80. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010 Nov;126(5):898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 8-9. [DOI] [PubMed] [Google Scholar]
- 55.Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999 Apr;29(4):1314–23. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 56.Hardenberg G, van Bostelen L, Hahne M, Medema JP. Thymus-independent class switch recombination is affected by APRIL. Immunol Cell Biol. 2008 Aug-Sep;86(6):530–4. doi: 10.1038/icb.2008.17. [DOI] [PubMed] [Google Scholar]
- 57.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. Journal of immunology. 2007 Aug 15;179(4):2282–8. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 58.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010 Sep;11(9):836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007 Apr 1;109(7):2961–7. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- 60.Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009 Feb 15;182(4):2113–23. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 61.Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol. 2012;32(4):287–305. doi: 10.1615/critrevimmunol.v32.i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reijmers RM, Groen RW, Kuil A, Weijer K, Kimberley FC, Medema JP, et al. Disruption of heparan sulfate proteoglycan conformation perturbs B-cell maturation and APRIL-mediated plasma cell survival. Blood. 2011 Jun 9;117(23):6162–71. doi: 10.1182/blood-2010-12-325522. [DOI] [PubMed] [Google Scholar]
- 63.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008 Mar 1;111(5):2755–64. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 64.Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H, et al. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia. 2006 Jul;20(7):1313–5. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 65.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011 Feb;12(2):151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 66.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008 Aug;118(8):2887–95. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008 Mar 15;180(6):3655–9. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 68.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 69.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 70.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000 Oct 2;192(7):931–42. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman ZS, Manser T. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J Immunol. 2004 Nov 15;173(10):6179–88. doi: 10.4049/jimmunol.173.10.6179. [DOI] [PubMed] [Google Scholar]
- 72.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. The Journal of experimental medicine. 2003 Oct 20;198(8):1157–69. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, et al. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003 Jul 15;171(2):547–51. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 74.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007 Mar 1;446(7131):83–7. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 75.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011 Jun 24;34(6):947–60. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J Immunol. 2011 Aug 1;187(3):1091–5. doi: 10.4049/jimmunol.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. The Journal of experimental medicine. 2010 Feb 15;207(2):365–78. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010 Feb 15;207(2):353–63. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klinman NR. In vitro analysis of the generation and propagation of memory B cells. Immunol Rev. 1996 Apr;150:91–111. doi: 10.1111/j.1600-065x.1996.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 80.Diaz M, Verkoczy LK, Flajnik MF, Klinman NR. Decreased frequency of somatic hypermutation and impaired affinity maturation but intact germinal center formation in mice expressing antisense RNA to DNA polymerase zeta. J Immunol. 2001 Jul 1;167(1):327–35. doi: 10.4049/jimmunol.167.1.327. [DOI] [PubMed] [Google Scholar]
- 81.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996 Dec 20;274(5295):2094–7. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]