Abstract
Venous stenosis, secondary to venous neointimal hyperplasia (VNH), at the arteriovenous anastomosis (AV) is a major etiology of vascular access failure in AV fistulas (AVF) and AV grafts (AVG). Recently, our group has reported that severe VNH also occurs prior to vascular access placement. The objective of this study was to perform a comparison of the cellular phenotypes within the neointima from veins collected from subjects at the time of new vascular access creation and stenotic veins from subjects with failed AVGs and AVFs.
Vein samples, collected at the time of new access surgery, and stenotic vein segments, collected at access revision, were evaluated for expression of α-smooth muscle actin (SMA), vimentin, and desmin within the neointima, and quantified using semi-quantitative scoring.
Within the neointima, the majority of cells from vein samples collected at the time of new access surgery were contractile smooth muscle cells, and veins from stenotic AVF and AVG were predominately myofibroblasts.
Our results suggests the possibility of different mechanistic pathways in response to vascular injury that occurs prior to vascular access creation vs after access creation, and that divergent therapeutic approaches may be needed for treating vascular injury in these two settings.
Keywords: End Stage Renal Disease, Hemodialysis, Vascular Access, Arteriovenous Fistula, Arteriovenous Graft, Neointimal Hyperplasia
Introduction
Venous neointimal hyperplasia (VNH) at the AV anastomosis is a major cause of AVF and AVG failure after vascular access creation1, 2. Recently, several studies have also reported that VNH is present at different severities prior to new vascular access creation3–6, suggesting that significant vascular injury from uremia and vascular complications of advanced chronic kidney disease (CKD) occurs before vascular access placement. A major feature within the VNH from stenotic AVF and AVG and preexisting VNH are smooth muscle cells and myofibroblasts2, 3, 7. Understanding the differences in the composition of cellular phenotypes within the neointima may provide valuable information on how cells proliferate, migrate, and transform before and after AV access creation; and may influence the approach to the development of targeted therapies that can be administered prior to and after AV access creation. Thus, the aim of this study was to perform a comparison of cellular phenotypes from venous tissue samples collected from subjects at the time of new vascular access creation and stenotic vein samples collected from subjects with failed AVF and AVG.
Subjects and Methods
Specimen Collection and Processing
Institutional Review Board approval was obtained to conduct this study. Vein samples were collected from subjects who had: (1) new vascular access creation and (2) surgical revision for a failed vascular access. Discarded tissue from the venous segments of 25 AVF and 8 AVG were collected at the time of vascular access revision surgery. 63 vein samples from patients requiring new vascular access placement were additionally collected.
For collection of vein segments at the time of new vascular access surgery, an approximately 8–10mm circumferential segment of vein was removed near the planned anastomosis site in each patient and immediately fixed in formalin. Each venous tissue sample, fixed in formalin, was embedded and cut into 2–3 tissue blocks of 3–4 mm thickness using previously described techniques2, 8. Each piece was paraffin-embedded and then sliced into 4μm sections for histological and immunohistochemistry studies. For collection of vein segments from stenotic AVF and AVG, discarded samples from the venous segments of AVF and AVG were collected at the time of vascular access revision surgery, fixed in formalin, embedded using standard techniques, and histologic and immunohistochemistry studies performed as previously described2, 7.
Histological and Immunohistochemistry Studies
Sections from each tissue block were evaluated for expression of alpha-smooth muscle actin (SMA, DAKO; 1A4, 1:200), desmin (DAKO; 1:100), and vimentin (DAKO V9, 1:200) using immunohistochemistry techniques previously described2, 3, 7. A brown color on the specimen indicated a positive stain. Negative controls were performed with each assay, by omitting the primary antibody. In addition, positive control tissue (lymph node, small bowel, tonsil or artery) was used to document the efficacy of each antibody.
Semiquantitative Immunohistochemical Scoring Analysis
Immunohistochemistry was performed to assess cellular phenotypes within the neointima by staining for SMA, desmin, and vimentin. Sections were graded, using a semiquantitative scoring scale from 0 to 4, which indicated the percentage of total cells that were positive for the specific marker in different regions of the vessel wall (0 indicates 0–10% positive; 1+ = 11–25% positive; 2+ = 26–50% positive; 3+ = 51–75% positive and 4+ = 76–100% positive) as previously published2, 3. Mean values for the cellular markers for all patients were calculated. These markers were used to identify the relative contributions of myofibroblasts and contractile smooth muscle cells, by using the schema described in table 1.
Table 1.
Scheme for Cellular Phenotyping
| SMA | Vimentin | Desmin | |
|---|---|---|---|
| SMC | + | − | + |
| Myofibroblasts | + | + | − |
Statistical Analysis
The distribution of study variables was characterized according to means ± S.E. and proportions. Semi-quantitative score analyses were performed using ANOVA. A p value <0.05 was considered to be statistically significant. All statistical analyses were performed using JMP® 9.0 (Cary, NC) statistical software package.
Results
Patient Population
Clinical information was available for 28 samples (9 grafts and 19 fistulas) from the stenotic vein sample group. Among the AVFs, 8 were radiocephalic, 6 were brachiocephalic, and 5 were basilic vein transpositions. Among the 63 vein samples collected at the time of new access creation, 35% came from subjects who had advanced CKD not currently on hemodialysis and over 90% of vein specimens had pre-existing VNH present.
Analysis of Cellular Phenotypes from Vein Samples Collected at Time of Surgery vs Stenotic AVFs and AVGs
Our semiquantitative cellular phenotype scoring showed similar distributions of SMA, vimentin, and desmin from advanced chronic kidney disease (CKD) non-dialysis subjects and prevalent end stage renal disease (ESRD) subjects, respectively, 2.38±0.21 vs 2.47±0.30 (p=0.799), 1.64±0.29 vs 1.72±0.22 (p=0.834), and 2.14±0.30 vs 1.79±0.23 (p=0.360), suggesting similar cellular phenotypes from vein specimens from advanced non-hemodialysis CKD subjects and prevalent ESRD subjects. The next sections describe the analysis of cellular phenotypes comparing vein samples collected at time of new vascular access surgery with vein samples from stenotic AVFs and stenotic AVGs.
Analysis of Cellular Phenotypes in AVFs
Using semi-quantitative cellular phenotyping scoring within the neointima the scores for SMA, vimentin, and desmin, between vein tissue collected at the time of surgery vs. vein tissue obtained from stenotic AVF were 2.37±0.14 vs 3.60±0.22 (p<0.0001), 1.68±0.16 vs. 2.84±0.23 (p=0.0002), and 1.96±0.15 vs. 0.24±0.20 (p<0.0001), respectively (see Figure 1). The majority of cells within the neointima from vein samples collected at the time of surgery were, therefore, SMA +ve and desmin +ve contractile smooth muscle cells (Figure 2), while stenotic veins from AVFs were predominately SMA +ve and vimentin +ve myofibroblasts (Figure 3). Figure 3 describes the histology of representative vein sample from a stenotic AVF vein sample and demonstrates the presence of predominately myofibroblasts within the neointima.
Figure 1. Comparison of Cellular Phenotypes within the Venous Neointima in Stenotic AVF (only) and Vein Collected at the Time of Surgical Access Creation.
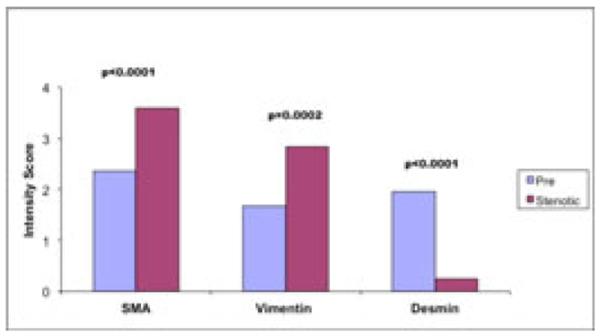
The predominant cellular phenotype within the neointima of vein samples collected at time of new access surgery (pre) were SMA (+), Desmin (+) contractile smooth muscle cells, while in vein segments from stenotic AVF were predominately, SMA(+), vimentin (+) myofibroblasts.
Figure 2. Cellular Phenotyping of Neointimal Cells from Representative Vein Samples Collected at Time of New Surgery.
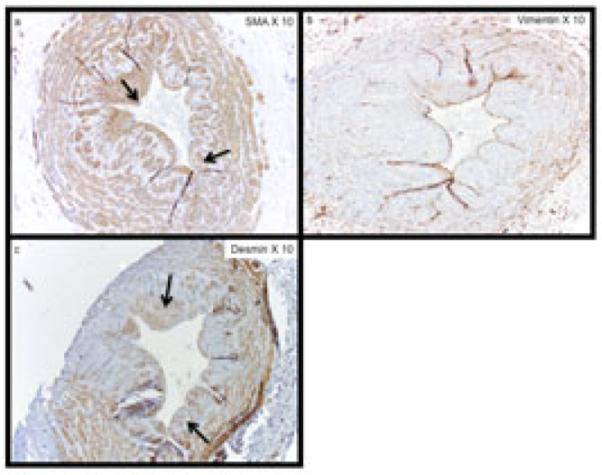
The expression of (a) α-SMA, (b) vimentin, and (c) desmin within sequential sections of a patient with pre-existing neointimal hyperplasia. The majority of cells within the neointima are SMA (+), vimentin (−), and desmin (+) contractile smooth muscle cells. Arrows show representative areas with SMA(+) and desmin (+) staining within the neointima. Note panel (b) with very little vimentin staining.
Figure 3. Cellular Phenotyping of Neointimal Cells from Representative Vein Sample from Stenotic AVF.
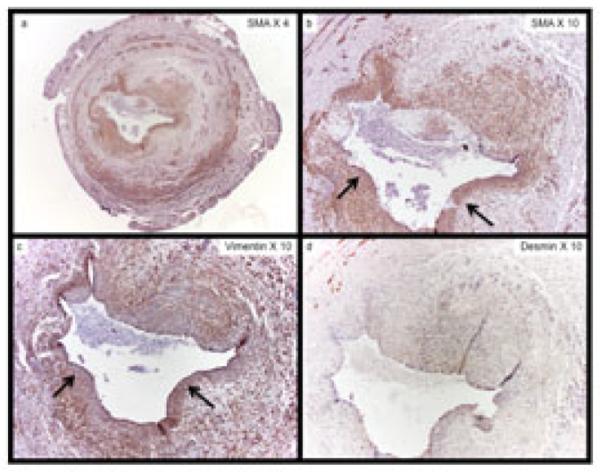
The expression of (a) and (b) α-SMA, (b) vimentin, and (c) desmin within sequential sections of a patient with a failed AVF. Note the presence of aggressive venous neointimal hyperplasia. The majority of cells within the neointima are SMA (+), vimentin (+), and desmin (−) myofibroblasts. Arrows show representative areas that SMA(+) and vimentin (+) staining within the neointima. Note panel (d) with minimal desmin staining.
Analysis of Cellular Phenotype in AVG
We also performed semi-quantitative scoring for SMA, vimentin, and desmin from vein tissue collected at time of surgery vs stenotic vein samples from failed AVGs. Semi-quantitative scores for SMA, vimentin, and desmin, between vein tissue collected at the time of new access surgery vs. downstream vein tissue from stenotic AVGs were 2.37±0.14 vs 3.75±0.16 (p=0.0013), 1.68±0.16 vs. 2.50±0.27 (p=0.049), and 1.96±0.15 vs. 0.38±0.18 (p=0.0006), respectively (Figure 4). In keeping with the results from the analyses above, the majority of cells within the neointima from the vein samples collected at the time of surgery are SMA +ve and desmin +ve contractile smooth muscle cells, compared to stenotic veins from AVGs which were predominately SMA +ve and vimentin +ve myofibroblasts, similar to stenotic veins from AVF.
Figure 4. Comparison of Cellular Phenotypes within the Venous Neointima in Stenotic AVG (only) and Vein Collected at the Time of Surgical Access Creation.
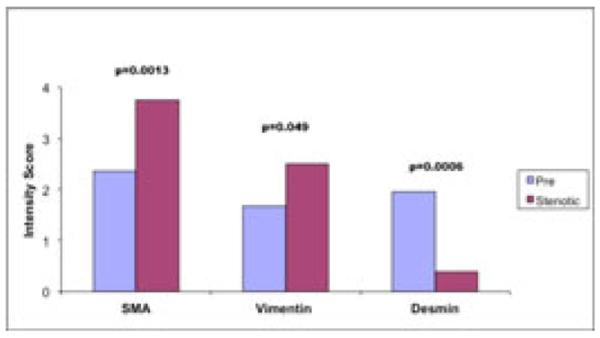
The predominant cellular phenotype within the neointima of vein samples collected at time of new access surgery (pre) were SMA (+), Desmin (+) contractile smooth muscle cells, while in vein segments from stenotic AVG, collectively were predominately, SMA(+), vimentin (+) myofibroblasts, similar to stenotic AVFs.
Discussion
Our results from this study show that the predominant cellular phenotype within the neointima of veins from failed vascular accesses is primarily composed of SMA+ve, vimentin +ve, and desmin -ve myofibroblasts, while the predominant cellular phenotype within the neointima of veins obtained at the time of new access creation is primarily SMA+ve, desmin +ve, vimentin −ve contractile smooth muscle cells. Understanding the identity of the cells that populate the neointima in both settings is important because it may: (1) provide novel information about the response to different vascular injuries prior to and after vascular access creation and (2) lay the groundwork for development of future therapies to improve vascular access dysfunction that target specific cellular phenotypes in these two different settings.
The natural history of vascular access dysfunction likely involves a series of progressive vascular injuries before and after vascular access creation. After creation of an AVF and AVG, two major processes are believed to play important roles in vascular access dysfunction in response to increased blood flow and hemodynamic shear stress: (1) inadequate vasodilation in the venous segment of the anastomosis (particularly critical in AVF non-maturation) and (2) development of aggressive VNH9. However, recently, the role of vascular injury to the vein prior to AV access creation, from factors such as uremia and vascular complications of CKD, has been the focus of a number of investigations, particularly in the pathophysiology of AVF maturation3–5, 10. Our results suggests that vein specimens collected at the time of new vascular access surgery demonstrated a predominance of contractile smooth muscle cells in the neointima, while those collected from failed AVF and AVG demonstrated a predominance of myofibroblasts. Thus, it is possible that different vascular injuries before and after vascular access creation may lead to the predominance and acquisition of different cellular phenotypes seen within the neointima. Furthermore, while our results demonstrate a predominance of contractile smooth muscle cells from vein segments collected at the time of new access surgery and myofibroblasts from vein segments of failed AVF and AVGs, we also found a mix of different cellular phenotypes within the venous neointima (myofibroblasts and contractile smooth muscle cells) from each group. Therefore, it is possible that some level of phenotypic switching occurs both within the neointima from veins collected at the time of new vascular access surgery and from stenotic veins from failed AVF and AVG, demonstrating the plasticity of myofibroblasts and contractile smooth muscle cells.
While our group of vein samples collected at the time of new vascular access creation included both advanced CKD non-hemodialysis subjects and prevalent ESRD subjects, the staining patterns and scoring for SMA, vimentin, and desmin were similar when comparing these two groups, suggesting similar cellular phenotypic characteristics and possibly a similar cellular response to vascular injury for both of these groups of vein samples collected prior to vascular access creation. In addition, vein specimens collected from the stenotic AVFs and AVGs also showed similar staining patterns and scoring with one another for the same cellular markers, SMA, vimentin, and desmin, possibly suggesting a similar cellular response to vascular injury after AV access creation in both AVF and AVG.
The potential implications of different cellular phenotypes within the neointima from veins collected at the time of new vascular access creation and from stenotic failed AVF and AVG are that: (1) vascular injuries prior to vascular access creation (from factors such as uremia, oxidative stress, and inflammation) and during and after vascular access creation (from factors such as hemodynamic sheer stress, surgical injury, and routine needle cannulation) may lead to different cellular phenotypic switching patterns and biological response to vascular injury and (2) therapies to treat vascular access dysfunction may need to differ before and after vascular access creation. The majority of research to-date in vascular access dysfunction from experimental and clinical studies has primarily focused on the events and biology that result after placement of an AV access, specifically, alteration in blood flow, vessel vasodilation, and hemodynamic sheer stress patterns. Furthermore, the main biological mechanisms examined in vascular access dysfunction after AV access creation has focused on mediators of oxidative stress and inflammation11, 12. These mediators are believed to play a major role in adventitial fibroblast activation and migration and cellular transformation into contractile smooth muscle cells and myofibroblasts, which later synthesize extracellular components that lead to neointimal hyperplasia formation13–15. Recently, the role of vein health prior to vascular access creation and its role in AVF maturation has become an emerging area of research in this field. Initial results from the multicenter National Institutes of Health Hemodialysis Fistula Maturation (HFM) Study has reported that 87.8% of samples examined to date (n=204; total planned enrollment 600 subjects) have VNH present prior to AVF creation, and within the neointima all contained the presence of myofibroblasts and/or contractile smooth muscle cells10. Furthermore, a recent clinical study by Owens et. al. reported that impairment of vascular health and endothelial function in CKD Stage IV and V patients receiving new AVF, as measured by brachial artery flow-mediated vasodilation, was associated with decreased arterial remodeling and final venous lumen diameter attained at three months16. The mechanisms that lead to endothelial dysfunction and pre-existing VNH are likely a consequence of factors such as uremia, oxidative stress, and inflammation, and remain poorly understood; and may be critically important in understanding vascular access maturation and development of future stenosis. Further investigation is needed to determine whether modulation of endothelial function prior to vascular access creation can improve AVF maturation and prevent AVG stenosis and whether the type of therapies administered prior to vascular access creation may need differ from those administered after vascular access creation. A recent example of the potential importance of therapies to modify the vascular endothelium and to improve vascular remodeling prior to AV access creation comes from an experimental study by Janardhanan et al.17. This study demonstrated that systemic delivery of an anti-oxidant and anti-inflammatory agent, simvastatin, given prior to AVF creation in uremic mice, reduced neointimal hyperplasia formation and expression of oxidative stress and inflammation genes, and decreased migration and proliferation of α-SMA positive cells (contractile smooth muscle cells and myofibroblasts)17.
We recognize that our study has several limitations. First, this is a single-center study and our results may not generalize to all medical centers. Second, the vein samples that represent the stenotic vein group are not from the same patient vein collected at the time of initial access surgery. However, we do believe that collectively our stenotic vein samples are an accurate representation of the histology of vascular access failure. Third, we do not have data on previous venipunctures and intravenous line placements, including peripherally inserted central catheters, and recognize that these factors are potentially important etiologies of vascular injury and neointimal hyperplasia development, particularly prior to access creation. However, all patients receiving a new vascular access have pre-operative vein mapping or venography as standard of care, and vessels with inadequate size and detected stenosis at an imaging level are not used for access creation in our practice. In addition, the presence of at least some degree of preexisting VNH in over 85% of a large sample size in the ongoing National Institutes of Health HFM study suggests that this is more likely to be a true biological response to injury as compared to an iatrogenic, site-specific problem.
Conclusions
Our results (a) demonstrate that the predominant cellular phenotype within the neointima from vein specimens collected at the time of initial vascular access creation are contractile smooth muscle cells while those from failed vascular accesses are myofibroblasts, (b) underscore the need to improve the understanding of the pathophysiologic changes that result from vascular injury prior to and after access creation and the need to better elucidate the role contractile smooth muscle cells and myofibroblasts play in this process, and (c) suggest that targeting of specific cellular phenotypes and phenotypic switching before and after AV access creation could serve as potential future novel therapies that improve vascular access dysfunction.
Acknowledgements
We would like to thank Maheshika Somarathna with help in preparing this manuscript.
Support and Financial Disclosures Dr. Lee is supported by NIH 5K23DK083528-04 and National Kidney Foundation Franklin McDonald/Fresenius Medical Care Young Investigator Clinical Research Award.
Dr. Roy-Chaudhury was supported by NIH 5U01-DK82218, NIH 5U01-DK82218S (ARRA), NIH 5R01-EB004527, NIH 1R21-DK089280-01, a VA Merit Review, two University of Cincinnati NIH/NCCR UL1RR026314 CTSA grants, and industry grants during the duration of this work.
Dr. Lee is a consultant for Proteon Therapeutics. Dr. Roy-Chaudhury is on the advisory board/consultant for Shire, Proteon Therapeutics, WL Gore, Bioconnect Systems, Bard Peripheral Vascular and Medtronic and has received research support from BioConnect Systems, Shire, WL Gore and Bard Peripheral Vascular. These funding sources had no involvement in the design and execution of this study or interpretation of results.
Footnotes
Portions of this work were presented in abstract form at the Vascular Access Society Meeting, Istanbul, Turkey, May 2011 and American Society of Nephrology Renal Week, Philadelphia, PA, November 2011.
References
- 1.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. The journal of vascular access. 2011:0. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasse H, Rivera AA, Huang R, Martinson DE, Long Q, McKinnon W, Naqvi N, Husain A. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Seminars in dialysis. 2011;24:688–693. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wali MA, Eid RA, Dewan M, Al-Homrany MA. Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Ann Thorac Cardiovasc Surg. 2006;12:341–348. [PubMed] [Google Scholar]
- 7.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011. Severe venous neointimal hyperplasia prior to dialysis access surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 10.Alpers CE. Prominent Neointimal Hyperplasia Is Common in Veins Utilized for AV Fistula Creation: The Hemodialysis Fistula Maturation (HFM) Study [Abstract] J Am Soc Nephrol. 2012;23:14A. [Google Scholar]
- 11.Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA. MCP-1 contributes to arteriovenous fistula failure. Journal of the American Society of Nephrology : JASN. 2011;22:43–48. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int. 2008;74:47–51. doi: 10.1038/ki.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Annals of biomedical engineering. 2011;39:1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos B, Wang Y, Mistry MJ, Woodle BK, Cornea V, Lee T, Roy Chaudhury P. Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis. J Am Soc Nephrol. 2011;22:78A. doi: 10.3390/ijms222212285. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Terry CM, Blumenthal DK, Kuji T, Masaki T, Kwan BC, Zhuplatov I, Leypoldt JK, Cheung AK. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm415. [DOI] [PubMed] [Google Scholar]
- 16.Owens CD, Wake N, Kim JM, Hentschel D, Conte MS, Schanzer A. Endothelial function predicts positive arterial-venous fistula remodeling in subjects with stage IV and V chronic kidney disease. The journal of vascular access. 2010;11:329–334. doi: 10.5301/jva.2010.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, Mandrekar J, Kong H, Leof EB, Mukhopadhyay D, Misra S. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013 doi: 10.1038/ki.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]


