Abstract
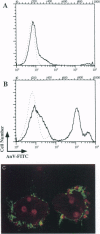
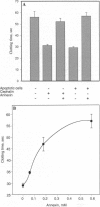
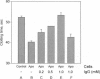
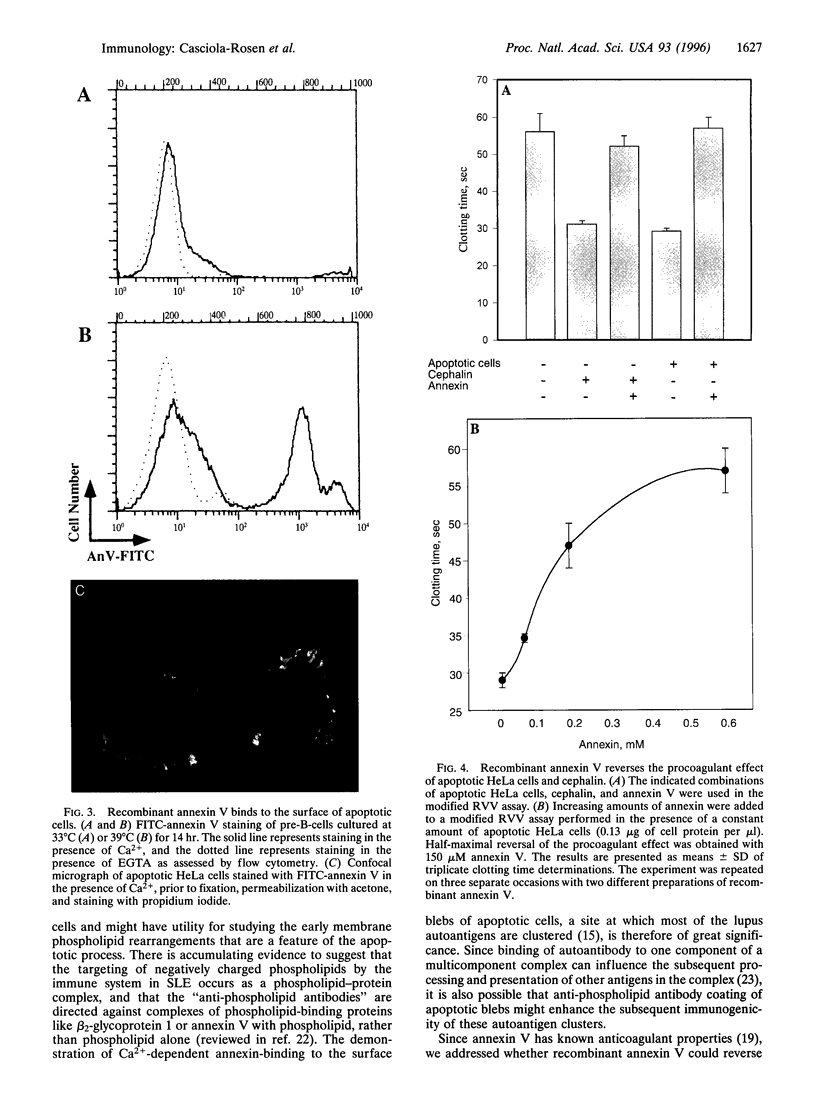
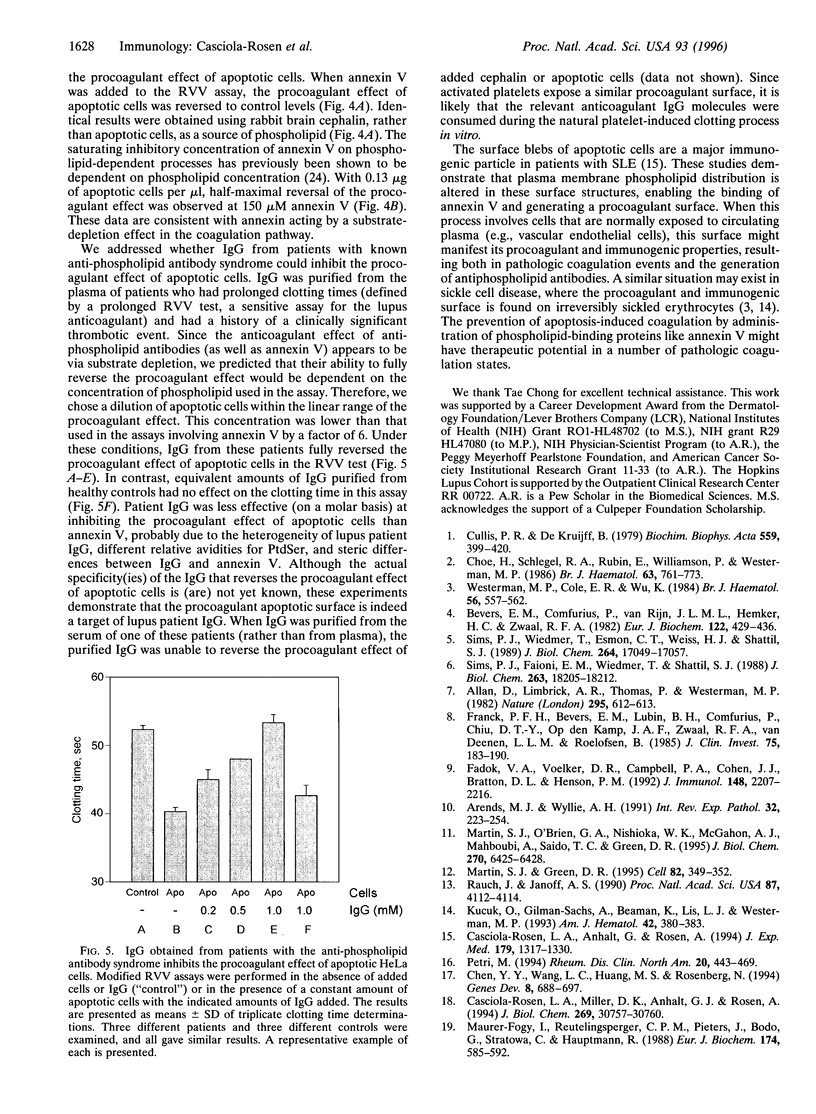
The restriction of phosphatidylserine (PtdSer) to the inner surface of the plasma membrane bilayer is lost early during apoptosis. Since PtdSer is a potent surface procoagulant, and since there is an increased incidence of coagulation events in patients with systemic lupus erythematosus (SLE) who have anti-phospholipid antibodies, we addressed whether apoptotic cells are procoagulant and whether anti-phospholipid antibodies influence this. Apoptotic HeLa cells, human endothelial cells, and a murine pre-B-cell line were markedly procoagulant in a modified Russell viper venom assay. This procoagulant effect was entirely abolished by addition of the PtdSer-binding protein, annexin V, confirming that it was PtdSer-dependent. The procoagulant effect was also abolished by addition of IgG purified from the plasma of three patients with anti-phospholipid antibody syndrome, but not IgG from normal controls. Confocal microscopy of apoptotic cells stained with fluorescein-isothiocyanate-conjugated-annexin V demonstrated (Ca2+)-dependent binding to the surface of membrane blebs o apoptotic cells, but not to intracellular membranes. Recent data indicate that the surface blebs of apoptotic cells constitute an important immunogenic particle in SLE. We propose that the PtdSer exposed on the outside of these blebs can induce the production of anti-phospholipid antibodies, which might also enhance the immunogenicity of the bleb contents. When apoptosis occurs in a microenvironment in direct contact with circulating plasma, the unique procoagulant consequences of the apoptotic surface may additionally be expressed. This might explain the increased incidence of pathological intravascular coagulation events that occur in some lupus patients who have anti-phospholipid antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Limbrick A. R., Thomas P., Westerman M. P. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature. 1982 Feb 18;295(5850):612–613. doi: 10.1038/295612a0. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Comfurius P., van Rijn J. L., Hemker H. C., Zwaal R. F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982 Feb;122(2):429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Miller D. K., Anhalt G. J., Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994 Dec 9;269(49):30757–30760. [PubMed] [Google Scholar]
- Chang C. P., Zhao J., Wiedmer T., Sims P. J. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J Biol Chem. 1993 Apr 5;268(10):7171–7178. [PubMed] [Google Scholar]
- Chen Y. Y., Wang L. C., Huang M. S., Rosenberg N. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 1994 Mar 15;8(6):688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- Choe H. R., Schlegel R. A., Rubin E., Williamson P., Westerman M. P. Alteration of red cell membrane organization in sickle cell anaemia. Br J Haematol. 1986 Aug;63(4):761–773. doi: 10.1111/j.1365-2141.1986.tb07560.x. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992 Apr 1;148(7):2207–2216. [PubMed] [Google Scholar]
- Franck P. F., Bevers E. M., Lubin B. H., Comfurius P., Chiu D. T., Op den Kamp J. A., Zwaal R. F., van Deenen L. L., Roelofsen B. Uncoupling of the membrane skeleton from the lipid bilayer. The cause of accelerated phospholipid flip-flop leading to an enhanced procoagulant activity of sickled cells. J Clin Invest. 1985 Jan;75(1):183–190. doi: 10.1172/JCI111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994 Sep 1;84(5):1415–1420. [PubMed] [Google Scholar]
- Kucuk O., Gilman-Sachs A., Beaman K., Lis L. J., Westerman M. P. Antiphospholipid antibodies in sickle cell disease. Am J Hematol. 1993 Apr;42(4):380–383. doi: 10.1002/ajh.2830420409. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. How can cryptic epitopes trigger autoimmunity? J Exp Med. 1995 Jun 1;181(6):1945–1948. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Green D. R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995 Aug 11;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., O'Brien G. A., Nishioka W. K., McGahon A. J., Mahboubi A., Saido T. C., Green D. R. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995 Mar 24;270(12):6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Maurer-Fogy I., Reutelingsperger C. P., Pieters J., Bodo G., Stratowa C., Hauptmann R. Cloning and expression of cDNA for human vascular anticoagulant, a Ca2+-dependent phospholipid-binding protein. Eur J Biochem. 1988 Jul 1;174(4):585–592. doi: 10.1111/j.1432-1033.1988.tb14139.x. [DOI] [PubMed] [Google Scholar]
- McNeil H. P., Simpson R. J., Chesterman C. N., Krilis S. A. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A. 1990 Jun;87(11):4120–4124. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri M. Diagnosis of antiphospholipid antibodies. Rheum Dis Clin North Am. 1994 May;20(2):443–469. [PubMed] [Google Scholar]
- Rauch J., Janoff A. S. Phospholipid in the hexagonal II phase is immunogenic: evidence for immunorecognition of nonbilayer lipid phases in vivo. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4112–4114. doi: 10.1073/pnas.87.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanat C., Archipoff G., Beretz A., Freund G., Cazenave J. P., Freyssinet J. M. Use of annexin-V to demonstrate the role of phosphatidylserine exposure in the maintenance of haemostatic balance by endothelial cells. Biochem J. 1992 Feb 15;282(Pt 1):7–13. doi: 10.1042/bj2820007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Faioni E. M., Wiedmer T., Shattil S. J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988 Dec 5;263(34):18205–18212. [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T., Esmon C. T., Weiss H. J., Shattil S. J. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989 Oct 15;264(29):17049–17057. [PubMed] [Google Scholar]
- Triplett D. A. Antiphospholipid-protein antibodies: laboratory detection and clinical relevance. Thromb Res. 1995 Apr 1;78(1):1–31. doi: 10.1016/0049-3848(95)00001-1. [DOI] [PubMed] [Google Scholar]
- Westerman M. P., Cole E. R., Wu K. The effect of spicules obtained from sickle red cells on clotting activity. Br J Haematol. 1984 Apr;56(4):557–562. doi: 10.1111/j.1365-2141.1984.tb02180.x. [DOI] [PubMed] [Google Scholar]