Abstract
Objective
Evaluate a computerized intervention supporting antiretroviral therapy (ART) adherence and HIV transmission prevention.
Design
Longitudinal RCT.
Settings
An academic HIV clinic and a community-based organization in Seattle.
Subjects
240 HIV-positive adults on ART; 209 completed nine-month follow-up (87% retention).
Intervention
Randomization to computerized counseling or assessment-only, 4 sessions over 9 months.
Main Outcome Measures
HIV-1 viral suppression, and self-reported ART adherence, and transmission risks, compared using generalized estimating equations.
Results
Overall, intervention participants had reduced viral load (VL): mean 0.17 log10 decline, versus 0.13 increase in controls, p = 0.053, and significant difference in ART adherence baseline to 9 months (p = 0.046). Their sexual transmission risk behaviors decreased (OR = 0.55, p = 0.020), a reduction not seen among controls (OR = 1.1, p = 0.664), and a significant difference in change (p = 0.040).
Intervention effect was driven by those most in need: among those with detectable virus at baseline (>30 copies/milliliter, n=89), intervention effect was mean 0.60 log10 VL decline versus 0.15 increase in controls, p=0.034. ART adherence at the final follow-up was 13 points higher among intervention participants versus controls, p = 0.038.
Conclusions
Computerized counseling is promising for integrated ART adherence and safer sex, especially for individuals with problems in these areas. This is the first intervention to report improved ART adherence, viral suppression, and reduced secondary sexual transmission risk behavior.
Keywords: HIV, computerized counseling, prevention with positives, viral load, antiretroviral adherence
INTRODUCTION
Antiretroviral therapy (ART) reduces morbidity and mortality related to human immunodeficiency virus (HIV) infection. Sustained adherence to ART improves individual outcomes and reduces secondary transmission, since low viral load is associated with reduced HIV transmission1-3 and earlier ART initiation reduces sexual transmission by 96%.4 It is important to identify efficient ways to support medication adherence over a lifetime, as ART is now recommended in the United States (US) for all persons living with HIV (PLWH) regardless of CD4 count.5 However, only an estimated 77% of US patients on ART have suppressed viral loads.6 Reducing transmission risk behaviors among PLWH (‘prevention with positives’) is a longstanding public health goal.7 The chronicity of HIV infection may be accompanied by continued or increased sexual risk behaviors for some individuals; however, not all providers routinely address HIV transmission risk reduction with their HIV-positive patients.8-10
Scalable strategies are needed to optimize ART adherence and to reduce secondary transmission of HIV. Meta-analyses show that interventions to support ART adherence,11,12 and to reduce secondary HIV transmission risk,13,14 are efficacious. Because these interventions have been largely research-based, and staff- and resource-intensive,15,16 population-level implementation may not occur17.18
We hypothesized that a computer-delivered intervention could support ART adherence and reduce HIV transmission risk by PLWH. We evaluated such an intervention called Computer Assessment & Rx Education for HIV-positive people (CARE+).
METHODS
Participants
Study participants were recruited from a university-affiliated public HIV clinic and a large AIDS service organization in Seattle, Washington. Eligibility criteria included age ≥18 years, on ART, able to understand spoken English; exclusions included thought disorders and current participation in ART adherence or prevention-with-positives studies. Written consent was obtained prior to randomized assignment. All procedures were approved by University of Washington Human Subjects Division, 06-1198-C. Participants received $20 at the first three, and $40 at the final, session.
Intervention
Design
The computerized-counseling intervention was evaluated in a prospective two-arm randomized controlled trial (RCT). The study sample of n=240 was assigned via an automated pseudo-random number generation algorithm, disallowing any exposure to intervention by controls. The experimental group received CARE+ (audio-narrated assessment, tailored feedback, skill-building videos, health plan, and printout) on a tablet computer and standard care, while controls received assessment only on tablets and standard care. Each group underwent four sessions specific to assigned arm at three-month intervals over nine months. Sessions were scheduled on same day as clinic visits wherever possible.
CARE+ is a .NET-based (Microsoft, Redmond, WA) custom software application on touchscreen computers. Intervention content is based on theoretical frameworks: information-motivation-behavior including ‘importance’ and confidence’ scales around ART use and transmission risk-reduction,19 transtheoretical including stage of change questions around condoms,20 social cognitive role-modeling with peers demonstrating healthy behaviors in videos,21 and motivational interviewing including messages acknowledging ambivalence around behavior change and highlighting user’s commitment22). The tool incorporates evidence-based elements shown in RCTs to improve ART adherence or reduce sexual risk,23,24 such as feedback including consequence-framed messages (e.g., “Unprotected sex may expose you to STDs”)25 and videos.26 Content recommendations were obtained through 30 qualitative interviews conducted with PLWH.27 Final CARE+ content was reviewed by an expert panel for face validity.
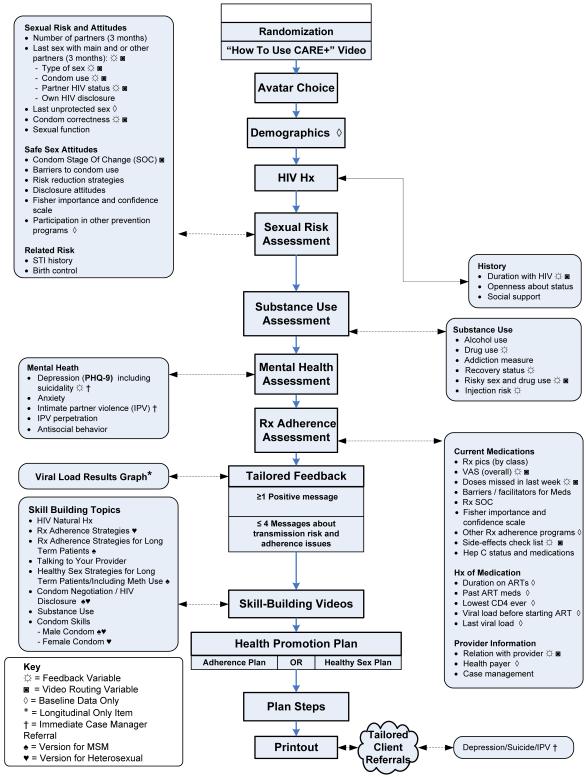
Figure 1 summarizes the CARE+ session. Users received tailored feedback based on risk assessment responses, and viewed video versions for heterosexuals or for men who have sex with men (MSM) showcasing skills around HIV disclosure, ART adherence, safer sex, substance abuse, male/ female condoms, condom use negotiation, working with providers, and HIV natural history and ART mechanisms. Users develop a plan for ART adherence or safer sex (user choice at 1st session, and switched at 3rd session). A personalized printout summarized feedback, health plan, and referral phone numbers.
Figure 1.
CARE+ Computerized Counseling Intervention Content By Session
The control condition comprised computerized risk-assessment only (sexual behaviors, substance use, mental health, ART regimen, side effects, adherence in last 7 and 30 days).
In both study arms, the tool flagged reports of severe depression by Patient Health Questionnaire (PHQ-9 score of ≥20),28 intimate partner violence (IPV), or suicidal ideation; as outlined in the consent, study staffers notified case managers for appropriate follow-up. At repeat sessions, these participants were asked how referrals went. All intervention participants were reminded of their last plan and asked to continue or make a new health plan. Software usability was evaluated among an additional 30 HIV-positive clients, and one week test-retest reliability assessment was done to establish psychometric performance of key tool variables.29
Outcome Measures
The primary biological outcome was HIV-1 RNA viral load (VL), determined using a 500 microliter (mL) plasma specimen in a TaqMan real-time polymerase chain reaction assay with 30 copies/microliter (mL) as the lower limit of quantification for detectable HIV-1. HIV-1 viral load was assessed using specimens drawn on day of study interview or as part of patient care within a month prior to study visit. The same laboratory was used for all study and clinical viral loads and was determined by personnel blinded to study arm. Primary behavioral outcome measures collected by self-report in both arms consisted of a composite variable of sexual transmission risk – no condom use (unprotected sex) or condom use with problems/errors (i.e., vaginal or anal sex either without a condom or where a condom was used but HIV exposure may have occurred due to mechanical or user failure) – and ART adherence by 30-day visual analog scale (VAS).30,31. Accurate reporting was encouraged during enrollment, consent, and sessions through normalizing language and reiteration that the study would not share self-reported data to providers, with sole exception of IPV, severe depression, and suicidality, which (as noted in the consent) prompted appropriate provider referral. Secondary outcome measures included changes in ART/condom stage of change and HIV disclosure.
Statistical Methods
Fisher’s exact and Wilcoxon rank sum tests assessed differences between intervention and control groups and between study sites in baseline study population characteristics. We utilized generalized estimating equation (GEE) models with a Gaussian link and an unstructured correlation structure to compare changes in viral load (log10-transformed) and ART adherence (30-day VAS) between intervention and control groups and from baseline to nine-month follow-up. GEE models with a logit link and an unstructured correlation structure compared odds of undetectable viral load and sexual transmission risks between intervention and control groups and from baseline to nine-month follow-up. All GEE models included main effects for intervention condition and linear trend as well as an interaction between these terms to capture differences in change between intervention conditions. The analysis was intent-to-treat. Covariates in the models included likely depression diagnosis by PHQ-9 since this was the only variable that differed significantly between study arms at baseline, as well as education, condom use with main partner at baseline, and study site. These analyses were done for the whole sample, and for the subgroup who had detectable viral load at baseline. Estimates for these ‘detectables’ were obtained by including relevant main and interaction effects for an indicator variable specifying whether each participant had detectable viral load at baseline. When modeling odds of viral load being undetectable within this subgroup, only the three follow-up assessments were included. Analyses were performed using R,32 including geepack33 for GEE, doBy34 for post-estimation, and ggplot235 for result visualization.
RESULTS
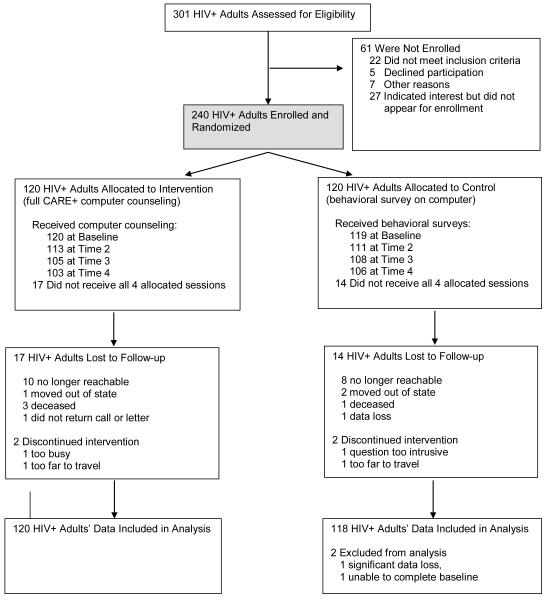
We approached 301 individuals at two study sites; 240 enrolled (80% acceptance), 239 completed baseline, and 87% (209/239) were retained for nine-month study duration (Fig. 2). Participants were consented, enrolled, then randomized. The 30 lost to follow-up had similar numbers and reasons across arms, suggesting non-differential dropout (p = .56 for attrition by arm).
Figure 2.
CARE+ Computerized Counseling Intervention Trial, Four Sessions Over 9 Months
HIV denotes human immunodeficiency virus-1.
Table 1 shows participant characteristics at baseline by study arm. At baseline intervention participants were less likely than controls to obtain a positive screening result for likely depression diagnosis (n=14 vs. 25, p = 0.032). There were several significant differences at baseline between clinic- and organization-recruited participants including proportion of MSM, proportion incarcerated more than one night, condom use, self-reported ART adherence (VAS), proportion with resistant virus, and proportion victimized by intimate partner violence [Supplemental Table 1].
Table 1.
Baseline Characteristics of CARE+ Intervention and Control Conditions and by Academic and Community-Based Study Sites.
| CARE+ (n=120) Study Arm |
CONTROL (n=119) Study Arm |
Difference by Study Arm |
Difference by Study Site |
|
|---|---|---|---|---|
| p-value | p-value | |||
| Female Gender | 12 | 9 | 0.6732 | NS |
| Age | 45 (11) | 45 (9.75) | 0.7759 | NS |
| Hispanic/Latino | 9 | 8 | 0.8161 | NS |
| American Indian or Alaska Native | 12 | 12 | 1.0000 | NS |
| Asian | 1 | 3 | 0.2109 | NS |
| Black or African-American | 28 | 31 | 0.7765 | NS |
| Native Hawaiian or Other Pacific Islander | 0 | 2 | 0.2448 | NS |
| White | 57 | 53 | 0.6020 | NS |
| Other Race | 10 | 8 | 0.6488 | NS |
| No High School (HS) Diploma/GED | 15 | 14 | 1.0000 | NS |
| HS Diploma/GED Only | 50 | 39 | 0.0919+ | NS |
| More than High School | 35 | 47 | 0.0862+ | NS |
| Man Who Has Sex with Men | 72 | 77 | 0.3738 | 0.0118 * |
| Incarcerated More Than One Night | 45 | 53 | 0.2452 | 0.0280 * |
| Injecting Drug Use Past 3 Months | 17 | 10 | 0.1831 | NS |
| Any Methamphetamine | 20 | 13 | 0.1610 | NS |
| Any Cocaine | 19 | 22 | 0.6338 | NS |
| Any Methamphetamine or Cocaine | 34 | 28 | 0.3279 | NS |
| Any Sex Past 3 Months | 61 | 59 | 0.7926 | NS |
| No Condom with Main Partner | 8 | 15 | 0.0688+ | NS |
| No Condom with Other Partner | 12 | 16 | 0.4648 | NS * |
| Unprotected Discordant Sex with Main Partner |
2 | 1 | 1.0000 | NS |
| Unprotected Discordant Sex with Other Partner |
2 | 5 | 0.3333 | NS |
| Condom Use with Problems | 14 | 11 | 0.5588 | NS |
| Any Sex without Condoms | 19 | 27 | 0.1693 | 0.0310 * |
| Any Sex without Condoms or with Condom Problems |
29 | 34 | 0.4879 | 0.0508 + |
| Adherence Visual Analog Scale (VAS) | 94.5 (12.25) | 93 (15) | 0.4189 | 0.0357 * |
| 96%+ VAS Adherence | 43 | 44 | 0.8952 | 0.0173 * |
| 90%+ VAS Adherence | 69 | 70 | 0.8874 | 0.0152 * |
| 86%+ VAS Adherence | 76 | 74 | 0.8798 | 0.0150 * |
| 80%+ VAS Adherence | 83 | 83 | 1.0000 | NS |
| 70%+ VAS Adherence | 87 | 90 | 0.5463 | NS |
| Missed Doses Past 7 Days | 0 (1) | 0 (1) | 0.6648 | NS |
| CD4 Nadir | 125.5 (214.5) | 91 (188.75) | 0.4800 | NS |
| Viral Load Before Medication | 4.95 (2.24) | 4.88 (2.39) | 0.5201 | NS |
| Ever Told Resistant Virus | 16 | 17 | 0.8627 | 0.0348 * |
| Years Since HIV Diagnosis | 12 (9.25) | 11 (9.75) | 0.3634 | 0.0503 + |
| Depression Severity (PHQ-9) | 6 (10.5) | 7 (14) | 0.0969+ | NS |
| Depression Diagnosis (PHQ-9) | 14 | 25 | 0.0319* | NS |
| Anxiety More than Half of Past 7 Days | 31 | 29 | 0.7774 | NS |
| Intimate Partner Violence (IPV) Perpetration | 2 | 3 | 0.4462 | NS |
| IPV Victimization | 9 | 7 | 0.6336 | 0.0031 ** |
| Past Adherence Intervention | 7 | 3 | 0.3753 | NS |
| Past Prevention with Positives Intervention | 12 | 10 | 0.8359 | NS |
| log10 HIV-1 Viral Load | 1.48 (1.49) | 1.48 (0.99) | 0.1149 | NS |
| Detectable Viral Load | 44 | 33 | 0.1102 | NS |
| CD4 | 394 (347.5) | 341 (364.5) | 0.4872 | NS |
Notes: Percentage or Median (Interquartile Range); comparison by Fisher’s exact test or Wilcoxon rank-sum test.
p <.10;
p <.05;
p <.01. A log10 viral load of 1.48 is “undetectable”.
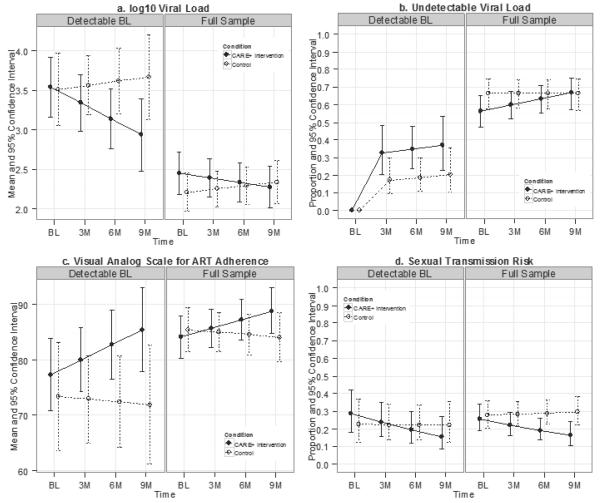
Figure 3 shows 95% confidence intervals for outcome means by time and study condition. Detailed GEE results are in Supplemental Digital Content Tables 2-4 showing impact on VL, proportion with undetectable VL, ART adherence, and sexual transmission risks in the full sample [Supplemental Table 2] and the subset of participants with detectable VL at baseline [Supplemental Table 3], as well as contrasts between intervention and control conditions at each time point and between baseline and nine-month time points within each condition, for full and subset samples [Supplemental Table 4].
Figure 3. Adjusted Means* by Time and Treatment Condition.
* NOTE: For these independent 95% confidence intervals, non-overlap indicates p < .05, but overlap does not reliably indicate that p > .05 (Cumming, 2009). See Fig. 4 for contrasts. Detectable BL: had detectable HIV1 viral load at first baseline (BL) session Full sample: All participants regardless of viral load status at baseline
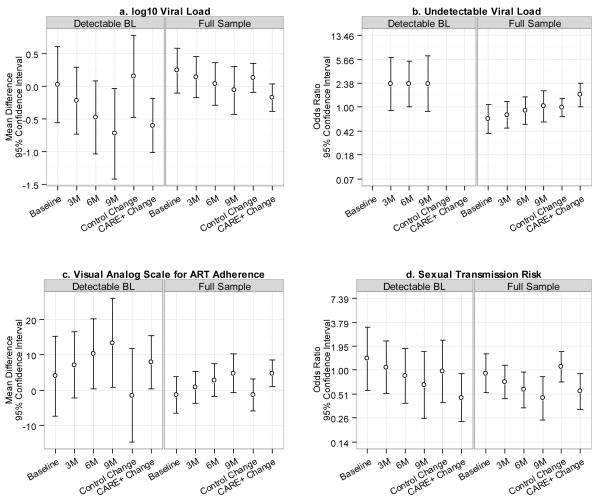
Figure 4 summarizes main outcomes of interest. The first four contrasts in each figure are intervention versus control at each time point while the last two contrasts in each figure compare baseline with nine-month follow-up within each study arm.
Figure 4.
Comparison of Viral Load, ART Adherence, and Unprotected Sex for Each Follow-up Point and Changes within each Treatment Condition.
HIV-1 Viral Load Effect
Figure 4a shows 95% confidence intervals for log10 viral load point-in-time study condition mean differences and change within each group from baseline to nine-month follow-up. Figure 4b shows 95% confidence intervals for point-in-time study condition differences and change within each group from baseline to nine-month follow-up in the odds of having undetectable viral load. There were marginally significant differences in change from baseline to the nine-month follow-up between study arms in log10 viral load (p = 0.053) and in the odds of having undetectable viral load (p = 0.090). CARE+ intervention participants overall had an average decrease of 0.17 log10 viral load (p = 0.112; 95% CI: −0.39–0.04) while control participants had an increase of 0.13 (p = 0.250; 95% CI: −0.09–0.35) [Fig. 4a Right]. Relative to baseline, the odds of having undetectable viral load at the nine-month follow-up were increased in the CARE+ condition (OR = 1.57; p = 0.037; 95% CI: 1.03–2.39) but reduced in the control condition (OR = 0.98; p = 0.925; 95% CI: 0.71–1.37) [Fig. 4b Right]. At the nine-month follow-up, CARE+ intervention participants were lower than controls in log10 viral load (−0.06; p = 0.741; 95% CI: −0.4 −0.30) and had increased odds of undetectable viral load (OR = 1.03; p = 0.920; 95% CI: 0.58–1.81), but neither of these differences were significant.
Among the subgroup who had detectable viral load at baseline, CARE+ intervention participants had an average decrease of 0.60 log10 viral load (p = 0.004; 95% CI: −1.01– −0.19) [Fig. 4a Left] while control participants had an increase of 0.15 log10 viral load (p = 0.641; 95% CI: −0.48–0.78). At the nine-month follow-up, CARE+ intervention participants were lower than controls in log10 viral load (−0.73; 95% CI: −1.42– −0.03), a significant difference (p = 0.041) [Fig. 4a Left]. CARE+ intervention participants also had higher odds of undetectable viral load than controls at the nine-month follow-up (OR= 2.32; 95% CI: 0.85–6.34), although this difference was only marginally significant (p = 0.101) [Fig. 4b Left]. For the subgroup with detectable viral load at baseline, in Figure 4b we do not show odds ratios for the baseline time point nor changes from baseline to the nine-month follow-up time point within each group, because all of the participants in this subgroup had detectable viral load at baseline. For the log10 viral load outcome, the three-way interaction between baseline detectable VL, study arm, and time was significant (p = 0.034), indicating CARE+ was more effective than control for those with detectable viral load at baseline [Supp. 6].
ART Adherence Effect
Figure 4c shows 95% confidence intervals for point-in-time VAS mean differences between groups and mean change within each group from baseline to nine-month follow-up. There was a statistically significant difference in change from baseline to the nine-month follow-up between study arms (p = 0.046) in self-reported ART adherence by 30-day VAS [Supplemental Content 2]. CARE+ intervention participants had an average increase of 4.71 points in the percentage of medication doses taken (p = 0.014; 95% CI: 0.95–8.48) while control participants had a decrease of 1.39 points (p = 0.556; 95% CI: −6.03–3.24) [Fig. 4c Right]. At the nine-month follow-up, CARE+ intervention participants were higher than controls in ART adherence (4.77; 95% CI: − 0.79–10.33), but this difference was not significant (p = 0.093) [Fig. 4c Right].
Among those with detectable viral load at baseline, CARE+ intervention participants had an average VAS adherence increase of 8.00 points (p = 0.040; 95% CI: 0.37–15.62) while control participants had a decrease of 1.53 points (p = 0.822; 95% CI: −14.84–11.78) [Fig. 4c Left]. At the nine-month follow-up, CARE+ intervention participants were higher than controls in ART adherence (13.44; 95% CI: 0.73–26.14), a significant difference (p = .038) [Fig. 4c Left].
Secondary HIV Transmission Risk Effect
Figure 4d shows confidence intervals for point-in-time study condition differences and change within each group from baseline to nine-month follow-up in self-reported transmission risks, defined as sex without a condom or condom use with errors. There was a statistically significant difference in change from baseline to the nine-month follow-up between study arms in self-reported transmission risks (p = 0.040). Among CARE+ intervention participants, the odds of transmission risks were 0.55 times lower at the nine-month follow-up than at baseline (p = 0.020; 95% CI: 0.34–0.91) while for control participants the odds of transmission risks increased over time (OR = 1.10; p = 0.664; 95% CI: 0.72–1.67) [Fig. 4d Right]. At the nine-month follow-up, CARE+ intervention participants had a reduced odds of transmission risks when compared with controls (OR = 0.46; 95% CI: 0.25–0.84), a significant difference (p = .012) [Fig. 4d Right].
Clinic Site and Detectable Viral Load at Baseline as Effect Modifiers
None of the three-way interactions involving study arm, linear trend, and clinic site were statistically significant, suggesting similar intervention effects in the university-affiliated and community-based organization sites (Supplement 5). Detectable viral load at baseline was a modifier of study arm effects on log10 viral load, as indicated by a significant three-way interaction between study arm, linear trend, and detectable viral load at baseline (p = 0.034). Three-way interactions involving study arm, linear trend, and detectable viral load at baseline were not statistically significant for other outcomes (Supplement 6).
Health Promotion Behavior Plans
CARE+ intervention participants made a concrete plan for ART adherence or transmission risk reduction (controls did not make plans). Many individuals (78%) indicated at baseline that they had an approach that was working for them, which they detailed with specific steps in the CARE+ session; 12% made a new plan. Common plans for ART adherence were to ‘keep doing what I am doing’ (n = 32) ‘use reminders’ (31), and ‘get support’ (25). Common plans for transmission risk reduction were to ‘not have sex’ (31), ‘use condoms’ (27), ‘have fewer or only 1 sex partner(s)’ (22), or ‘only have sex with people who are also positive’ (7).
Intervention participants’ confidence in their plan success increased over time, from 66% at three months to 80% at nine months (McNemar’s χ2 p = .02). Confidence in their ability to not transmit HIV increased over time: 0-10 ascending scale for confidence, mean 8.43 (SD 2.27) at baseline and 9.14 (SD 1.53) at nine months, p = .02.
At baseline, 41 referrals were made for intervention participants and 52 for controls for reported severe depression (37%), IPV (9%), or suicidal ideation (53%); at nine months total referrals for these conditions were 21 and 32, respectively (p = .10).
Intervention Acceptability
Nearly all (97%) CARE+ intervention participants found the tool easy to use; 99% rated session length as “just right”; 97% felt they had “enough privacy” during the session. Most (93%) felt the CARE+ session helped them as much or more than face-to-face counseling with a staff person, and 75% said they would prefer the computer over a human counselor in the future. No harms or unintended effects were noted in either arm of the study.
DISCUSSION
We found that a computerized counseling tool was effective at helping PLWH improve ART adherence and reduce HIV transmission risk behaviors, as measured by improvement in self-reported adherence, reduction in viral load, and improvement in reported correct and consistent condom use, compared to controls receiving usual care. The adherence effect was most pronounced among those whose plasma HIV-1 was not suppressed at baseline. The reduced viral load and fewer sexual transmission risk behaviors seen among those undergoing the intervention both may contribute to decreasing HIV transmission to sexual partners.
In our study population area, chart audits found that fewer than half of HIV-positive clients were assessed for sexual risks, STD testing or referral.36 Another study assessing 26 HIV clinics across the US found that providers reported delivering prevention-with-positives counseling at 67% of initial visits but only 53% of subsequent regular visits.37 Computerized counseling may lack some advantages offered by a highly-skilled human counselor, but it is delivered consistently with fidelity,38 without need for staff time or training. In our study it proved highly acceptable and had an efficacious impact on priority behaviors and objective measures of viral load response.
Multiple studies have utilized computers to assess ART non-adherence,39-41 or HIV transmission risk42-48 among PLWH, but fewer have been used to influence patient behavior.49,50 Lightfoot found that computer-assisted self-monitoring of transmission risk behaviors can be a strategy for PLWH.51,52 Fisher et al. found that computerized counseling supported ART adherence though this study did not find a viral load impact.53 Others have used computerized counseling to reduce HIV acquisition risk,54-56 which meta-analyses have found to be effective.57
Economic evaluation models have found that adherence interventions with modest effectiveness may provide survival benefit to patients and be cost-effective.58 The intervention we tested that does not require staff time, training, and monitoring may be easier to introduce into busy practice settings.
Study limitations include the fact that two-thirds of our population already had suppressed VL at baseline and 60% did not engage in sexual activities at any timepoint. Both limitations present conservative biases to the null. Mirroring the Seattle HIV epidemic, the sample was predominantly male. This makes extension of these results to females, or to those living outside the US, less generalizable. These were heavily treatment- and intervention-experienced populations. Half the sample was from an HIV clinic whose approach to ART adherence support was itself found to reduce log10 viral load.59 The potential for detecting intervention effect, and magnitude of effect, may be greater in populations with lower ART adherence or higher sexual risk at baseline.60 The study was well-powered to detect a clinically meaningful intervention effect, i.e., a mean half-log10 change in viral load. However, given that this is one study, examining intervention effects in additional diverse HIV-positive samples would contribute to important next steps of replication and generalization. Future research could include examination of the interplay of multiple HIV behaviors such as nonadherence and sexual risk. The intervention may have influenced risk behavior reporting, though computerized approaches can reduce social desirability bias61 and the VL differences seen is consistent with differences in reported adherence behavior. In previous work with the CARE platform users reported that it was easier to be honest with the computer and that the session allowed them to reflect on recent risk behaviors.62 Self-reports of sexual transmission risk outcomes and of ART adherence are limitations. Follow-up of nine months did not allow evaluation of longer-term impact or effect duration. Though the study was conducted in a period when there were fewer ART regimens available, inconsistent ART adherence even to current simplified regimens continues to be a major challenge.63
The study was strengthened by including community- and clinic-based samples, which had similar intervention effects, increasing generalizability.
CONCLUSION
Computer-delivered counseling had a modest, but significant, positive impact on HIV-1 viral load—a primary driver of morbidity and genital compartment infectivity64—and on self-reported HIV transmission risks. This was particularly the case for those who had non-suppression of viral load at baseline – precisely the highest-need group in whom an intervention can have impact. This group’s average VL at baseline declined by a clinically meaningful reduction of approximately 0.5 log10 a reduction that has implications for the person’s own health as well as infectiousness. Their reported ART adherence increased by around 10% (76% at baseline to 85% at 9-months), whereas controls started at 74% mean adherence and showed no improvement over time. The intervention’s relatively modest absolute changes were enough to get this vulnerable group into better ranges of medication adherence, as seen by viral load impact. The importance of supporting treatment adherence has been highlighted by Gardner and others who have shown how few HIV-positive individuals even in care are virally suppressed in the US.65 Interventions to support adherence have tended to show relatively small effects, highlighting the need for efficacious interventions that can be implemented without straining health system resources,63 as is the promise of computerized counseling tools such as CARE+.
As far as we know this is the first ART adherence and secondary HIV transmission risk intervention to find biological effect (viral load) and behavioral impact among persons living with HIV. The computer format was highly acceptable and facilitated delivery in busy settings. Such an approach warrants further evaluation to determine utility in improving HIV treatment outcomes and reducing secondary HIV transmission among persons living with HIV.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Jim Larkin and Tycen Hopkins of Resources Online, CARE+ software developers; and J. Dennis Fortenberry, MD, MS and C. Kevin Malotte, DrPH for their contribution to the original CARE platform. We appreciate the assistance of Carol Glenn, RN, Robert D. Harrington, MD, and Thomas M. Hooton, MD of the Harborview Medical Center HIV Clinic affiliated with the University of Washington; David Richart and Hal Garcia-Smith of Lifelong AIDS Alliance; and Peter Tarczy-Hornoch, University of Washington Biomedical and Health Informatics. We thank Nok Chhun for her expert assistance with manuscript production.
Conflicts of Interest and Source of Funding: Kurth and Spielberg were co-developers of underlying software (‘CARE’) adapted for use in this intervention, with media company Resources Online, Seattle WA, from an original SBIR grant by the Centers for Disease Control and Prevention (CDC). For the remaining authors, no conflicts were declared. This study was funded by a Health Promotion Research Initiative Mentored Scientist Award to Kurth (5 K01 PS000066), CDC. Holmes’ and Simoni’s time was supported by the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757) that is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC or the NIH. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Trial Registration: ClinicalTrials.gov NCT00443378, http://clinicaltrials.gov/ct2/show/NCT00443378?term=04-3810-C+01&rank=1.
Author contributions: Dr. Kurth had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kurth, Spielberg, Bangsberg, Holmes.
Intervention content: Kurth, Clausen, Spielberg, Frick, Simoni.
Acquisition of data: Severynen, Clausen, Lambdin.
Analysis and interpretation of data: Kurth, Cleland.
Statistical analysis: Lockhart, Lambdin, Norman, Cleland.
Drafting of the manuscript: Kurth, Lambdin, Cleland.
Critical revision of the manuscript for important intellectual content: All authors.
List of Supplemental Digital Content: Supplemental Digital Content 1.doc
Supplemental Digital Content 2.doc
Supplemental Digital Content 3.doc
Supplemental Digital Content 4.doc
Supplemental Digital Content 5.doc
Supplemental Digital Content 6.doc
References
- 1.Barroso PF, Schechter M, Gupta P, Bressan C, Bomfim A, Harrison LH. Adherence to antiretroviral therapy and persistence of HIV RNA in semen. J Acquir Immune Defic Syndr. 2003 Apr 1;32(4):435–440. doi: 10.1097/00126334-200304010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. The New England journal of medicine. 2000 Mar 30;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; 2013. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 6.CDC Vital Signs: HIV Prevention through Care and Treatment - United States. Morbidity and Mortality Weekly Report (MMWR) 2011;60(47):1621. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6047a4.htm. [PubMed] [Google Scholar]
- 7.Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2003 Jul 18;52(RR-12):1–24. [PubMed] [Google Scholar]
- 8.Marks G, Richardson JL, Crepaz N, et al. Are HIV care providers talking with patients about safer sex and disclosure?: A multi-clinic assessment. Aids. 2002 Sep 27;16(14):1953–1957. doi: 10.1097/00002030-200209270-00013. [DOI] [PubMed] [Google Scholar]
- 9.Gilliam PP, Straub DM. Prevention with positives: a review of published research, 1998-2008. The Journal of the Association of Nurses in AIDS Care : JANAC. 2009 Mar-Apr;20(2):92–109. doi: 10.1016/j.jana.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Rose CD, Koester KA, Kang Dufour MS, et al. Messages HIV clinicians use in prevention with positives interventions. AIDS care. 2012;24(6):704–711. doi: 10.1080/09540121.2011.644232. [DOI] [PubMed] [Google Scholar]
- 11.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006 Mar;41(3):285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 12.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006 Dec 1;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual risk reduction for persons living with HIV: research synthesis of randomized controlled trials, 1993 to 2004. J Acquir Immune Defic Syndr. 2006 Apr 15;41(5):642–650. doi: 10.1097/01.qai.0000194495.15309.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006 Jan 9;20(2):143–157. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 15.Bell SG, Newcomer SF, Bachrach C, et al. Challenges in replicating interventions. J Adolesc Health. 2007 Jun;40(6):514–520. doi: 10.1016/j.jadohealth.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007 Dec 15;46(5):574–580. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon CM. Commentary on meta-analysis of randomized controlled trials for HIV treatment adherence interventions. Research directions and implications for practice. J Acquir Immune Defic Syndr. 2006 Dec 1;43(Suppl 1):S36–40. doi: 10.1097/01.qai.0000248347.87512.ba. [DOI] [PubMed] [Google Scholar]
- 18.Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Ann Behav Med. 2004 Feb;27(1):3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006 Jul;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 20.Prochaska JO, Redding CA, Harlow LL, Rossi JS, Velicer WF. The transtheoretical model of change and HIV prevention: a review. Health Educ Q. 1994 Winter;21(4):471–486. doi: 10.1177/109019819402100410. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004 Apr;31(2):143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 22.Miller WR, Rollnick S. Motivational interviewing. Guildford Press; NY: 1991. [Google Scholar]
- 23.Haddad M, Inch C, Glazier RH, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2000;3 doi: 10.1002/14651858.CD001442. [DOI] [PubMed] [Google Scholar]
- 24.Fisher JD, Cornman DH, Osborn CY, Amico KR, Fisher WA, Friedland GA. Clinician-Initiated HIV Risk Reduction Intervention for HIV-Positive Persons: Formative Research, Acceptability, and Fidelity of the Options Project. J Acquir Immune Defic Syndr. 2004 Oct 1;37:S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 25.Richardson JL, Milam J, McCutchan A, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: a multi-clinic assessment. Aids. 2004 May 21;18(8):1179–1186. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell CR, O’Donnell L, San Doval A, Duran R, Labes K. Reductions in STD infections subsequent to an STD clinic visit. Using video-based patient education to supplement provider interactions. Sex Transm Dis. 1998;25(3):161–168. doi: 10.1097/00007435-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kurth A, Clausen M, Moore A. Formative research for a computer counseling intervention to support antiretroviral adherence. Paper presented at: A State of the Science Meeting on Intervention Research to Improve Anti-Retroviral Adherence, Yale University; New Haven, CT. November 2005. [Google Scholar]
- 28.Thibault JM, Steiner RW. Efficient identification of adults with depression and dementia. Am Fam Physician. 2004 Sep 15;70(6):1101–1110. [PubMed] [Google Scholar]
- 29.Skeels MM, Kurth A, Clausen M, Severynen A, Garcia-Smith H. CARE+ User Study: Usability and Attitudes Towards a Tablet PC Computer Counseling Tool for HIV+ Men and Women. AMIA Annu Symp Proc. 2006:729–733. [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. Aids. 2002;16(2):269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Safren SA, Skolnik PR, et al. Optimal Recall Period and Response Task for Self-Reported HIV Medication Adherence. AIDS Behav. 2007 Jun 19; doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 32.Core TR. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. ISBN: 3-900051-07-0. Available at http://www.R-project.org. http://www.R-project.org. [Google Scholar]
- 33.Halekoh U, Højsgaard S, Yan J. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2006;15(2):1–11. [Google Scholar]
- 34.Højsgaard S, Halekoh U, Robison-Cox J, Wright K, Leidi AA. doBy - Groupwise summary statistics, general linear contrasts,population means (least-squares-means), and other utilities. (R package version 4.5-5) 2012 Available at http://CRAN.R-project.org/package=doBy.
- 35.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York, NY: 2009. [Google Scholar]
- 36.Kahle E, Zhang Q, Golden M, Goldbaum G, Buskin S. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007 Dec;34(12):940–946. doi: 10.1097/olq.0b013e31813e0a48. [DOI] [PubMed] [Google Scholar]
- 37.Myers JJ, Rose CD, Shade SB, et al. Sex, risk and responsibility: provider attitudes and beliefs predict HIV transmission risk prevention counseling in clinical care settings. AIDS Behav. 2007 Sep;11(5 Suppl):S30–38. doi: 10.1007/s10461-007-9269-9. [DOI] [PubMed] [Google Scholar]
- 38.Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of Web-based vs. non-Web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res. 2004 Nov 10;6(4):e40. doi: 10.2196/jmir.6.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003 Mar-Apr;14(2):52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 40.Johnson MO, Catz SL, Remien RH, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care STDS. 2003 Dec;17(12):645–656. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- 41.Bangsberg DR, Bronstone A, Chesney MA, Hecht FM. Computer-assisted self-interviewing (CASI) to improve provider assessment of adherence in routine clinical practice. J Acquir Immune Defic Syndr. 2002 Dec 15;31(Suppl 3):S107–111. doi: 10.1097/00126334-200212153-00004. [DOI] [PubMed] [Google Scholar]
- 42.Grimley DM, Bachmann LH, Jenckes MW, Erbelding EJ. Provider-delivered, Theory-based, Individualized Prevention Interventions for HIV Positive Adults Receiving HIV Comprehensive Care. AIDS Behav. 2006 Dec 6; doi: 10.1007/s10461-006-9196-1. [DOI] [PubMed] [Google Scholar]
- 43.Zuniga ML, Baldwin H, Uhler D, et al. Supporting Positive Living and Sexual Health (SPLASH): a clinician and behavioral counselor risk-reduction intervention in a university-based HIV clinic. AIDS Behav. 2007 Sep;11(5 Suppl):S58–71. doi: 10.1007/s10461-007-9255-2. [DOI] [PubMed] [Google Scholar]
- 44.Smith DM, Drumright LN, Frost SD, et al. Characteristics of recently HIV-infected men who use the Internet to find male sex partners and sexual practices with those partners. J Acquir Immune Defic Syndr. 2006 Dec 15;43(5):582–587. doi: 10.1097/01.qai.0000243100.49899.2a. [DOI] [PubMed] [Google Scholar]
- 45.Wight RG, Rotheram-Borus MJ, Klosinski L, Ramos B, Calabro M, Smith R. Screening for transmission behaviors among HIV-infected adults. AIDS Educ Prev. 2000 Oct;12(5):431–441. [PubMed] [Google Scholar]
- 46.Gross M, Holte SE, Marmor M, Mwatha A, Koblin BA, Mayer KH. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J Acquir Immune Defic Syndr. 2000;24(4):393–398. doi: 10.1097/00126334-200008010-00015. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert P, Ciccarone D, Gansky SA, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PloS one. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serovich JM, Reed S, Grafsky EL, Andrist D. An intervention to assist men who have sex with men disclose their serostatus to casual sex partners: results from a pilot study. AIDS education and prevention : official publication of the International Society for AIDS Education. 2009 Jun;21(3):207–219. doi: 10.1521/aeap.2009.21.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naar-King S, Outlaw AY, Sarr M, et al. Motivational Enhancement System for Adherence (MESA): Pilot Randomized Trial of a Brief Computer-Delivered Prevention Intervention for Youth Initiating Antiretroviral Treatment. Journal of pediatric psychology. 2013 Jan 28; doi: 10.1093/jpepsy/jss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remien RH, Mellins CA, Robbins RN, et al. Masivukeni: Development of a Multimedia Based Antiretroviral Therapy Adherence Intervention for Counselors and Patients in South Africa. AIDS and behavior. 2013 Mar 7; doi: 10.1007/s10461-013-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lightfoot M, Rotheram-Borus MJ, Comulada S, Gundersen G, Reddy V. Self-monitoring of behaviour as a risk reduction strategy for persons living with HIV. AIDS Care. 2007 Jul;19(6):757–763. doi: 10.1080/09540120600971117. [DOI] [PubMed] [Google Scholar]
- 52.Lightfoot M, Rotheram-Borus MJ, Comulada WS, Reddy VS, Duan N. Efficacy of brief interventions in clinical care settings for persons living with HIV. J Acquir Immune Defic Syndr. 2010 Mar;53(3):348–356. doi: 10.1097/QAI.0b013e3181c429b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher JD, Amico KR, Fisher WA, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS and behavior. 2011 Nov;15(8):1635–1646. doi: 10.1007/s10461-011-9926-x. [DOI] [PubMed] [Google Scholar]
- 54.Grimley DM, Hook EW., 3rd A 15-minute interactive, computerized condom use intervention with biological endpoints. Sexually transmitted diseases. 2009 Feb;36(2):73–78. doi: 10.1097/OLQ.0b013e31818eea81. [DOI] [PubMed] [Google Scholar]
- 55.Marsch LA, Grabinski MJ, Bickel WK, et al. Computer-assisted HIV prevention for youth with substance use disorders. Substance use & misuse. 2011;46(1):46–56. doi: 10.3109/10826084.2011.521088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peipert JF, Redding CA, Blume JD, et al. Tailored intervention to increase dual-contraceptive method use: a randomized trial to reduce unintended pregnancies and sexually transmitted infections. American journal of obstetrics and gynecology. 2008 Jun;198(6):630, e631–638. doi: 10.1016/j.ajog.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noar SM. Computer technology-based interventions in HIV prevention: state of the evidence and future directions for research. AIDS care. 2011 May;23(5):525–533. doi: 10.1080/09540121.2010.516349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freedberg KA, Hirschhorn LR, Schackman BR, et al. Cost-effectiveness of an intervention to improve adherence to antiretroviral therapy in HIV-infected patients. J Acquir Immune Defic Syndr. 2006 Dec 1;43(Suppl 1):S113–118. doi: 10.1097/01.qai.0000248334.52072.25. [DOI] [PubMed] [Google Scholar]
- 59.Frick PTK, Grant P, Novotny M, Kerzee J. The effect of a multidisciplinary program on HAART adherence. AIDS Patient Care and STDs. 2006;20(7):511–524. doi: 10.1089/apc.2006.20.511. [DOI] [PubMed] [Google Scholar]
- 60.Remien RH, Exner TM, Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: implications for transmission of resistant virus. AIDS Behav. 2007 Sep;11(5):663–675. doi: 10.1007/s10461-006-9201-8. [DOI] [PubMed] [Google Scholar]
- 61.Beauclair R, Meng F, Dreprez N, et al. Evaluating audio computer assisted self-interviews in urban South African communities: evidence for good suitability and reduced social desirability bias of a cross sectional survey on sexual behaviour. BMC Med Res Methodol. 2013 Jan;13:11. doi: 10.1186/1471-2288-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackenzie SL, Kurth AE, Spielberg F, et al. Patient and staff perspectives on the use of a computer counseling tool for HIV and sexually transmitted infection risk reduction. J Adolesc Health. doi: 10.1016/j.jadohealth.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Current HIV/AIDS reports. 2010 Feb;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; 2007 Jun;40(6):572.e9–16. [Google Scholar]
- 64.Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008 Jan;35(1):55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 65.Mugavero MJ, Amico KR, Horn T, Thompson MA. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Jun 23; doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.