Abstract
Background
The American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) collaborate annually to provide updates on cancer incidence and death rates and trends in these outcomes for the U.S. This year’s report includes the prevalence of comorbidity at time of first cancer diagnosis among patients with lung, colorectal, breast or prostate cancer and the survival among cancer patients based on comorbidity level.
Methods
Data on cancer incidence were obtained from NCI, CDC, and NAACCR, and on mortality from CDC. Long- (1975/92-2010) and short- (2001-2010) term trends in age-standardized incidence and death rates for all cancers combined and for the leading cancers among men and among women were examined by joinpoint analysis. Through linkage with Medicare claims, the prevalence of comorbidity among cancer patients diagnosed between 1992 through 2005 residing in 11 Surveillance, Epidemiology, and End Results (SEER) areas were estimated and compared to those among a 5% random sample of cancer-free Medicare beneficiaries. Among cancer patients, survival and the probabilities of dying of their cancer and of other causes by comorbidity level, age, and stage were calculated.
Results
Death rates continued to decline for all cancers combined for men and women of all major racial and ethnic groups and for most major cancer sites; rates for both sexes combined decreased by 1.5% per year from 2001 through 2010. Overall incidence rates decreased in men and stabilized in women. The prevalence of comorbidity was similar among cancer-free Medicare beneficiaries (31.8%), breast cancer patients (32.2%), and prostate cancer patients (30.5%), highest among lung cancer patients (52.8%), and intermediate among colorectal cancer patients (40.7%). Among all cancer patients and especially for patients diagnosed with local and regional disease, age and comorbidity level were important influences on the probability of dying of other causes and consequently on overall survival. For patients diagnosed with distant disease, the probability of dying of cancer was much higher than the probability of dying of other causes, and age and comorbidity had a smaller effect on overall survival.
Conclusions
Cancer death rates in the U.S. continue to decline. Estimates of survival that include the probability of dying of cancer and other causes stratified by comorbidity level, age and stage can provide important information to facilitate treatment decisions.
Keywords: cancer, comorbidity, multiple chronic conditions, multiple health conditions, incidence, mortality, survival, trends, SEER, NPCR, NAACCR, SEER-Medicare, United States (U.S.)
INTRODUCTION
The Annual Report to the Nation is a collaborative effort among the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) to provide updated cancer incidence and mortality data for the United States (U.S.). The first report, published in 1998, documented the first sustained decline in cancer death rates since the 1930s.1 Subsequent reports have featured in-depth analyses of selected special topics.2-15 This current report provides updated cancer rates and trends for all cancers combined, childhood cancer and the 15 most prevalent cancers for each of the major racial and ethnic groups by sex. In addition, this report describes the prevalence and impact of comorbidities on crude probabilities of dying from cancer or other causes for patients diagnosed with one of the four major cancer sites in men and women aged 66 years or older. The data source for the comorbidity analysis is the linked SEER-Medicare database, the most comprehensive source of population-based information with cancer treatment and outcomes data in the U.S.16
Comorbidities are co-existing non-cancer medical conditions that are distinct from the principal cancer diagnosis.17 Prior studies have shown that the number and severity of comorbidities at the time of cancer diagnosis strongly influences the probability of dying from non-cancer causes and also may influence cancer-specific survival.18-21 Population-based measures of cancer survival have typically focused on relative survival,22 which is the ratio of observed survival to expected survival for a given cohort, accounting for age, sex, race and year of diagnosis based on U.S. life tables. However, overall and stage-specific estimates of relative survival for cancer patients may be unrepresentative if their health status differs from that of the general population.23 One strategy to have potentially more accurate estimates is using the SEER-Medicare linked data to provide tailored survival estimates based on both cancer stage at diagnosis and comorbidity levels. This is particularly pertinent for older patients who often have multiple or serious comorbidities, and for persons diagnosed with cancers such as low-grade prostate cancer, who have a relatively good prognosis in the absence of treatment.21 , 24 These estimates25 may be useful for cancer treatment planning which often requires balancing treatment toxicity and complications with the expected benefit of potential healthy life years gained.26 In addition to providing contemporary cancer rates and trends, this report highlights the considerable prevalence of comorbidities and their impact on overall health and quality of life27 among cancer patients aged 65 or older in whom 53% of all new cancer cases occur.28
MATERIALS AND METHODS
Cancers and Cancer Deaths
Population-based data on newly diagnosed invasive cancers were obtained from registries that participate in the NCI’s SEER Program and/or CDC’s National Program of Cancer Registries (NPCR) and submit their data to NAACCR. Site and histology were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis and converted to the third edition coding,29 and categorized according to SEER anatomic site groups.30 Incidence rates were calculated for all cancer sites combined, childhood cancers (ages 0-14 years and 0-19 years), and for the 15 most prevalent cancers among men and women for each of the major racial and ethnic groups (white, black, Asian Pacific Islander [API], American Indian/Alaska Native [AI/AN] and Hispanic). Hispanic ethnicity includes individuals from all races identified as Hispanic. Rates for AI/ANs were based on cases and deaths occurring in counties covered by the Indian Health Service Contract Health Service Delivery Areas (CHSDA); these areas have better race/ethnicity ascertainment leading to more accurate rates for this population.10, 31
Incidence data were not available uniformly for every calendar year, geographic area, and racial and ethnic group in the U.S. Long-term (1992-2010) incidence trends for all racial and ethnic groups combined were based on SEER 13 registries covering approximately 13% of the U.S. population.32, 33 Five-year (2006-2010) average annual age-adjusted incidence rates and short-term (2001-2010) incidence trends for each of the five major racial and ethnic groups and all races combined were calculated using combined data from NPCR and SEER registries (November 2012 submissions) and provided by NAACCR (December 2012 submission). U.S. population coverage was 90.1% and 85.4% for the rates and trends, respectively.
Cause of death was based on death certificate information reported to state vital statistics offices and compiled into a national file for the entire U.S. by the CDC National Center for Health Statistics’ National Vital Statistics System.34 The underlying causes of death were selected according to the International Classification of Disease (ICD) codes and selection rules in use at the time of death (ICD-8 through ICD-10) and categorized according to SEER anatomic site groups to maximize comparability between ICD and ICD-O versions.30, 35-37 Death rates were calculated for all cancer sites combined, childhood cancers (ages 0-14 years and 0-19 years) and for the 15 most prevalent cancers among men and women for each of the major racial and ethnic groups. We examined long-term (1975-2010) mortality trends for all racial and ethnic groups combined, five-year (2006-2010) average annual age-adjusted death rates and short-term (2001-2010) mortality trends for each of the five major racial and ethnic groups.
Population Estimates
A modified version of the annual time series of July 1 county population estimates by age, sex, race and ethnicity produced by the U.S. Census Bureau38 was used. These population estimates incorporated both the 2000 and 2010 Census results. Other modifications incorporated bridged single-race estimates that were derived from multiple-race categories in the 2000 Census.39 For most states, population estimates as of July 1 were used to calculate annual incidence and death rates because it is presumed that these estimates reflect the average population of a defined geographic area for a calendar year. Certain county estimates were adjusted to account for populations along the Gulf coasts of Louisiana, Alabama, Mississippi and Texas displaced during 2005 by Hurricanes Katrina and Rita.38 Additional information was used to more accurately estimate the native Hawaiian population40 and to derive population estimates for newly created counties. These modified county-level population estimates were summed to the state and national levels, and used as denominators in the rate calculations.
Comorbidity among Older Cancer Patients and the Medicare Population
The comorbidity analyses included a cohort of cancer patients diagnosed between 1992 and 2005 who resided in 11 SEER areas and whose data have been linked to Medicare claims data, and a 5% random sample of cancer-free Medicare beneficiaries.41 More information is available on the linked SEER-Medicare database and the 5% cancer-free sample.16 Medicare claims were available in the same format for cancer patients and individuals without cancer (cancer-free patients). Comorbidities were identified from Medicare Part A hospitalization claims and Part B physician/supplier and outpatient facility claims. The analysis excluded individuals who were enrolled in health maintenance organizations (HMOs), individuals not enrolled continuously in both Parts A and B of Medicare between 1992 and 2005, and patients whose cancer was diagnosed by death certificate or autopsy only. HMO enrollment in the Medicare population is about 25%.41 The analysis also included only individuals 66 years or older to ensure that comorbidities in the year prior to cancer diagnosis could be identified in Medicare claims.
Sixteen comorbid conditions identified by Charlson et al.42, 43 from medical records comprised the comorbidity measure used in this analysis. Charlson’s original algorithm was adapted for use with ICD-9 codes from administrative databases43 and in SEER Medicare studies of cancer patients.44, 45 The 16 conditions are: acute myocardial infarction, acquired immunodeficiency syndrome (AIDS), cerebrovascular disease, chronic renal failure, cirrhosis/chronic hepatitis, congestive heart failure, chronic obstructive pulmonary disease (COPD), dementia, diabetes, diabetes with sequelae, history of myocardial infarction, liver disease, paralysis, rheumatologic disease, ulcer disease and vascular disease. In the current analyses, diabetes (ICD-9: 250.0x - 250.3x, 250.7x) and diabetes with sequelae (ICD-9: 250.4x - 250.6x, 250.8x -250.9x) were grouped together. While the Charlson index typically includes solid tumors and lymphoma/leukemia, these conditions were not included in our analysis because of our focus on non-cancer comorbidity. The presence or absence of the 16 comorbid conditions was determined using the clinical modification of ICD-9 codes and Current Procedural Terminology codes recorded in Medicare claims data according to an algorithm developed by Klabunde et al.44-46 and used in Mariotto et al.23 to estimate comorbid condition weights. Consistent with prior work,44, 45 a rule-out algorithm was used so that only conditions appearing on more than one physician claim were included, thereby ensuring that diagnoses recorded only in Part B claims were not transient episodes.
Patients with female breast, prostate, lung or colorectal cancer were selected from the SEER-Medicare database. These four cancer sites were chosen because they are the most common cancers (about half of all new cancer cases and all cancer deaths) in the U.S and because prevalence of comorbidities and the probability of dying from other causes have been shown to vary by these cancer types.20 For each cancer patient, the presence or absence of the 16 comorbidities was identified in the year prior to their first cancer diagnosis, excluding the month of diagnosis. The month of cancer diagnosis was excluded to minimize the misclassification of complications potentially related to cancer diagnosis or its treatment as comorbid conditions.
Non-cancer controls were randomly selected from the 5% random sample of cancer-free Medicare recipients in the SEER areas for each calendar year and were frequency matched to cancer cases by sex and age.47 Comorbid conditions were identified in the year prior to the birthday of the matched calendar year.
STATISTICAL METHODS
Incidence and Mortality Rates and Trends
Age-adjusted rates were expressed per 100,000 persons based on the 2000 U.S. standard population and generated using SEER*Stat software, version 8.0.4.48 Corresponding 95% confidence intervals were calculated as modified gamma intervals.49 Rates were not reported if there were fewer than 10 cases.
Trends in age-adjusted cancer incidence and death rates were estimated using joinpoint regression which involves fitting a series of joined straight lines on a logarithmic scale to the trends in the annual age-adjusted rates.50, 51 A maximum of three joinpoints was allowed in models for the period 1992 through 2010, up to five joinpoints were allowed in models for the period 1975 through 2010, and up to two joinpoints were allowed in models for the period 2001 through 2010. The resulting trends were described by the slope of the line segment or the annual percent change (APC). The average APC (AAPC) was estimated as a weighted geometric average of the APCs, with the weights equal to the length of each line segment during the pre-specified fixed interval.52 Long-term incidence trends were calculated using both observed and delay-adjusted SEER 13 data; descriptions of these trends were based on the delay-adjusted data. Delay adjustment53 is a statistical method that corrects for the unreported (delayed) or updated cases and mostly affects cancers diagnosed in recent years and in non-hospital settings, e.g. melanoma of the skin or leukemia. The t-test was used to test whether the APC was statistically different from zero and the Z test was used to test whether the AAPC was statistically different from zero. All statistical tests were two-sided. In describing trends, the terms increase or decrease were used when the slope (APC or AAPC) of the trend was statistically significant (two-sided P<0.05). For nonstatistically significant trends, terms such as stable, nonsignificant increase and nonsignificant decrease were used.
Comorbidity Scores and Levels of Severity
For each individual in the study cohort, a comorbidity score was calculated by multiplying previously estimated condition weights by comorbid condition indicators and summing over the 16 conditions. The weights represented the effect of comorbid conditions on survival for other causes of death and were estimated by fitting a Cox proportional hazards model to non-cancer survival time, controlling for age, sex, and race.23 Because individuals may have more than one comorbid condition, interactions among the most prevalent conditions (i.e., diabetes, COPD and congestive heart failure) were included in the model. Comorbidity was grouped into three levels based on comorbidity scores and clinical judgment.20 Specifically, having no comorbidity (none) refers to a zero comorbidity score and no identified comorbid conditions. The low and moderate groups with a comorbidity score of 0-0.66 were combined because of small sample sizes in the low group. Low comorbidity refers to conditions that usually do not require adjusting cancer treatment, such as ulcer or rheumatologic disease. Moderate comorbidity refers to conditions that may sometimes require modifying cancer treatment, including vascular disease, diabetes, paralysis, and AIDS. Severe comorbidity refers to a comorbidity score > 0.66 or severe illnesses that frequently lead to organ failure or systemic dysfunction and usually require adjusting cancer treatment, such as COPD, liver dysfunction, chronic renal failure, dementia and congestive heart failure. Most individuals with more than one comorbid condition fell into the severe comorbidity group.
Survival Measures and Analyses
To provide the most recent survival estimates, the survival analysis was restricted to patients diagnosed with cancer between 1999 through 2005. Survival measures by comorbidity level, taking into account competing risks of death, were calculated as the crude probabilities of death due to cancer, death due to other causes, and survival.18, 54 Two competing endpoints/outcomes were considered: death due to a specific first diagnosis of cancer and death to other (non-cancer) causes. These events were considered to be mutually exclusive and the occurrence of one precluded the occurrence of the other, i.e., the probability of death was partitioned into the two causes. Survival was calculated as 1 minus the probabilities of dying from cancer and dying from other causes and is exactly the same as all-cause survival. We used the SEER cause-specific death classification variable19, 55 which has been shown to more accurately classify cancer deaths than cause of death reported from death certificates. In addition to cause of death this variable incorporates sequence of tumor occurrence, site of original diagnosis, and comorbidities. Probabilities of dying from cancer, dying from other causes and surviving five years from diagnosis were calculated by cancer site, sex, age group (66-74, 75-84, 85+ years), stage at cancer diagnosis (localized, regional and distant) and comorbidity level using SEER*Stat software.48 Stage was based on summary stage 2000.56, 57
RESULTS
Long-Term (1992-2010) Cancer Incidence Trends for All Racial and Ethnic Groups Combined
Trend analysis based on SEER 13 data showed that overall delay-adjusted and age-adjusted cancer incidence rates for all persons combined decreased by 0.4% per year from 2001 through 2010, continuing a trend from 1998 (Table 1). The majority of this decrease occurred among men, for whom a 0.6% annual decrease was documented during the past decade. In contrast, the cancer incidence rate among women remained stable. For children, rates increased by 0.8% per year during the past decade, for both children aged 0 through 14 years and 0 through 19 years, continuing a trend dating to 1992.
Table 1.
Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Rate Trends With Joinpoint Analyses from 1992 to 2010 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Joinpoint analyses (1992-2010)b |
||||||||||
| Trend 1 |
Trend 2 |
Trend 3 |
Trend 4 |
AAPCc |
||||||
| Sex/Cancer Site or Type | Years | APCd | Years | APCd | Years | APCd | Years | APCd | 2001 - 2010 |
2006 - 2010 |
| All sitese | ||||||||||
| Both sexes | 1992 - 1994 | −3.1 | 1994 - 1999 | 0.3 | 1999 - 2010 | −0.6 f | −0.6 g | −0.6 g | ||
| (Delay-adjusted) | 1992 - 1994 | −3.2 f | 1994 - 1998 | 0.4 | 1998 - 2010 | −0.4 f | −0.4 g | −0.4 g | ||
| Males | 1992 - 1994 | −5.8 f | 1994 - 2007 | −0.5 f | 2007 - 2010 | −2.2 f | −1.1 g | −1.8 g | ||
| (Delay-adjusted) | 1992 - 1994 | −5.6 f | 1994 - 2010 | −0.6 f | −0.6 g | −0.6 g | ||||
| Females | 1992 - 1998 | 0.7 f | 1998 - 2010 | −0.3 f | −0.3 g | −0.3 g | ||||
| (Delay-adjusted) | 1992 - 1994 | −0.4 | 1994 - 1998 | 1.2 | 1998 - 2003 | −0.8 f | 2003 - 2010 | 0.1 | −0.1 | 0.1 |
| Children (ages 0-14) | 1992 - 2010 | 0.8 f | 0.8 g | 0.8 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.8 f | 0.8 g | 0.8 g | ||||||
| Children (ages 0-19) | 1992 - 2010 | 0.7 f | 0.7 g | 0.7 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.8 f | 0.8 g | 0.8 g | ||||||
| Top 17 cancers for malesh | ||||||||||
| Prostate | 1992 - 1995 | −11.2 f | 1995 - 2000 | 2.2 | 2000 - 2010 | −2.2 f | −2.2 g | −2.2 g | ||
| (Delay-adjusted) | 1992 - 1995 | −11.2 f | 1995 - 2000 | 2.2 | 2000 - 2010 | −2.0 f | −2.0 g | −2.0 g | ||
| Lung and bronchus | 1992 - 2010 | −2.0 f | −2.0 g | −2.0 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −1.9 f | −1.9 g | −1.9 g | ||||||
| Colon and rectum | 1992 - 1995 | −2.6 f | 1995 - 1998 | 1.4 | 1998 - 2008 | −2.5 f | 2008 - 2010 | −4.7 f | −3.0 g | −3.6 g |
| (Delay-adjusted) | 1992 - 1995 | −2.6 f | 1995 - 1998 | 1.4 | 1998 - 2008 | −2.5 f | 2008 - 2010 | −4.2 f | −2.9 g | −3.3 g |
| Urinary bladder | 1992 - 2010 | −0.1 | −0.1 | −0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.0 | 0.0 | 0.0 | ||||||
| Melanoma of the skin | 1992 - 2010 | 2.4 f | 2.4 g | 2.4 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | 2.4 f | 2.4 g | 2.4 g | ||||||
| Non-Hodgkin lymphoma | 1992 - 1995 | 2.8 | 1995 - 1998 | −2.1 | 1998 - 2010 | 0.5 f | 0.5 g | 0.5 g | ||
| (Delay-adjusted) | 1992 - 1995 | 2.8 | 1995 - 1998 | −2.2 | 1998 - 2010 | 0.7 f | 0.7 g | 0.7 g | ||
| Kidney and renal pelvis | 1992 - 2004 | 1.9 f | 2004 - 2008 | 4.5 f | 2008 - 2010 | −2.8 | 1.9 g | 0.8 | ||
| (Delay-adjusted) | 1992 - 2004 | 1.9 f | 2004 - 2008 | 4.7 f | 2008 - 2010 | −2.0 | 2.2 g | 1.3 | ||
| Oral cavity and pharynx | 1992 - 2003 | −1.5 f | 2003 - 2010 | 0.5 | 0.0 | 0.5 | ||||
| (Delay-adjusted) | 1992 - 2003 | −1.5 f | 2003 - 2010 | 0.7 | 0.2 | 0.7 | ||||
| Leukemia | 1992 - 2010 | −0.1 | −0.1 | −0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.4 f | 0.4 g | 0.4 g | ||||||
| Pancreas | 1992 - 2003 | 0.0 | 2003 - 2007 | 2.5 f | 2007 - 2010 | −1.2 | 0.7 | −0.3 | ||
| (Delay-adjusted) | 1992 - 2001 | 0.0 | 2001 - 2010 | 1.3 f | 1.3 g | 1.3 g | ||||
| Liver and intrahepatic bile duct | 1992 - 2010 | 3.5 f | 3.5 g | 3.5 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | 3.7 f | 3.7 g | 3.7 g | ||||||
| Stomach | 1992 - 2010 | −1.7 f | −1.7 g | −1.7 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −1.7 f | −1.7 g | −1.7 g | ||||||
| Esophagus | 1992 - 2010 | −0.1 | −0.1 | −0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.0 | 0.0 | 0.0 | ||||||
| Brain and other nervous system | 1992 - 2010 | −0.3 f | −0.3 g | −0.3 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −0.3 f | −0.3 g | −0.3 g | ||||||
| Myeloma | 1992 - 2010 | 0.5 f | 0.5 g | 0.5 g | ||||||
| (Delay-adjusted) | 1992 - 2006 | 0.4 | 2006 - 2010 | 3.5 f | 1.8 g | 3.5 g | ||||
| Larynx | 1992 - 2003 | −3.2 f | 2003 - 2010 | −1.6 f | −1.9 g | −1.6 g | ||||
| (Delay-adjusted) | 1992 - 2003 | −3.2 f | 2003 - 2010 | −1.5 f | −1.8 g | −1.5 g | ||||
| Thyroid | 1992 - 1996 | −1.0 | 1996 - 2010 | 5.4 f | 5.4 g | 5.4 g | ||||
| (Delay-adjusted) | 1992 - 1996 | −0.9 | 1996 - 2010 | 5.4 f | 5.4 g | 5.4 g | ||||
| Top 18 cancers for femalesh | ||||||||||
| Breast | 1992 - 1999 | 1.3 f | 1999 - 2004 | −2.2 f | 2004 - 2010 | 0.1 | −0.7 | 0.1 | ||
| (Delay-adjusted) | 1992 - 1999 | 1.3 f | 1999 - 2004 | −2.2 f | 2004 - 2010 | 0.2 | −0.6 | 0.2 | ||
| Lung and bronchus | 1992 - 2007 | 0.0 | 2007 - 2010 | −2.6 f | −0.9 g | −1.9 g | ||||
| (Delay-adjusted) | 1992 - 1998 | 0.8 f | 1998 - 2001 | −1.3 | 2001 - 2005 | 0.7 | 2005 - 2010 | −1.2 f | −0.4 | −1.2 g |
| Colon and rectum | 1992 - 1995 | −1.8 f | 1995 - 1998 | 1.8 | 1998 - 2008 | −2.0 f | 2008 - 2010 | −4.7 f | −2.6 g | −3.3 g |
| (Delay-adjusted) | 1992 - 1995 | −1.8 f | 1995 - 1998 | 1.8 | 1998 - 2008 | −1.9 f | 2008 - 2010 | −4.1 f | −2.4 g | −3.0 g |
| Corpus and uterus, NOS | 1992 - 2006 | −0.2 | 2006 - 2010 | 2.8 f | 1.1 g | 2.8 g | ||||
| (Delay-adjusted) | 1992 - 2006 | −0.2 | 2006 - 2010 | 2.9 f | 1.2 g | 2.9 g | ||||
| Thyroid | 1992 - 1998 | 3.9 f | 1998 - 2010 | 6.5 f | 6.5 g | 6.5 g | ||||
| (Delay-adjusted) | 1992 - 1998 | 3.9 f | 1998 - 2010 | 6.5 f | 6.5 g | 6.5 g | ||||
| Non-Hodgkin lymphoma | 1992 - 2003 | 1.3 f | 2003 - 2010 | −0.2 | 0.1 | −0.2 | ||||
| (Delay-adjusted) | 1992 - 2003 | 1.3 f | 2003 - 2010 | 0.0 | 0.3 | 0.0 | ||||
| Melanoma of the skin | 1992 - 1997 | 4.0 f | 1997 - 2010 | 1.6 f | 1.6 g | 1.6 g | ||||
| (Delay-adjusted) | 1992 - 1997 | 3.9 f | 1997 - 2010 | 1.7 f | 1.7 g | 1.7 g | ||||
| Ovarye | 1992 - 2010 | −1.0 f | −1.0 g | −1.0 g | ||||||
| (Delay-adjusted) ‡ | 1992 - 2010 | −0.9 f | −0.9 g | −0.9 g | ||||||
| Kidney and renal pelvis | 1992 - 1999 | 1.4 f | 1999 - 2008 | 3.4 f | 2008 - 2010 | −4.7 | 1.5 g | −0.7 | ||
| (Delay-adjusted) | 1992 - 2000 | 1.6 f | 2000 - 2008 | 3.6 f | 2008 - 2010 | −4.0 | 1.9 g | −0.2 | ||
| Pancreas | 1992 - 1999 | −0.2 | 1999 - 2010 | 1.1 f | 1.1 g | 1.1 g | ||||
| (Delay-adjusted) | 1992 - 2000 | −0.1 | 2000 - 2010 | 1.4 f | 1.4 g | 1.4 g | ||||
| Leukemia | 1992 - 2010 | 0.1 | 0.1 | 0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.6 f | 0.6 g | 0.6 g | ||||||
| Urinary bladder | 1992 - 2004 | −0.2 | 2004 - 2010 | −1.3 f | −0.9 g | −1.3 g | ||||
| (Delay-adjusted) | 1992 - 2010 | −0.4 f | −0.4 g | −0.4 g | ||||||
| Cervix uteri | 1992 - 2010 | −2.5 f | −2.5 g | −2.5 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −2.5 f | −2.5 g | −2.5 g | ||||||
| Oral cavity and pharynx | 1992 - 2010 | −0.9 f | −0.9 g | −0.9 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −0.9 f | −0.9 g | −0.9 g | ||||||
| Brain and other nervous system | 1992 - 2010 | −0.1 | −0.1 | −0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.0 | 0.0 | 0.0 | ||||||
| Myeloma | 1992 - 2010 | 0.1 | 0.1 | 0.1 | ||||||
| (Delay-adjusted) | 1992 - 2010 | 0.5 f | 0.5 g | 0.5 g | ||||||
| Stomach | 1992 - 2010 | −0.8 f | −0.8 g | −0.8 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | −0.7 f | −0.7 g | −0.7 g | ||||||
| Liver and intrahepatic bile duct | 1992 - 2010 | 2.8 f | 2.8 g | 2.8 g | ||||||
| (Delay-adjusted) | 1992 - 2010 | 2.9 f | 2.9 g | 2.9 g | ||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: Surveillance, Epidemiology, and End Results (SEER) 13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico, the Alaska Native Tumor Registry, rural Georgia, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound).
Joinpoint analyses with up to 3 joinpoints yielding up to 4 trend segments (Trends 1-4) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.0.3, April 2013; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is a weighted average of the APCs that is calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130).
All sites excludes myelodysplastic syndromes and borderline tumors; ovary excludes borderline tumors.
The APC is statistically significantly different from zero (2-sided t test; P < .05).
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2006 through 2010 for all racial and ethnic groups combined (using data from the National Program of Cancer Registries [NPCR] and SEER Program areas reported by the North American Association of Central Cancer Registries [NAACCR] as meeting high-quality incidence data standards for 2006-2010). More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Among men, delay-adjusted and age-adjusted incidence rates from 2001 through 2010 decreased for six of the 17 most common cancer sites: prostate, lung and bronchus (lung), colon and rectum (colorectal), stomach, brain and other nervous system (brain), and larynx. Rates increased for eight other cancer sites: melanoma of the skin (melanoma), non-Hodgkin lymphoma (NHL), kidney and renal pelvis (kidney), leukemia, pancreas, liver and intrahepatic bile duct (liver), myeloma, and thyroid. Colorectal cancer showed the largest percent decrease and thyroid cancer the largest increase. The trends were similar for the 18 most common sites among women, with six sites decreasing and eight sites increasing. The decreasing sites were colorectal, ovary, urinary bladder (bladder), cervix uteri (cervix), oral cavity and pharynx (oral), and stomach; the increasing sites were corpus and uterus NOS (uterus), thyroid, melanoma, kidney, pancreas, leukemia, myeloma, and liver. As with men, the largest increase was for thyroid cancer; the largest decrease was for cervical cancer. Rates for women were stable for all other sites, including breast cancer.
Long-Term (1975-2010) Cancer Mortality Trends for All Racial and Ethnic Groups Combined
Following many years of sustained increase in cancer mortality, in the early 1990s rates began to stabilize and then decline among both men and women (Table 2) and among children since the 1970s. From 2001 through 2010, the average annual decline was slightly larger for men (1.8% per year) compared to women (1.4% per year). Death rates declined for 11 of the 17 most common cancers in men (lung, prostate, colorectal, leukemia, NHL, esophagus, kidney, stomach, myeloma, oral, larynx) and for 15 of the 18 most common cancers in women (lung, breast, colorectal, ovary, leukemia, NHL, brain, myeloma, kidney, stomach, cervix, bladder, esophagus, oral, gallbladder) from 2001 through 2010. During this same period pancreas and liver cancer death rates increased among men and women, melanoma and cancer of soft tissue including heart (primarily sarcomas) increased in men and cancer of the uterus increased in women. Long-term trends have varied, such as more recent declines that followed persistent increases (e.g., cancer of the lung, kidney, prostate, female breast, brain, and myeloma), or declining trends with an intermittent period of an increase (e.g. ovarian cancer). Several of the long-term mortality trends have stabilized following a decline, such as bladder cancer in men, or have shifted from decreasing mortality to an increase as seen with cancer of the uterus.
Table 2.
US Cancer Death Rate Trends With Joinpoint Analyses From 1975 to 2010 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
| Joinpoint analyses (1975-2010)b |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 |
Trend 2 |
Trend 3 |
Trend 4 |
Trend 5 |
Trend 6 |
AAPCc |
||||||||
| Sex/Cancer Site or Type |
Years | AP Cd |
Years | APC d |
Years | APC d |
Years | APC d |
Years | AP Cd |
Years | AP Cd |
2001 - 2010 |
2006 - 2010 |
| All sites | ||||||||||||||
| Both sexes | 1975 - 1984 |
0.5 e | 1984 - 1991 |
0.3 e | 1991 - 1994 |
−0.5 | 1994 - 1998 |
−1.3 e | 1998 - 2001 |
−0.8 | 2001 - 2010 |
−1.5 e | −1.5 f | − 1.5 f |
| Males | 1975 - 1979 |
1.0 e | 1979 - 1990 |
0.3 e | 1990 - 1993 |
−0.5 | 1993 - 2001 |
−1.5 e | 2001 - 2010 |
−1.8 e | −1.8 f | − 1.8 f |
||
| Females | 1975 - 1990 |
0.6 e | 1990 - 1994 |
−0.2 | 1994 - 2002 |
−0.8 e | 2002 - 2010 |
−1.4 e | −1.4 f | − 1.4 f |
||||
| Children (ages 0-14) |
1975 - 1998 |
−2.9 e | 1998 - 2003 |
0.2 | 2003 - 2010 |
−2.4 e | −1.9 f | − 2.4 f |
||||||
| Children (ages 0-19) |
1975 - 1998 |
−2.7 e | 1998 - 2002 |
0.3 | 2002 - 2010 |
−2.4 e | −2.1 f | − 2.4 f |
||||||
| Top 17 cancers for malesg |
||||||||||||||
| Lung and bronchus |
1975 - 1978 |
2.5 e | 1978 - 1984 |
1.2 e | 1984 - 1990 |
0.4 e | 1990 - 1993 |
−1.1 | 1993 - 2005 |
−1.9 e | 2005 - 2010 |
−2.9 e | −2.5 f | − 2.9 f |
| Prostate | 1975 - 1987 |
0.9 e | 1987 - 1991 |
3.1 e | 1991 - 1994 |
−0.8 | 1994 - 2004 |
−3.8 e | 2004 - 2010 |
−3.1 e | −3.3 f | − 3.1 f |
||
| Colon and rectum |
1975 - 1978 |
0.8 | 1978 - 1985 |
−0.4 e | 1985 - 1990 |
−1.4 e | 1990 - 2002 |
−2.0 e | 2002 - 2005 |
−4.0 e | 2005 - 2010 |
−2.5 e | −2.9 f | − 2.5 f |
| Pancreas | 1975 - 1986 |
−0.8 e | 1986 - 2001 |
−0.3 e | 2001 - 2010 |
0.5 e | 0.5 f | 0.5 f | ||||||
| Leukemia | 1975 - 1980 |
0.5 | 1980 - 1987 |
−0.7 e | 1987 - 1995 |
0.1 | 1995 - 2010 |
−0.9 e | −0.9 f | − 0.9 f |
||||
| Liver and intrahepatic bile duct |
1975 - 1985 |
1.5 e | 1985 - 1996 |
3.8 e | 1996 - 1999 |
0.5 | 1999 - 2010 |
2.5 e | 2.5 f | 2.5 f | ||||
| Non-Hodgkin lymphoma |
1975 - 1996 |
2.5 e | 1996 - 2010 |
−2.6 e | −2.6 f | − 2.6 f |
||||||||
| Urinary bladder | 1975 - 1983 |
−1.4 e | 1983 - 1987 |
−2.8 e | 1987 - 1993 |
0.2 | 1993 - 1997 |
−1.1 | 1997 - 2010 |
0.1 | 0.1 | 0.1 | ||
| Esophagus | 1975 - 1985 |
0.7 e | 1985 - 1994 |
1.2 e | 1994 - 2005 |
0.4 e | 2005 - 2010 |
−1.1 e | −0.4 f | − 1.1 f |
||||
| Kidney and renal pelvis |
1975 - 1991 |
1.1 e | 1991 - 2001 |
−0.1 | 2001 - 2010 |
−0.9 e | −0.9 f | − 0.9 f |
||||||
| Brain and other nervous system |
1975 - 1977 |
4.4 | 1977 - 1982 |
−0.4 | 1982 - 1991 |
1.3 e | 1991 - 2007 |
−1.0 e | 2007 - 2010 |
0.7 | −0.4 | 0.3 | ||
| Stomach | 1975 - 1987 |
−2.4 e | 1987 - 1990 |
−0.3 | 1990 - 2010 |
−3.4 e | −3.4 f | − 3.4 f |
||||||
| Myeloma | 1975 - 1994 |
1.5 e | 1994 - 2010 |
−1.1 e | −1.1 f | − 1.1 f |
||||||||
| Melanoma of the skin |
1975 - 1989 |
2.2 e | 1989 - 2010 |
0.3 e | 0.3 f | 0.3 f | ||||||||
| Oral cavity and pharynx |
1975 - 1993 |
−1.9 e | 1993 - 2000 |
−3.0 e | 2000 - 2010 |
−1.2 e | −1.2 f | − 1.2 f |
||||||
| Larynx | 1975 - 1994 |
−0.8 e | 1994 - 2010 |
−2.5 e | −2.5 f | − 2.5 f |
||||||||
| Soft tissue including heart |
1975 - 1980 |
7.6 e | 1980 - 1997 |
1.2 e | 1997 - 2002 |
−3.6 e | 2002 - 2010 |
1.4 e | 0.8 f | 1.4 f | ||||
| Top 18 cancers for femalesg |
||||||||||||||
| Lung and bronchus |
1975 - 1982 |
6.0 e | 1982 - 1990 |
4.2 e | 1990 - 1995 |
1.7 e | 1995 - 2004 |
0.3 e | 2004 - 2010 |
−1.4 e | −0.8 f | −1.4 f | ||
| Breast | 1975 - 1990 |
0.4 e | 1990 - 1995 |
−1.8 e | 1995 - 1998 |
−3.2 e | 1998 - 2010 |
−1.9 e | −1.9 f | − 1.9 f |
||||
| Colon and rectum |
1975 - 1984 |
−1.0 e | 1984 - 2001 |
−1.8 e | 2001 - 2010 |
−2.9 e | −2.9 f | − 2.9 f |
||||||
| Pancreas | 1975 - 1984 |
0.8 e | 1984 - 2002 |
0.1 | 2002 - 2010 |
0.5 e | 0.4 f | 0.5 f | ||||||
| Ovary | 1975 - 1982 |
−1.2 e | 1982 - 1992 |
0.3 e | 1992 - 1998 |
−1.2 e | 1998 - 2002 |
1.0 | 2002 - 2010 |
−1.9 e | −1.6 f | − 1.9 f |
||
| Leukemia | 1975 - 1980 |
0.8 | 1980 - 2000 |
−0.4 e | 2000 - 2010 |
−1.3 e | −1.3 f | − 1.3 f |
||||||
| Non-Hodgkin lymphoma |
1975 - 1994 |
2.2 e | 1994 - 1997 |
0.9 | 1997 - 2010 |
−3.2 e | −3.2 f | − 3.2 f |
||||||
| Corpus and uterus, NOS |
1975 - 1989 |
−1.6 e | 1989 - 1997 |
−0.7 e | 1997 - 2010 |
0.4 e | 0.4 f | 0.4 f | ||||||
| Brain and other nervous system |
1975 - 1992 |
0.9 e | 1992 - 2010 |
−0.9 e | −0.9 f | − 0.9 f |
||||||||
| Liver and intrahepatic bile duct |
1975 - 1987 |
0.8 e | 1987 - 1995 |
3.8 e | 1995 - 2000 |
0.3 | 2000 - 2010 |
1.6 e | 1.6 f | 1.6 f | ||||
| Myeloma | 1975 - 1993 |
1.5 e | 1993 - 2001 |
−0.4 | 2001 - 2010 |
−2.3 e | −2.3 f | − 2.3 f |
||||||
| Kidney and renal pelvis |
1975 - 1994 |
1.1 e | 1994 - 2010 |
−0.9 e | −0.9 f | − 0.9 f |
||||||||
| Stomach | 1975 - 1987 |
−2.8 e | 1987 - 1990 |
−0.4 | 1990 - 2010 |
−2.7 e | −2.7 f | −2.7 f | ||||||
| Cervix uteri | 1975 - 1982 |
−4.3 e | 1982 - 1996 |
−1.6 e | 1996 - 2003 |
−3.7 e | 2003 - 2010 |
−1.1 e | −1.7 f | − 1.1 f |
||||
| Urinary bladder | 1975 - 1986 |
−1.7 e | 1986 - 2010 |
−0.4 e | −0.4 f | − 0.4 f |
||||||||
| Esophagus | 1975 - 2000 |
0.1 | 2000 - 2010 |
−1.5 e | −1.5 f | −1.5 f | ||||||||
| Oral cavity and pharynx |
1975 - 1990 |
−0.9 e | 1990 - 2005 |
−2.4 e | 2005 - 2010 |
−0.9 | −1.6 f | − 0.9 |
||||||
| Gallbladder | 1975 - 2002 |
−2.7 e | 2002 - 2010 |
−1.2 e | −1.3 f | − 1.2 f |
||||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total US, 1975 through 2010.
Joinpoint analyses with up to 5 joinpoints yielding up to 6 trend segments (Trends 1-6) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups:ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.0.3, April 2013; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is a weighted average of the APCs calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 U.S. standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, and 85 years; Census publication p25-1130).
The APC is statistically significantly different from zero (2-sided t test; P <.05).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2006 through 2010 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Cancer Incidence Rates (2006-2010) and Short-Term (2001-2010) Trends by Race and Ethnicity
Five-year (2006-2010) average annual age-adjusted incidence rates and short-term (2001-2010) trends were based on combined data from SEER and NPCR registries submitted to NAACCR (Table 3); these data were not adjusted for delayed reporting. For all cancer sites combined and all racial and ethnic groups, cancer incidence rates during 2006 through 2010 were higher in men (532.7 per 100,000 population) versus women (412.6). Black men had the highest overall cancer incidence rate (593.9) of any racial and ethnic group. Prostate and breast cancers were the most common cancers in each racial and ethnic group among men and women, respectively. Incident lung and colorectal cancers ranked second and third among men and women in all racial and ethnic groups except for Hispanic men, Hispanic women and API women, for whom rates of colorectal cancer were higher than lung cancer. Beyond these common cancers, cancer rankings varied by race and ethnicity.
Table 3.
Incidence rates for 2006–2010 and fixed-interval trends for 2001–2010 for the top 15 cancers by sex, race, and ethnicity, for areas in the United States with high-quality incidence dataa
| All races/ethnicities |
Whiteb | Blackb | APIb | AI/AN (CHSDA)b |
Hispanicb | Non-Hispanicb | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/cancer site or type |
R a n k |
Ratec | 2001– 2010 AAPC d |
2006– 2010 AAPC d |
R a n k |
Ratec | 2001– 2010 AAPC d |
R a n k |
Ratec | 2001– 2010 AAP Cd |
R a n k |
Ratec | 2001– 2010 AAPC d |
R a n k |
Ratec | 2001 – 2010 AA PCd |
R a n k |
Rate c |
2001– 2010 AAPC d |
R a n k |
Ratec | 2001– 2010 AAPC d |
| All sitese | ||||||||||||||||||||||
| Both sexes | 462.6 | −1.1 f | −1.6 f | 463.2 | −1.2 f | 471.7 | −0.9 f | 293.5 | −0.8 f | 393.8 | −0.5 | 361.7 | −1.4 f | 473.4 | −1.0 f | |||||||
| Males | 532.7 | −1.6 f | −2.5 f | 526.3 | −1.7 f | 593.9 | −1.8 f | 319.3 | −1.7 f | 435.1 | −0.8 | 419.2 | −2.0 f | 544.4 | −1.6 f | |||||||
| Females | 412.6 | −0.3 f | −0.3 f | 418.6 | −0.4 f | 390.6 | −0.1 | 278.4 | 0.0 | 367.2 | −0.2 | 325.1 | −0.8 f | 422.3 | −0.2 | |||||||
| Children (ages 0-14) |
15.9 | 0.5 f | 0.5 f | 16.5 | 0.4 | 12.2 | 1.1 f | 12.5 | −0.2 | 11.1 | −2.7 | 15.7 | 0.2 | 15.9 | 0.6 f | |||||||
| Children (ages 0-19) |
17.2 | 0.3 | 0.3 | 18.1 | 0.3 | 12.8 | 1.0 f | 13.2 | 0.2 | 12.3 | −2.5 f | 16.7 | 0.2 | 17.4 | 0.4 f | |||||||
| Malesg | ||||||||||||||||||||||
| Prostate | 1 | 146.6 | −3.6 f | −5.2 f | 1 | 136.6 | −4.0 f | 1 | 220.0 | −2.6 f | 1 | 75.0 | −3.9 f | 1 | 104.1 | −2.3 f | 1 | 124.2 | −3.1 f | 1 | 148.9 | −3.5 f |
| Lung and bronchus |
2 | 80.0 | −2.4 f | −3.2 f | 2 | 79.6 | −2.3 f | 2 | 94.7 | −2.7 f | 2 | 48.8 | −1.5 f | 2 | 70.2 | −1.4 | 3 | 45.9 | −2.7 f | 2 | 83.1 | −2.2 f |
| Colon and rectum |
3 | 51.7 | −3.8 f | −4.5 f | 3 | 50.5 | −4.0 f | 3 | 62.5 | −2.0 f | 3 | 40.8 | −2.8 f | 3 | 51.7 | −1.4 | 2 | 47.3 | −2.9 f | 3 | 52.2 | −3.8 f |
| Urinary bladder |
4 | 36.9 | −0.9 f | −1.7 f | 4 | 39.0 | −1.0 f | 5 | 19.0 | 0.0 | 6 | 15.5 | −0.7 | 5 | 18.3 | 0.1 | 4 | 20.8 | −1.7 f | 4 | 38.2 | −0.8 f |
| Melanoma of the skin |
5 | 24.7 | 1.6 f | 1.6 f | 5 | 27.5 | 1.6 f | 25 | 1.1 | −0.4 | 20 | 1.5 | −1.8 | 13 | 7.1 | 1.0 | 17 | 4.7 | −1.7 | 5 | 26.9 | 1.8 f |
| Non- Hodgkin lymphoma |
6 | 23.3 | −0.1 | −0.8 | 6 | 23.9 | −0.2 | 6 | 16.8 | −0.2 | 7 | 15.0 | −0.4 | 7 | 16.5 | −0.7 | 6 | 20.1 | −0.3 | 6 | 23.6 | 0.0 |
| Kidney and renal pelvis |
7 | 21.4 | 1.3 f | −0.2 | 7 | 21.5 | 1.4 f | 4 | 23.0 | 2.1 f | 9 | 10.6 | 3.1 f | 4 | 30.6 | 5.1 f | 5 | 20.5 | 1.2 f | 7 | 21.5 | 1.5 f |
| Oral cavity and pharynx |
8 | 16.5 | 0.2 | 0.2 | 9 | 16.8 | 0.5 f | 9 | 15.2 | −3.0 f | 8 | 10.7 | 0.1 | 8 | 14.0 | 2.1 | 11 | 10.6 | −1.4 f | 8 | 17.3 | 0.4 |
| Leukemia | 9 | 16.4 | −0.8 f | −0.8 f | 8 | 16.9 | −0.9 f | 1 2 |
12.4 | −0.8 f | 11 | 8.9 | −0.7 | 10 | 11.7 | −1.7 | 9 | 12.8 | −0.7 | 9 | 16.6 | −0.8 f |
| Pancreas | 10 | 13.7 | 0.7 f | 0.7 f | 10 | 13.6 | 0.7 f | 7 | 16.7 | 0.8 f | 10 | 9.7 | 0.1 | 11 | 10.8 | 0.4 | 10 | 12.0 | 0.2 | 10 | 13.9 | 0.8 f |
| Liver and intrahepatic bile duct |
11 | 10.8 | 3.9 f | 3.9 f | 11 | 9.6 | 4.1 f | 10 | 14.9 | 4.3 f | 4 | 21.3 | −0.9 | 6 | 17.8 | 4.4 f | 7 | 18.8 | 3.0 f | 11 | 10.1 | 3.8 f |
| Stomach | 12 | 9.4 | −1.7 f | −1.7 f | 13 | 8.4 | −1.8 f | 8 | 15.7 | −1.8 f | 5 | 15.6 | −3.3 f | 9 | 13.1 | −4.8 f | 8 | 13.9 | −2.0 f | 12 | 9.0 | −1.8 f |
| Esophagus | 13 | 8.5 | −1.0 f | −2.3 f | 12 | 8.6 | −0.5 | 14 | 8.7 | −5.0 f | 15 | 3.9 | −0.9 | 12 | 7.2 | −2.2 | 15 | 5.4 | −1.6 f | 13 | 8.7 | −0.8 |
| Brain and other nervous system |
14 | 7.8 | −0.6 f | −1.2 f | 14 | 8.4 | −0.4 f | 15 | 4.6 | −0.5 | 13 | 4.3 | −0.9 | 16 | 5.3 | −0.9 | 13 | 6.0 | −1.2 f | 14 | 8.1 | −0.3 |
| Myeloma | 15 | 7.4 | 0.1 | 0.1 | 15 | 6.8 | −0.2 | 11 | 13.9 | 0.2 | 14 | 4.3 | 1.9 f | 15 | 6.0 | −4.9 f | 12 | 7.0 | −0.5 | 15 | 7.4 | 0.1 |
| Larynx | 16 | 6.6 | −2.4 f | −2.4 f | 17 | 6.4 | −2.5 f | 13 | 9.9 | −3.3 f | 18 | 2.3 | −1.9 | 14 | 6.2 | −0.3 | 14 | 5.6 | −3.0 f | 16 | 6.8 | −2.3 f |
| Thyroid | 17 | 6.3 | 6.3 f | 6.3 f | 16 | 6.7 | 6.4 f | 18 | 3.3 | 5.4 f | 12 | 5.5 | 5.2 f | 20 | 3.3 | 3.8 | 16 | 4.7 | 4.8 f | 17 | 6.6 | 6.5 f |
| Femalesg | ||||||||||||||||||||||
| Breast | 1 | 122.2 | −1.0 | 0.0 | 1 | 123.5 | −1.1 | 1 | 118.4 | 0.5 f | 1 | 84.7 | 0.4 | 1 | 90.3 | −0.5 | 1 | 91.1 | −0.6 | 1 | 125.6 | −0.9 |
| Lung and bronchus |
2 | 55.1 | −0.7 f | −1.8 f | 2 | 56.8 | −0.6 f | 2 | 50.4 | −0.1 | 3 | 28.0 | 0.0 | 2 | 52.1 | −0.3 | 3 | 26.6 | −1.0 f | 2 | 57.7 | −0.5 f |
| Colon and rectum |
3 | 39.1 | −3.2 f | −4.0 f | 3 | 38.0 | −3.3 f | 3 | 46.7 | −2.9 f | 2 | 31.0 | −2.2 f | 3 | 42.7 | −1.3 f | 2 | 32.6 | −2.8 f | 3 | 39.7 | −3.1 f |
| Corpus and uterus, NOS |
4 | 24.6 | 0.4 | 1.1 f | 4 | 25.0 | 0.3 | 4 | 23.0 | 2.0 f | 5 | 16.9 | 2.3 f | 4 | 22.4 | 1.8 | 4 | 20.1 | 1.0 f | 4 | 25.0 | 0.4 |
| Thyroid | 5 | 18.5 | 6.4 f | 5.1 f | 5 | 19.6 | 6.4 f | 8 | 11.2 | 6.1 f | 4 | 18.5 | 6.0 f | 7 | 11.6 | 3.3 f | 5 | 17.4 | 6.0 f | 5 | 18.9 | 6.4 f |
| Non- Hodgkin lymphoma |
6 | 16.3 | −0.2 | −0.9 f | 7 | 16.8 | −0.3 | 7 | 11.7 | 0.1 | 6 | 10.4 | −0.7 | 6 | 14.2 | −1.0 | 6 | 15.2 | −0.2 | 7 | 16.4 | −0.2 |
| Melanoma of the skin |
7 | 15.6 | 1.4 f | 1.4 f | 6 | 18.0 | 1.6 f | 27 | 1.0 | −1.0 | 22 | 1.1 | −2.2 | 14 | 5.5 | 0.1 | 18 | 4.0 | −1.5 | 6 | 17.3 | 1.8 f |
| Ovary | 8 | 12.3 | −2.2 f | −2.9 f | 8 | 12.8 | −2.3 f | 11 | 9.4 | −1.5 f | 8 | 9.0 | −1.6 f | 8 | 11.6 | −2.0 | 9 | 10.9 | −2.1 f | 8 | 12.4 | −2.2 f |
| Kidney and renal pelvis |
9 | 11.2 | 1.6 f | −0.1 | 9 | 11.3 | 1.9 f | 6 | 12.2 | 3.0 f | 13 | 5.1 | 2.0 | 5 | 17.5 | 2.1 | 7 | 11.5 | 1.5 f | 9 | 11.2 | 1.7 f |
| Pancreas | 10 | 10.7 | 0.7 f | 0.1 | 10 | 10.4 | 0.7 f | 5 | 13.9 | 0.4 | 9 | 8.3 | 0.8 | 9 | 10.0 | −0.5 | 10 | 10.2 | 0.2 | 10 | 10.8 | 0.8 f |
| Leukemia | 11 | 10.0 | −0.5 f | −0.5 f | 11 | 10.3 | −0.5 f | 13 | 7.8 | −0.9 f | 12 | 6.0 | 0.7 | 12 | 8.0 | −0.1 | 11 | 8.8 | −0.5 | 11 | 10.0 | −0.5 f |
| Urinary bladder |
12 | 9.1 | −1.2 f | −1.2 f | 12 | 9.6 | −1.3 f | 14 | 6.6 | −0.9 | 15 | 3.8 | −1.0 | 17 | 4.7 | −1.6 | 14 | 5.2 | −2.4 f | 12 | 9.5 | −0.9 |
| Cervix uteri | 13 | 8.0 | −1.9 f | −1.2 f | 13 | 7.7 | −1.5 f | 9 | 10.3 | −2.9 f | 11 | 6.7 | −3.5 f | 10 | 9.7 | 0.4 | 8 | 10.9 | −4.2 f | 13 | 7.6 | −1.6 f |
| Oral cavity and pharynx |
14 | 6.2 | 0.0 | 0.0 | 14 | 6.3 | 0.3 | 15 | 5.2 | −1.4 f | 14 | 4.8 | −1.7 f | 15 | 5.0 | −4.1 | 17 | 4.2 | 0.2 | 14 | 6.5 | 0.1 |
| Brain and other nervous system |
15 | 5.7 | −0.5 | −1.6 f | 15 | 6.1 | −0.5 | 17 | 3.6 | −0.4 | 16 | 3.0 | −0.8 | 18 | 4.2 | −0.7 | 16 | 4.7 | −1.2 f | 15 | 5.8 | −0.3 |
| Myeloma | 16 | 4.8 | 0.0 | 0.0 | 16 | 4.2 | −0.4 f | 10 | 10.2 | 0.5 | 17 | 2.9 | 0.1 | 16 | 4.9 | −4.2 | 15 | 4.9 | −1.5 f | 16 | 4.8 | 0.1 |
| Stomach | 17 | 4.7 | −1.1 f | −1.1 f | 17 | 4.0 | −1.4 f | 12 | 8.1 | −1.5 f | 7 | 9.0 | −2.8 f | 13 | 6.9 | −2.6 | 12 | 8.2 | −2.1 f | 17 | 4.3 | −1.3 f |
| Liver and intrahepatic bile duct |
18 | 3.7 | 3.4 f | 3.4 f | 18 | 3.3 | 3.5 f | 16 | 4.4 | 3.5 f | 10 | 8.0 | −0.6 | 11 | 8.0 | 2.5 | 13 | 6.9 | 2.1 f | 18 | 3.4 | 3.3 f |
Abbreviations: API, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native; IHS, Indian Health Service; CHSDA, IHS Contract Health Services Delivery Area; AAPC, average annual percent change; NOS, not otherwise specified; NPCR, National Program of Cancer Registries; SEER, Surveillance, Epidemiology, and End Results; NAACCR, North American Association of Central Cancer Registries; APC, annual percent change.
Source: NPCR and SEER areas reported by NAACCR as meeting high-quality incidence data standards for the specified time periods.
White, black, API, and AI/AN (CHSDA 2012 counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive. AI/AN (CHSDA 2012) statistics exclude data from Kansas.
Rates are per 100,000 persons and were age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000)
AAPC is a weighted average of the APCs that is calculated by joinpoint regression over the time period 2001–2010 unless otherwise noted. Joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.0.3, April 2013; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analysis. Ovary excludes borderline tumors.
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Cancers are listed in descending rank order according to sex-specific, age-adjusted rates for 2006 through 2010 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
2006-2010 rates for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (46 states): Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, Wyoming.
2001-2010 AAPCs for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (42 states): Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, Wyoming.
Cancer incidence rates decreased from 2001 through 2010 in men of every racial and ethnic group although the decrease was not significant for AI/AN men. Cancer incidence rates decreased in white and Hispanic women but were stable among women of other racial and ethnic groups. For children aged 0 through 19 years, cancer incidence rates increased in blacks, decreased in AI/ANs and were stable for all other racial and ethnic groups. In men, incidence rates of the most common cancers (prostate, lung and colorectal) decreased from 2001 to 2010, although the decreases in lung and colorectal cancers were not significant for AI/AN men. In women, breast cancer incidence rates increased among black women but were stable for all other racial and ethnic groups. Lung and colorectal cancer incidence rates decreased among women of all racial and ethnic groups combined, although lung cancer incidence rates were stable in black, API, and AI/AN women. Melanoma incidence rates increased only in white men and women. Liver cancer incidence rates increased among men and women in every racial and ethnic group except API men and women and AI/AN women. Incidence rates of myeloma increased in API men, whereas they decreased in AI/AN men and white and Hispanic women and were stable in all other groups.
Current Cancer Death Rates (2006-2010) and Short-Term (2001-2010) Trends by Race and Ethnicity
For all cancer sites combined and all racial and ethnic groups, cancer death rates for 2006 through 2010 were higher in men (215.3 deaths per 100,000 population, Table 4) than women (149.7). Black men had the highest cancer death rate (276.6) of any racial or ethnic group. Lung, prostate and colorectal cancers were the three leading causes of cancer death for men in every racial and ethnic group except API, for whom liver cancer ranked second. For most women, the leading causes of cancer death were lung, breast and colorectal. However, among Hispanic women breast cancer was the leading cause of cancer death.
Table 4.
US Cancer Death Rates for 2006-2010 and Fixed-Interval Trends From 2001 to 2010 for the Top Cancers by Sex, Race, and Ethnicitya
| All Racial and Ethnic Groups Combined |
Whiteb |
Blackb |
APIb |
AI/AN (CHSDA Counties)b |
Hispanicb,c |
Non-Hispanicb,c |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Cancer Site or Typed |
Ra nk |
Ratee | 2001- 2010 AAP Cf |
2006- 2010 AAPCf |
Ran k |
Ratee | 2001- 2010 AAP Cf |
Ra nk |
Ratee | 2001- 2010 AAPC f |
Ra nk |
Ratee | 2001- 2010 AAP Cf |
Ran k |
Ratee | 2001- 2010 AAP Cf |
Ra nk |
Ratee | 2001- 2010 AAP Cf |
Ra nk |
Ratee | 200 1- 201 0 AA PCf |
| All Sites | ||||||||||||||||||||||
| Both Sexes | 176.4 | −1.5 g | −1.5 g | 175.8 | −1.4 g | 210.3 | −2.1 g | 108.8 | −1.2 g | 160.4 | −0.7 g | 121.9 | −1.4 g | 180.7 | −1.5 g | |||||||
| Men | 215.3 | −1.8 g | −1.8 g | 213.1 | −1.7 g | 276.6 | −2.6 g | 132.4 | −1.3 g | 191.0 | −0.4 | 152.2 | −1.6 g | 219.9 | −1.7 g | |||||||
| Women | 149.7 | −1.4 g | −1.4 g | 149.8 | −1.3 g | 171.2 | −1.7 g | 92.1 | −1.0 g | 139.0 | −1.1 g | 101.3 | −1.2 g | 153.7 | −1.3 g | |||||||
| Children (ages 0-14) |
2.2 | −2.0 g | −2.0 g | 2.3 | −1.9 g | 2.1 | −2.3 g | 1.9 | −0.8 | 1.6 | h | 2.3 | −2.3 g | 2.2 | −2.0 g | |||||||
| Children (ages 0-19) |
2.4 | −2.2 g | −2.2 g | 2.5 | −2.2 g | 2.3 | −2.3 g | 2.1 | −0.5 | 1.8 | −3.0 | 2.5 | −2.5 g | 2.4 | −2.2 g | |||||||
| Top 17 cancers for mend |
||||||||||||||||||||||
| Lung and Bronchus |
1 | 63.5 | −2.5 g | −2.8 g | 1 | 63.2 | −2.4 g | 1 | 78.5 | −3.3 g | 1 | 35.5 | −1.6 g | 1 | 49.6 | −0.5 | 1 | 31.3 | −2.8 g | 1 | 66.0 | −2.3 g |
| Prostate | 2 | 23.0 | −3.4 g | −3.4 g | 2 | 21.2 | −3.3 g | 2 | 50.9 | −3.8 g | 4 | 10.1 | −2.3 g | 2 | 20.7 | −1.4 | 2 | 19.2 | −3.0 g | 2 | 23.2 | −3.3 g |
| Colon and Rectum |
3 | 19.6 | −3.0 g | −3.0 g | 3 | 19.1 | −3.1 g | 3 | 28.7 | −2.4 g | 3 | 13.1 | −2.3 g | 3 | 18.7 | −1.5 | 3 | 16.1 | −1.4 g | 3 | 20.0 | −3.0 g |
| Pancreas | 4 | 12.5 | 0.4 g | 0.4 g | 4 | 12.5 | 0.5 g | 4 | 15.3 | 0.0 | 6 | 8.3 | 0.5 | 5 | 10.1 | 5.7 | 5 | 9.6 | 0.5 | 4 | 12.8 | 0.5 g |
| Leukemia | 5 | 9.5 | −1.0 g | −1.0 g | 5 | 9.8 | −0.9 g | 7 | 8.2 | −1.3 g | 8 | 5.0 | −0.2 | 8 | 7.0 | 3.7 g | 8 | 6.1 | −1.1 g | 5 | 9.7 | −0.9 g |
| Liver and Intrahepatic Bile Duct |
6 | 8.3 | 2.5 g | 2.5 g | 9 | 7.6 | 2.6 g | 5 | 11.8 | 3.0 g | 2 | 14.4 | −0.5 | 4 | 13.2 | 3.3 | 4 | 12.3 | 1.9 g | 7 | 8.0 | 2.5 g |
| Non- Hodgkin Lymphoma |
7 | 8.2 | −2.6 g | −2.6 g | 6 | 8.5 | −2.6 g | 10 | 5.9 | −2.0 g | 7 | 5.2 | −2.0 g | 10 | 5.4 | −1.8 | 7 | 6.5 | −1.4 g | 6 | 8.3 | −2.6 g |
| Urinary Bladder |
8 | 7.7 | 0.1 | 0.1 | 7 | 8.1 | 0.2 | 12 | 5.5 | 0.0 | 12 | 2.8 | −1.0 | 11 | 4.1 | h | 11 | 4.0 | −1.1 | 8 | 8.0 | 0.3 g |
| Esophagus | 9 | 7.6 | −0.5 g | −0.5 g | 8 | 7.8 | 0.1 | 9 | 7.7 | −4.6 g | 9 | 3.1 | −1.1 | 9 | 6.1 | −2.4 | 10 | 4.3 | −0.3 | 9 | 7.9 | −0.4 g |
| Kidney and Renal Pelvis |
10 | 5.8 | −1.0 g | −1.0 g | 10 | 5.9 | −0.9 g | 11 | 5.7 | −1.3 g | 11 | 3.0 | 3.3 g | 6 | 9.5 | 0.1 | 9 | 5.1 | −1.5 | 10 | 5.8 | −0.9 g |
| Brain and Other Nervous System |
11 | 5.2 | −0.5 | 0.5 | 11 | 5.6 | −0.4 | 15 | 3.0 | −0.8 | 13 | 2.3 | −1.5 | 14 | 2.8 | 1.0 | 13 | 3.3 | −0.1 | 11 | 5.4 | −0.5 |
| Stomach | 12 | 4.9 | −3.2 g | −3.2 g | 13 | 4.2 | −3.4 g | 6 | 9.8 | −3.1 g | 5 | 8.7 | −3.1 g | 7 | 8.1 | −5.7 g | 6 | 7.6 | −2.9 g | 12 | 4.6 | −3.4 g |
| Myeloma | 13 | 4.3 | −1.4 g | −1.4 g | 14 | 4.0 | −1.5 g | 8 | 7.9 | −1.3 g | 14 | 2.3 | 3.2 g | 12 | 3.6 | −3.6 g | 12 | 3.5 | −1.9 | 14 | 4.3 | −1.3 g |
| Melanoma of the Skin |
14 | 4.1 | 0.9 g | 0.9 g | 12 | 4.6 | 1.0 g | 22 | 0.5 | 1.9 | 20 | 0.4 | h | 16 | 1.7 | h | 17 | 1.1 | 2.2 | 13 | 4.4 | 1.0 g |
| Oral Cavity and Pharynx |
15 | 3.8 | −1.3 g | −1.3 g | 15 | 3.6 | −0.8 g | 13 | 5.2 | −3.7 g | 10 | 3.0 | −2.6 g | 13 | 3.4 | −3.4 | 14 | 2.5 | −1.9 g | 15 | 3.9 | −1.1 g |
| Larynx | 16 | 2.0 | −2.7 g | −2.7 g | 16 | 1.9 | −2.4 g | 14 | 3.9 | −4.0 g | 16 | 0.8 | −2.6 | 15 | 2.1 | h | 15 | 1.7 | −2.7 g | 16 | 2.1 | −2.6 g |
| Soft Tissue including Heart |
17 | 1.5 | 1.0 g | 1.0 g | 18 | 1.5 | 1.2 g | 16 | 1.4 | −0.8 | 15 | 0.9 | 1.2 | 17 | 1.3 | h | 16 | 1.1 | 1.4 | 18 | 1.5 | 1.0 g |
| Top 18 cancers for womend |
||||||||||||||||||||||
| Lung and Bronchus |
1 | 39.2 | −0.9 g | −1.5 g | 1 | 40.4 | −0.9 g | 1 | 37.2 | −1.0 g | 1 | 18.4 | −0.5 | 1 | 33.1 | −0.8 | 2 | 14.1 | −1.1 g | 1 | 41.3 | −0.8 g |
| Breast | 2 | 22.6 | −2.0 g | −2.0 g | 2 | 22.1 | −2.0 g | 2 | 30.8 | −1.6 g | 2 | 11.5 | −1.7 g | 2 | 15.5 | 1.3 | 1 | 14.8 | −1.5 g | 2 | 23.3 | −1.8 g |
| Colon and Rectum |
3 | 13.9 | −3.0 g | −3.0 g | 3 | 13.4 | −3.0 g | 3 | 19.0 | −3.3 g | 3 | 9.7 | −1.6 g | 3 | 15.4 | 0.4 | 3 | 10.2 | −2.1 g | 3 | 14.1 | −2.9 g |
| Pancreas | 4 | 9.6 | 0.4 g | 0.4 g | 4 | 9.4 | 0.6 g | 4 | 12.5 | −0.4 | 4 | 7.1 | 0.5 | 4 | 8.6 | 0.5 | 4 | 7.8 | 0.1 | 4 | 9.7 | 0.5 g |
| Ovary | 5 | 8.1 | −1.8 g | −1.8 g | 5 | 8.4 | −1.8 g | 6 | 6.7 | −1.2 | 7 | 4.8 | −0.6 | 5 | 7.1 | −2.3 | 5 | 5.8 | −1.2 g | 5 | 8.3 | −1.8 g |
| Leukemia | 6 | 5.3 | −1.3 g | −1.0 g | 6 | 5.5 | −1.2 g | 8 | 4.8 | −1.9 g | 9 | 3.1 | 0.7 | 11 | 3.3 | −3.4 | 9 | 4.0 | −0.6 | 6 | 5.4 | −1.3 g |
| Non- Hodgkin Lymphoma |
7 | 5.1 | −3.1 g | −2.8 g | 7 | 5.3 | −3.1 g | 12 | 3.6 | −3.0 g | 8 | 3.4 | −2.0 g | 8 | 4.3 | −3.8 | 7 | 4.4 | −1.5 g | 7 | 5.2 | −3.1 g |
| Corpus and Uterus, NOS |
8 | 4.3 | 0.5 | 0.5 | 8 | 4.0 | 0.4 | 5 | 7.4 | 0.7 g | 10 | 2.6 | 2.3 g | 12 | 3.2 | h | 10 | 3.3 | 0.6 | 8 | 4.3 | 0.5 g |
| Brain and Other Nervous System |
9 | 3.5 | −0.5 g | −0.5 g | 9 | 3.8 | −0.4 | 16 | 2.1 | −0.2 | 12 | 1.6 | 3.8 | 14 | 2.3 | h | 12 | 2.4 | −0.4 | 9 | 3.6 | −0.4 |
| Liver and Intrahepatic Bile Duct |
10 | 3.4 | 2.0 g | 2.2 g | 10 | 3.2 | 1.9 g | 11 | 4.1 | 0.8 | 5 | 6.0 | −1.5 | 6 | 6.1 | −1.1 | 6 | 5.4 | 0.9 g | 10 | 3.2 | 1.6 g |
| Myeloma | 11 | 2.7 | −2.3 g | −2.3 g | 12 | 2.5 | −2.3 g | 7 | 5.4 | −2.2 g | 13 | 1.3 | −2.5 | 13 | 2.4 | −6.2 | 13 | 2.3 | −2.3 g | 11 | 2.7 | −2.2 g |
| Kidney and Renal Pelvis |
12 | 2.6 | −1.3 g | −1.3 g | 11 | 2.6 | −1.0 g | 14 | 2.6 | −1.1 g | 14 | 1.2 | −0.3 | 7 | 4.4 | −0.7 | 14 | 2.3 | −0.4 | 12 | 2.6 | −1.3 g |
| Stomach | 13 | 2.5 | −2.7 g | −2.7 g | 14 | 2.2 | −2.7 g | 9 | 4.7 | −3.5 g | 6 | 5.1 | −3.7 g | 9 | 3.8 | −6.0 g | 8 | 4.4 | −2.6 g | 13 | 2.4 | −3.0 g |
| Cervix Uteri | 14 | 2.4 | −1.8 g | −1.8 g | 15 | 2.2 | −1.3 g | 10 | 4.2 | −2.4 g | 11 | 1.9 | −4.6 g | 10 | 3.5 | −0.4 | 11 | 2.9 | −2.9 g | 14 | 2.3 | −1.8 g |
| Urinary Bladder |
15 | 2.2 | −0.6 g | −0.6 g | 13 | 2.2 | −0.4 | 13 | 2.6 | −1.9 g | 16 | 0.9 | −1.1 | 18 | 1.2 | h | 16 | 1.3 | −1.9 | 15 | 2.3 | −0.5 |
| Esophagus | 17 | 1.6 | −1.5 g | −1.5 g | 17 | 1.6 | −0.9 g | 15 | 2.1 | −5.2 g | 18 | 0.8 | 0.1 | 16 | 1.6 | h | 18 | 0.8 | −2.6 g | 17 | 1.7 | −1.4 g |
| Oral Cavity and Pharynx |
18 | 1.4 | −1.5 g | −1.5 g | 18 | 1.4 | −1.3 g | 17 | 1.4 | −3.2 g | 15 | 1.2 | −2.4 | 17 | 1.5 | h | 19 | 0.8 | −1.6 | 18 | 1.4 | −1.4 g |
| Gallbladder | 20 | 0.8 | −1.4 g | −1.4 g | 20 | 0.7 | −1.6 g | 19 | 1.0 | −0.8 | 20 | 0.8 | −0.3 | 15 | 2.0 | −5.2 | 15 | 1.3 | −1.6 | 20 | 0.7 | −1.5 g |
Abbreviations: AAPC, average annual percent change; API, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total US, 1975-2010.
White, black, API, and AI/AN (CHSDA counties) populations include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Data for Hispanic and non-Hispanic exclude the District of Columbia, Minnesota, New Hampshire, North Dakota, and South Carolina.
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2006 to 2010 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Rates are per 100,000 persons and are age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, 85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000).
The AAPC is a weighted average of the annual percent change and is calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, 85 years; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.0.3, April 2013; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
The statistic could not be calculated. The average annual percent change is based on <10 cases for at least 1 year within the time interval.
Declines in cancer death rates from 2001 through 2010 were seen for men, women, and children in all racial and ethnic groups, although the decline was not statistically significant in API and AI/AN children (Table 4). Death rates declined for the most common cancers (lung, prostate, and colorectal) among men of all racial and ethnic groups during 2001 through 2010, although the declines were not statistically significant for AI/AN men. Death rates declined for the most common cancers (lung, breast, and colorectal) among women of most racial and ethnic groups, except for API women (in whom lung cancer rates were stable), and AI/AN women (stable for all three cancer sites). Death rates for liver cancer increased in white, Hispanic, and black men and in white and Hispanic women during 2001 through 2010. Pancreatic cancer death rates increased in white men and women. In addition, death rates for melanoma and soft tissue cancer increased in white men, kidney cancer in API men, leukemia in AI/AN men, and cancer of the uterus in black and API women.
Comorbidity Prevalence among Older Cancer Patients and the Medicare Population
Table 5 shows the prevalence of selected comorbid conditions among cancer patients for each of the four most common cancer sites, for all cancers combined and for the non-cancer control cohort of Medicare beneficiaries. The prevalence of comorbidity was similar among cancer-free Medicare beneficiaries (31.8%), breast cancer patients (32.2%), and prostate cancer patients (30.5%), highest among lung cancer patients (52.8%), and intermediate among colorectal cancer patients (40.7%). The most common conditions among cancer patients were diabetes (16.0%), COPD (15.5%), congestive heart failure (9.7%) and cerebrovascular disease (6.0%). Female breast and prostate cancer patients and the cohort of cancer-free patients were more likely than colorectal or lung cancer patients to have no comorbidity. Lung cancer patients had the highest prevalence of comorbidities and the most prevalent comorbidity was COPD (33.6%). The prevalence of congestive heart failure was high in lung cancer patients (12.4%) and colorectal cancer patients (11.6%) relative to the cancer-free cohort (6.9%). The prevalence of diabetes was high among colorectal cancer patients (17.2%). Demographic characteristics for cancer patients and the cancer-free cohort are shown in Table 6.
Table 5.
Prevalence of selected comorbidities for cancer patients, aged 66 years or older, diagnosed between 1992-2005 with the four most common cancers, all cancers combined and for people without cancera
| All cancers | Breast (female) | Colorectal | Lung | Prostate |
People
without cancer b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=1,056,534 |
N=123,680 |
N=137,536 |
N=166,053 |
N=213,311 |
N=100,000 |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Comorbidity | ||||||||||||
| Diabetes | 168,639 | 16.0 | 17,909 | 14.5 | 23,685 | 17.2 | 24,418 | 14.7 | 28,005 | 13.1 | 13,928 | 13.9 |
| Chronic obstructive pulmonary disease | 163,938 | 15.5 | 11,802 | 9.5 | 17,744 | 12.9 | 55,724 | 33.6 | 20,801 | 9.8 | 8,980 | 9.0 |
| Congestive heart failure | 102,049 | 9.7 | 8,576 | 6.9 | 15,908 | 11.6 | 20,502 | 12.4 | 12,065 | 5.7 | 6,864 | 6.9 |
| Cerebrovascular disease | 63,149 | 6.0 | 5,718 | 4.6 | 8,942 | 6.5 | 11,932 | 7.2 | 9,403 | 4.4 | 5,392 | 5.4 |
| Peripheral vascular disease | 45,436 | 4.3 | 3,306 | 2.7 | 5,767 | 4.2 | 11,350 | 6.8 | 6,229 | 2.9 | 3,440 | 3.4 |
| Chronic renal failure | 21,807 | 2.1 | 1,455 | 1.2 | 2,725 | 2.0 | 3,592 | 2.2 | 3,350 | 1.6 | 1,612 | 1.6 |
| Rheumathologic disease | 21,302 | 2.0 | 2,687 | 2.2 | 2,464 | 1.8 | 4,270 | 2.6 | 2,382 | 1.1 | 1,685 | 1.7 |
| History myocardial infarction | 21,347 | 2.0 | 1,286 | 1.0 | 2,856 | 2.1 | 4,382 | 2.6 | 3,777 | 1.8 | 1,641 | 1.6 |
| Ulcer disease | 19,489 | 1.8 | 1,245 | 1.0 | 2,911 | 2.1 | 3,246 | 2.0 | 2,428 | 1.1 | 1,223 | 1.2 |
| Dementia | 15,839 | 1.5 | 1,731 | 1.4 | 2,804 | 2.0 | 2,429 | 1.5 | 1,619 | 0.8 | 1,784 | 1.8 |
| Acute myocardial infarction | 13,898 | 1.3 | 931 | 0.8 | 2,280 | 1.7 | 2,628 | 1.6 | 2,248 | 1.1 | 1,088 | 1.1 |
| Paralysis | 7,764 | 0.7 | 638 | 0.5 | 1,176 | 0.9 | 1,325 | 0.8 | 1,052 | 0.5 | 674 | 0.7 |
| Various cirrhodites | 6,208 | 0.6 | 365 | 0.3 | 513 | 0.4 | 799 | 0.5 | 442 | 0.2 | 239 | 0.2 |
| Liver disease moderate/severe | 2,109 | 0.2 | 99 | 0.1 | 167 | 0.1 | 206 | 0.1 | 128 | 0.06 | 88 | 0.1 |
| Acquired immunodeficiency syndrome | 270 | 0.03 | 8 | 0.01 | 18 | 0.01 | 56 | 0.03 | 50 | 0.02 | 21 | 0.02 |
| No comorbidity | 631,457 | 59.8 | 83,791 | 67.8 | 81,559 | 59.3 | 78,301 | 47.1 | 148,170 | 69.5 | 68,231 | 68.2 |
| Only 1 condition | 266,928 | 25.2 | 27,766 | 22.4 | 34,579 | 25.1 | 50,931 | 30.7 | 45,627 | 21.4 | 20,930 | 20.9 |
| 2 or more conditions | 158,149 | 15.0 | 12,123 | 9.8 | 21,398 | 15.6 | 36,821 | 22.2 | 19,514 | 9.1 | 10,839 | 10.9 |
Source: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound). and 5% random sample of cancer-free Medicare beneficiaries.
Random sample of 100,000 persons (controls) chosen by frequency matching to all sites combined cancer cohort by calendar year, age and sex. Controls can be sampled only once in a calendar year, but can be sampled repeatedly across multiple years.
Table 6.
Demographic characteristics for cancer patients diagnosed between 1992-2005 with the four most common cancers, all cancers combined and for people without cancera
| All cancers | Breast (female) | Colorectal | Lung | Prostate |
People
without cancer b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=1108085 |
N=140873 |
N=135745 |
N=165345 |
N=200332 |
N=100000 |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Age | ||||||||||||
| 66-74 | 479,409 | 45.4 | 56,110 | 45.4 | 51,349 | 37.3 | 80,304 | 48.4 | 116,236 | 54.5 | 45,382 | 45.4 |
| 75-84 | 436,637 | 41.3 | 50,785 | 41.1 | 60,075 | 43.7 | 69,483 | 41.8 | 81,033 | 38.0 | 41,323 | 41.3 |
| 85+ | 140,488 | 13.3 | 16,785 | 13.6 | 26,112 | 19.0 | 16,266 | 9.8 | 16,042 | 7.5 | 13,295 | 13.3 |
| Sex | ||||||||||||
| Female | 490,430 | 46.4 | 123,680 | 100.0 | 74,873 | 54.4 | 77,249 | 46.5 | 46,414 | 46.4 | ||
| Male | 566,104 | 53.6 | 62,663 | 45.6 | 88,804 | 53.5 | 213,311 | 100.0 | 53,586 | 53.6 | ||
| Race | ||||||||||||
| Unknown | 3,609 | 0.3 | 314 | 0.3 | 491 | 0.4 | 719 | 0.4 | 441 | 0.2 | 242 | 0.2 |
| White | 928,230 | 87.9 | 111,449 | 90.1 | 120,220 | 87.4 | 145,548 | 87.7 | 182,425 | 85.5 | 87,551 | 87.6 |
| Black | 77,066 | 7.3 | 7,698 | 6.2 | 10,138 | 7.4 | 12,433 | 7.5 | 20,788 | 9.8 | 6,770 | 6.8 |
| Other | 47,629 | 4.5 | 4,219 | 3.4 | 6,687 | 4.9 | 7,353 | 4.4 | 9,657 | 4.5 | 5,437 | 5.4 |
Abbreviatons: SEER, Surveillance, Epidemiology, and End Results.
Source: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound). and 5% random sample of cancer-free Medicare beneficiaries.
Random sample of 100,000 persons (controls) chosen by frequency matching to all sites combined cancer cohort by calendar year, age and sex. Controls can be sampled only once in a calendar year, but can be sampled repeatedly across multiple years.
Survival Measures Considering Competing Risks of Death by Comorbidity Level
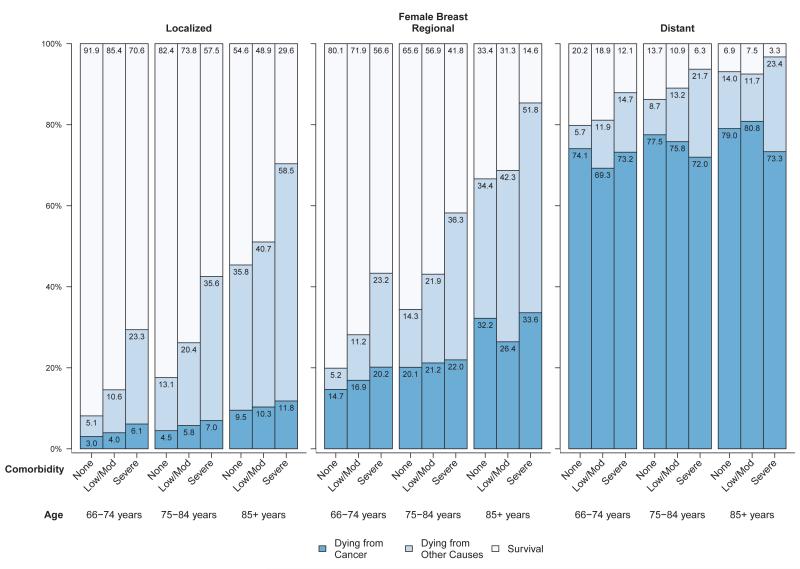
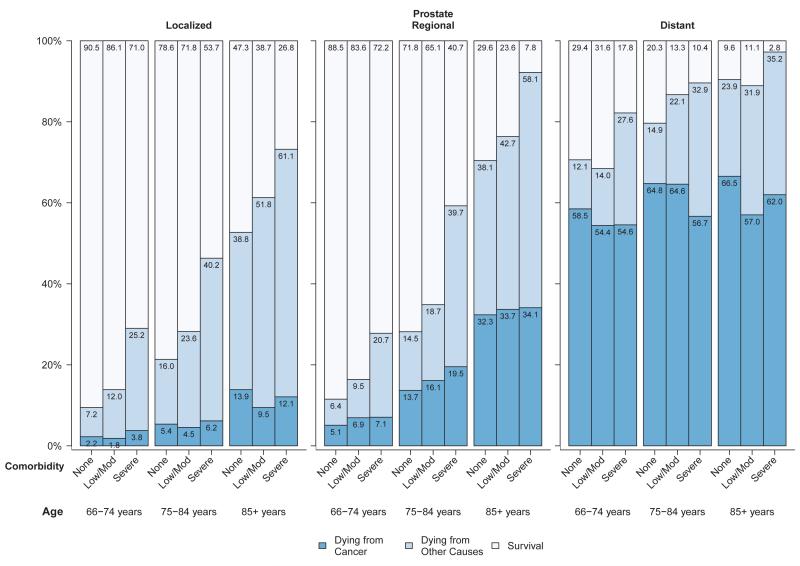
Figures 1-4 present the probability of dying from cancer, dying from causes other than cancer, and survival in the five years after diagnosis for each of the four major cancer sites stratified by stage and within each stage by age and comorbidity level. The cancer survival data are based on the survival experience of patients diagnosed with cancer from 1999 through 2005 and thus reflect the probability of survival given treatment patterns prevalent at the time, not the probability of survival in the absence of treatment or survival given a particular treatment. Among women diagnosed with breast cancer at a localized stage, the probability of dying from cancer was much lower than the probability of dying from non-cancer causes, and both age and comorbidity level were predictive of overall survival (Figure 1). For women diagnosed with breast cancer at regional stage, comorbidity and age were associated with overall survival. However, in women diagnosed with breast cancer at distant stage, about 69% or more died from cancer five years after diagnosis, in all age and comorbidity strata. Among men diagnosed with prostate cancer, probabilities of dying from cancer and non-cancer and survival by stage, age and comorbidity level were generally similar to those of breast cancer patients. Between 2% and 14% of men diagnosed with prostate cancer at a localized stage died from their cancer, in all age and comorbidity strata (Figure 2). In men diagnosed with prostate cancer at regional stage, especially those aged 75-84 years, the probability of both cancer and non-cancer death increased with comorbidity level. Men diagnosed with prostate cancer at distant stage had a high (>54%) probability of cancer death regardless of age and comorbidity level.
Figure 1.
Probability of dying from cancer, dying from other causes and survival stratified by stage and comorbidity status among women with breast cancer diagnosed between 1999 and 2005a
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
aSource: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound).
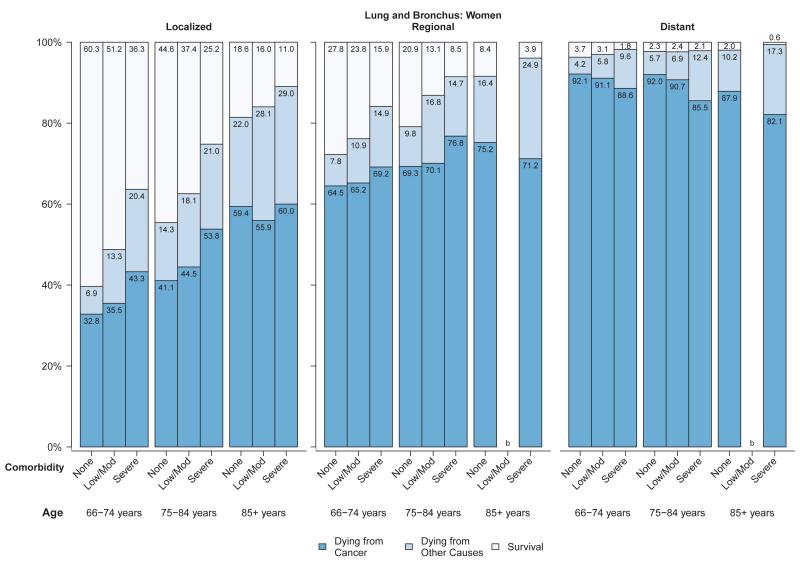
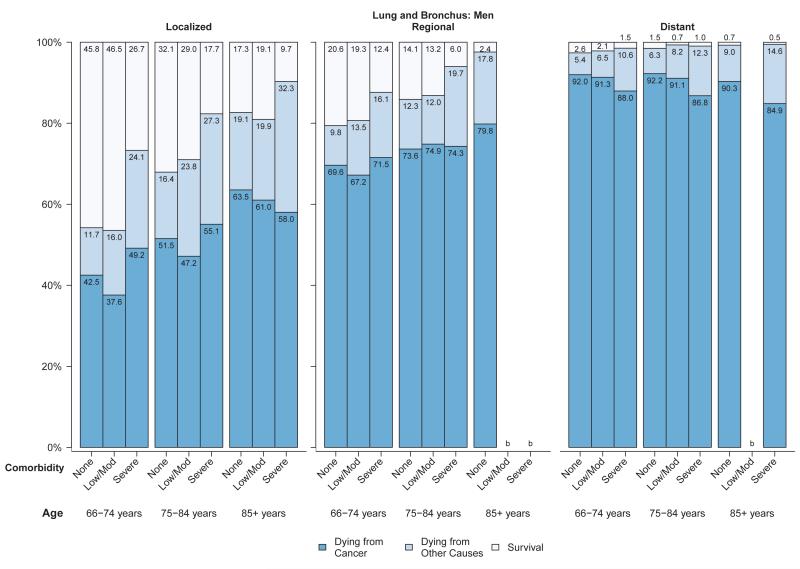
Figure 4.
a: Probabilities of dying from cancer, dying from other causes and survival stratified by selected stage and comorbidity status among women with lung and bronchus cancer diagnosed between 1999 and 2005a
b: Probabilities of dying from cancer, dying from other causes and survival stratified by selected stage and comorbidity status among men with lung and bronchus cancer diagnosed between 1999 and 2005a
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
aSource: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound).
bThe statistic could not be calculated.
Figure 2.
Probability of dying from cancer, dying from other causes and survival stratified by stage and comorbidity status among men with prostate cancer diagnosed between 1999 and 2005a
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
aSource: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound).
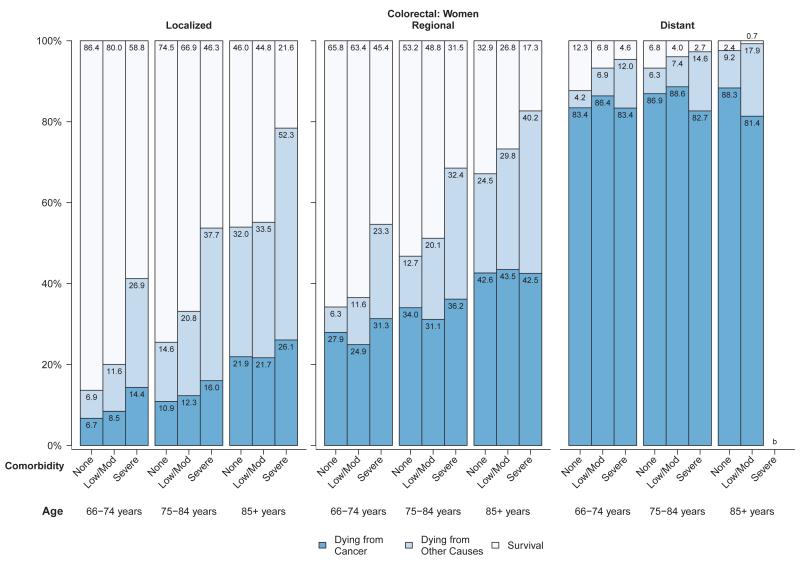
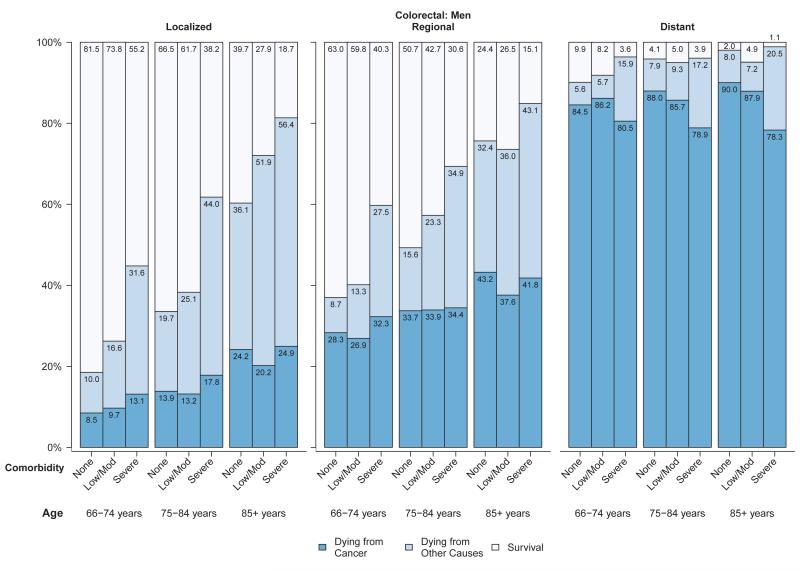
Among women (Figure 3a) and men (Figure 3b) diagnosed with colorectal cancer, approximately 7% to 26% of those diagnosed at localized stage died from their cancer in all age and comorbidity strata, compared with 25% to 44% of those with regional stage disease and generally over 80% of those with distant stage disease. Overall survival and probability of non-cancer death was strongly related to comorbidity level in men and women diagnosed with colorectal cancer at local or regional stage (Figures 3a,3b). The influence of comorbidities on the probability of both cancer and non-cancer death was smaller for lung cancer than for other cancers, due to the relatively poor prognosis even among individuals diagnosed at local stage (Figures 4a,4b). For older patients diagnosed with colorectal or lung cancer at regional or distant stage, the probability of dying from cancer for people with severe comorbidity was smaller than for people with less severe comorbidity. This shows the role of comorbidity in reducing the risk of dying from cancer (i.e. competing risk).
Figure 3.
a: Probabilities of dying from cancer, dying from other causes and survival stratified by selected stage and comorbidity status among women with colorectal cancer diagnosed between 1999 and 2005a
b: Probabilities of dying from cancer, dying from other causes and survival stratified by selected stage and comorbidity status among men with colorectal cancer diagnosed between 1999 and 2005a
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
aSource: National Cancer Institute SEER-Medicare linked database (Connecticut, Hawaii, Iowa, Utah, and New Mexico, and the metropolitan areas of Los Angeles, Greater Bay, Detroit, Atlanta, and Seattle-Puget Sound).
bThe statistic could not be calculated.
DISCUSSION
Overall cancer death rates continue to decrease in the U.S. Death rates declined for the most common sites including female breast, prostate, and lung and colorectal cancers among both men and women and in most racial and ethnic groups. Declines in death rates for these cancers were not statistically significant among AI/AN men or women or for lung cancer among API women. However, death rates increased for cancers of the liver and pancreas among men and women and for melanoma and cancer of soft tissue including heart (primarily sarcomas) among men and cancer of the uterus among women. The overall decreases in cancer death rates indicate progress in cancer control and reflect a combination of primary prevention by reductions of important risk factors, as well as improved early detection and treatment.2-15
Temporal trends in incidence rates require a more complex interpretation. In addition to factors which influence both incidence and mortality trends such as changes in exposure to environmental and endogenous risk factors, disease classification, data collection systems, and variability in population estimates (i.e., denominators for rates), incidence trends are also affected by changes in diagnostic practices. Overall incidence trends during 2001 through 2010 decreased for lung, colorectal, and prostate cancer, but stabilized for breast cancer among women. The decrease in lung cancer incidence rates reflects long-term reduction in smoking prevalence,11 while the decrease in colorectal cancer incidence rates may largely reflect increased use of screening that allows the detection and removal of adenomatous polyps.12 The decrease in prostate cancer incidence rates likely reflects declines in prostate-specific antigen (PSA) testing.58 In 2008, the US Preventive Services Task Force (USPSTF) recommended against prostate cancer screening for men ages 75 years and older.59 This change resulted in declines in both PSA testing58 and prostate cancer incidence rates among older men (75 years and older), especially early-stage prostate cancer which is most likely to be detected by screening.60 Indeed, from 2009 through 2010, we found that incidence rates of localized prostate cancer decreased while incidence rates of regional prostate cancer stabilized and rates of distant prostate cancer increased (Table 7). Although breast cancer incidence rates were stable during 2001-2010, they sharply decreased between 2002 and 2003 (most likely due to reductions in the use of postmenopausal hormone replacement therapy) after previously increasing for many decades, in part due to early detection through mammography, changes in reproductive factors, hormone replacement therapy and obesity.61-65
Table 7.
Cancer Incidence Rates for Prostate and Female Breast Cancer by Stage for 2009 and 2010a
| Rateb | |||
|---|---|---|---|
| Stage | 2009 | 2010 | Rate change |
| Prostate | |||
| All | 62.0 | 57.7 | −6.9% |
| Local | 48.5 | 44.5 | −8.3% |
| Regional | 6.1 | 6.1 | −0.7% |
| Distant | 2.4 | 2.4 | 2.5% |
| Unknown | 4.9 | 4.7 | −5.5% |
| Female Breast | |||
| All | 122.0 | 118.1 | −3.2% |
| Local | 75.0 | 72.8 | −3.0% |
| Regional | 36.2 | 34.8 | −3.8% |
| Distant | 6.5 | 6.6 | 2.3% |
| Unknown | 4.3 | 3.9 | −10.9% |
NPCR and SEER areas reported by NAACCR as meeting high-quality incidence data standards for the specified time periods. Rates based on data from all states except Minnesota and including District of Columbia and Puerto Rico.
Rates are per 100,000 persons and were age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1-4 years, 5-9 years, …, 80-84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000)
External factors in data collection or reporting may have also contributed to the decline in cancer incidence rates, such as the implementation of the Collaborative Staging algorithm (CSv2) for cases diagnosed in 2010 that might have resulted in longer reporting delays.66
However, a review of the delay-adjustment factors used in recent years suggests that reporting delay in the SEER Program has actually been diminishing.67 This Annual Report highlights the prevalence of comorbidity and its impact on survival among persons diagnosed with lung, colorectal, breast or prostate cancer. Data on individual comorbid conditions were combined into comorbidity indices or scores which are frequently used for research purposes to increase analytic efficiency with fewer variables. The comorbidity score was calculated by summarizing multiple chronic conditions into a single measure that reflects the burden of these conditions on health outcomes, such as survival that was presented in this report. During the past 20 years, many comorbidity measures have been developed and several reviews of these measures have been published.68, 69 The index (score) used in this Report 23 is an extension of Klabunde et al.44 and used claims from SEER-Medicare cancer patients diagnosed in 1992 through 2005. This score is based on the original Charlson prognostic index to predict one-year mortality using hospital data, modified for use with ICD-9-CM diagnostic and procedure codes from both inpatient hospitalization (Medicare Part A) and from physician and outpatient administrative claims (Medicare Part B), and to exclude cancer as a comorbidity. The index (score) is a weighted average of the 16 comorbid conditions with the weights estimated from the models of five-year survival.
Because of the importance of comorbidity in the care of cancer patients, prognostic and survival tools that incorporate comorbidity measures may be useful in aiding clinical decisions.70 New tools are being developed to improve survival estimates by taking into account comorbidity.20, 21, 23 However, it is important that clinicians and patients who use these tools understand that the probabilities of cancer and non-cancer death in observational registry data are based on outcomes of cancer patients treated during the years studied (1999 through 2005 for our study) and do not reflect the probability of death from the cancer if left untreated or the effect of the most current treatments. The usefulness of comorbidity-adjusted survival data for clinical decision making would be improved if analyses could be stratified by treatment and other prognostic markers, as in a recent analysis of prostate cancer mortality using SEER-Medicare data.71
Furthermore, it is becoming increasingly important to understand the major health problems in the U.S. and how they are changing.26, 72 Measures of the U.S. burden of disease, including cancer, rely on important data systems and surveys produced by local, state and federal agencies. Measures of comorbidity contribute to our understanding of cancer, other diseases, and their relationships. Improving health outcomes and care in all individuals with comorbidities72 will require response from the public and private health sectors, communities, individuals, and researchers.73-76,77, 78 Some research has used comorbidity as a surrogate measure of risk and disability79, 80 and global estimates of disability-adjusted life years.81 Although cancer registries do not routinely collect data on comorbidities, registry data may be supplemented with data on comorbid conditions from several sources. SEER Patterns of Care Studies82 review medical records to obtain more detailed data on treatment and comorbid conditions for a sample of individuals with selected cancers.83 CDC has funded 10 NPCR registries to collect detailed treatment data as well as information on comorbidities directly from medical records and through data linkages to enhance data collection capabilities for comparative effectiveness research.84, 85 The National Cancer Database, a hospital-based registry system, requires hospitals to code diagnoses listed on the face sheet of the inpatient hospital record.86 Registry data can also be linked with Medicaid records to obtain data on comorbid conditions.87 One of the most widely used data sources for research on cancer treatment and outcomes is the SEER-Medicare database used in this report, in which detailed information on cancer treatment, as well as comorbidity, can be derived from claims records of Medicare enrollees.44, 45 Each data source and study has advantages and limitations of size, generalizability, scope and content.
Limitations
High-quality cancer surveillance data in the U.S. are available: mortality for the entire population and incidence for 90% of the population (2006 through 2010). However, certain limitations in data sources, data collection, and analyses may have influenced the findings of this report. First, differences between the numerator (incidence data) and denominator (Census population data) can occur in the designation of characteristics such as age, race, ethnicity, and place of residence and these differences change over time. In general, data quality has improved over time (e.g., decreases in unknown stage and shorter reporting delays). Also, with the incorporation of the 2010 census in this report, the populations for the intercensal years are more accurate than in prior reports where these data were estimated based only on the 2000 census. We examined the possibility that the incorporation of information from the 2010 census in developing the population estimates influenced the changes in rates,88 but found the population estimates used in the previous Annual Report for calculation of rates through 2009 were very similar to those used in this report.89
Dynamic trends in racial and ethnic self identification (in particular, Hispanics and API as “some other race”) and changes in methodology for allocating “some other race” (resulting in more Hispanic AI/ANs) present difficulties for maintaining health statistics over long time series and make 2010 data less directly comparable to earlier years. Cancer statistics have generally used the 1977 federal definitions of race and ethnicity, whereby all persons belong to one of four races (white, black, API, and AI/AN) and are either of Hispanic or non-Hispanic ethnicity. The 2010 census revealed that Americans are self-identifying in ways that deviate considerably from these standards.90-95 While the official 2010 population estimate showed an increase of less than 1% over the 2009 estimate, it contained 19% more AI/AN, 9% more API, and 31% more individuals of multiple race. Additionally, in the last two censuses a sizable fraction of all Hispanics self-identified as “some other race.” In 2000 the Census Bureau reassigned nearly all of these as white, but in 2010 many were reassigned to other racial categories, particularly AI/AN and API.39 The unanticipated spike in the AI/AN and, to a lesser extent, API populations in 2010 means the cancer rates in these groups are lower relative to whites and blacks than they were previously. Rather than representing a meaningful difference, it is more likely that this is a reflection of differences in data collection between the census, changes in methodology and data inputs for post censal population estimates, revised intercensal population estimates, and racial/ethnic data recorded in medical settings.
Second, as noted in previous Annual Reports to the Nation,1-15 the broad racial and ethnic group categories used in our analyses may mask variations in the cancer burden by country of origin or by other unique characteristics of high- or low-risk populations. Also, as noted above, cancer rates for racial and ethnic groups may be affected by difficulties in ascertaining race and ethnicity information from medical records, death certificates, and census reports.96
Third, analyses of trends should be carefully interpreted for several additional reasons. Changes in incidence may result from changes in the prevalence of risk factors, the introduction or increased use of screening or diagnostic techniques or a combination of these factors. The AAPC was used as a summary measure to average trends over a five- or 10-year period using joinpoint regression. Joinpoint models identify recent changes in the magnitude and direction of trends but may give an impression of a continuous increase or decrease over time when trends actually may be more variable. Furthermore, delayed case reporting may affect incidence trends if the most recent joinpoint segments overestimate recent declines or underestimate recent increases. Methods to adjust for delayed reporting53 were used in our analysis only in the analysis of SEER 13 data. The largest effects of adjusting for delayed reporting are seen in cancers diagnosed in non-hospital settings such as melanoma and leukemia; however, SEER reporting delays have decreased over time. This report presents trends based both on data from the SEER 13 registries and on combined data from NAACCR which includes SEER and NPCR registries. Both datasets have strengths and limitations and provide valuable insight into cancer trends in the U.S. Longer-term trends can be examined using the SEER 13 registries and these data have also been delay-adjusted. However, the combined data from SEER and NPCR registries cover nearly the entire U.S. population and may better capture geographic and population differences in risk factors and incidence rates even though estimates for the most recent diagnosis years are attenuated without adjustments for reporting delays.
A 2007 policy change regarding the transfer of Veterans Health Administration (VA) cases to state cancer registries led to incomplete reporting of VA hospital cases. Since VA hospitals provide a critical source of data for cancers diagnosed among veterans who are eligible to receive care from these facilities, cancer incidence rates for men were underestimated. However, clarification of the policy and subsequent reporting of these cases has shown that underreporting from VA facilities diminished over time.
The SEER-Medicare linked database is a unique resource used extensively in health services research to study cancer screening, treatment, outcomes and costs. The comparison of the older adult population in SEER areas to the U.S. total shows that in the SEER areas there are a lower percentage of white persons and individuals living in poverty, and a higher percentage of urban-dwellers than the total U.S. population.41 Older persons in the SEER regions also have higher rates of HMO enrollment (about 25%) and lower rates of cancer mortality. Medicare claims are created for payment purposes, not research, and the absence of information about reasons and outcomes for a test or procedure can make it difficult to distinguish whether secondary diagnoses are complications or comorbidities.
Future Directions
This report brings attention to the prevalence (over 30%) of comorbidities (multiple chronic diseases) among individuals aged 65 years and older which increases with age and can be substantial for some cohorts of cancer patients.20, 97 Cancer patients with comorbidities have the additional challenge of coordinating both their cancer and non-cancer-related care.98, 99 Even in the absence of comorbidities, the challenge of coordinating multi-disciplinary cancer treatment and survivorship care is formidable.100-102 Studies using SEER-Medicare data have found that cancer survivors are less likely than age-matched controls to receive recommended clinical preventive services or appropriate treatment for acute and chronic conditions.103-105
The number of individuals diagnosed with cancer and living after a cancer diagnosis (cancer survivors)106 will continue to rise in the coming decades due to population aging and expansion107 as well as increasing success in treatment, even if incidence rates remain stable or decline. The ability of the US healthcare system to respond to the growing population of older adults, cancer survivors and patients with multiple comorbidities is uncertain.108 A national response by the public and private sectors has been initiated to improve the overall health status of individuals with multiple chronic conditions by fostering change within the health care system, providing information for health professionals and patients, and facilitating research to improve oversight and care.72 Public health actions to reduce disability and improve functioning and the quality of life of persons with chronic disease include an increased application of effective tobacco control measures and fostering behavioral changes such as a healthy diet and sufficient physical activity that contribute to healthier aging.78, 108, 109 Health system interventions that address the increasing need for healthcare in our aging population include the development of better systems for coordination of care.100 Research initiatives to improve the coordination of care and increase the receipt of recommended preventive and treatment services are ongoing110, 111 and include increasing use of electronic medical records, reminder systems, patient navigation programs, and monitoring of quality measures by payers and consumer advocates.112 Finally, a greater focus on risk factor prevention,109 coordination of care,100 chronic disease management,78 and multi-level interventions113 may lessen the future burden of such conditions.
Acknowledgments
We gratefully acknowledge the contributions of the state and regional cancer registry staffs for their work in collecting the data used in this study. In addition we thank Andrew Lake, Martin Krapcho, Rick Firth, Jessica Garshell, and Zhuoqiao Wang of Information Management Services, Inc., for assistance in compiling the data used in this report; Terri Harshman of the National Cancer Institute for editorial assistance with manuscript preparation; and Dr. Carrie N. Klabunde of the National Cancer Institute for consultation on measurement of comorbidity. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING This work was supported by the American Cancer Society, the Centers for Disease Control and Prevention, the National Cancer Institute, National Institutes of Health, and the North American Association of Central Cancer Registries.
Contributor Information
Brenda K. Edwards, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD.
Anne-Michelle Noone, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD.
Francis P. Boscoe, Surveillance Research Program, American Cancer Society, Atlanta, GA; NY State Registry; North American Association of Central Cancer Registries, Springfield, IL.
S. Jane Henley, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA.
Ahmedin Jemal, Surveillance Research Program, American Cancer Society, Atlanta, GA.
Hyunsoon Cho, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD.
Robert N. Anderson, Division of Vital Statistics, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD.
Christie R. Eheman, Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA.
Elizabeth M. Ward, Surveillance Research Program, American Cancer Society, Atlanta, GA.
References
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973-1995: a report card for the U.S. Cancer. 1998;82(6):1197–207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93(11):824–42. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 3.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91(8):675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 4.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 6.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 9.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–42. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 10.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;18(9):2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute [accessed November 6, 2013];SEER-Medicare linked database. Available from URL: http://www.appliedresearch.cancer.gov/seermedicare/
- 17.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases-a systematic review on existing multimorbidity indices. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2011;66(3):301–11. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 18.Cronin KA, Feuer EJ. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med. 2000;19(13):1729–40. doi: 10.1002/1097-0258(20000715)19:13<1729::aid-sim484>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–98. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–49. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feuer EJ, Lee M, Mariotto AB, et al. The Cancer Survival Query System: Making survival estimates drom the Surveillance, Epidemiology, and End Results Program more timely and relevant for recently diagnosed patients. Cancer. 2012;118(22):5652–62. doi: 10.1002/cncr.27615. [DOI] [PubMed] [Google Scholar]
- 22.Cho H, Howlader N, Mariotto AB, Cronin KA. Estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method. Technical Reports: National Cancer Institute; 2011. [Google Scholar]
- 23.Mariotto AB, Wang Z, Klabunde CN, Cho H, Das B, Feuer EJ. Life tables adjusted for comorbidity more accurately estimate noncancer survival for recently diagnosed cancer patients. J Clin Epidemiol. 2013 doi: 10.1016/j.jclinepi.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albertsen PC, Moore DF, Shih WC, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. Journal of Clinical Oncology. 2011;29(10):1335–41. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med. 2012;31(5):489–500. doi: 10.1002/sim.4454. [DOI] [PubMed] [Google Scholar]
- 26.Murray CJ, Abraham J, Ali MK, et al. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA. 2013 doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute [accessed November 6, 2013];Age distribution (%) of incidence cases by site, 2006-2010. Available from URL: http://seer.cancer.gov/csr/1975_2010/results_merged/topic_age_dist.pdf.
- 29.World Health Organization . International Classification of Diseases for Oncology. 3rd ed Geneva, Switzerland: 2000. [Google Scholar]
- 30.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 31.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 Suppl):1120–30. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute [accessed November 6, 2013];About the SEER Program. Available from URL: http://seer.cancer.gov/about/
- 33.National Cancer Institute [accessed November 6, 2013];SEER registry groupings for analyses. Available from URL: http://seer.cancer.gov/registries/terms.html.
- 34.Murphy SL, Xu J, Kochanek KD. Natl Vital Stat Rep. National Center for Health Statistics; Hyattsville: 2013. Deaths: Final data for 2010. [PubMed] [Google Scholar]
- 35.Manual of the international statistical classificiation of diseases, injuries, and causes of death, adapted for use in the United States. 8th Rev ed National Center for Health Statistics, Public Health Service, US Department of Health Education and Welfare; Washington, DC: 1968. [Google Scholar]
- 36.Manual of the international statistical classificiation of diseases, injuries, and causes of death, based on the recommendations of the Ninth Revision Conference, 1975; Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 37.Manual of the international statistical classificiation of diseases and health related problems. 10th Rev. ed World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- 38.National Cancer Institute [accessed May 8, 2013];Surveillance Epidemiology and End Results (SEER) Program. Population estimates used in NCI’s SEER*Stat software. Available from URL: http://seer.cancer.gov/popdata/methods.html.
- 39.Centers for Disease Control and Prevention NVSS [accessed May 8, 2013];U.S. Census populations with bridged race categories. Available from URL: http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 40.National Cancer Institute [accessed November 6, 2013];US Population Data -- 1969-2011. Available from URL: http://seer.cancer.gov/popdata/
- 41.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med.Care. 2002;40(8 Suppl):IV–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 42.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic co-morbidity in longitudinal studies - development and validation. Journal of Chronic Diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 43.Deyo RA, Cherkin DC, Ciol MA. Adapting a Clinical Comorbidity Index for Use with ICD-9-CM Administrative Databases. J Clin Epidemiol. 1992;45(6):613–19. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 44.Klabunde CN, Legler JM, Warren JL, Laura-Mae B, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–90. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 46.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44(10):921–8. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 47.Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of Surveillance, Epidemiology, and End Results-Medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol. 2011;174(7):860–70. doi: 10.1093/aje/kwr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surveillance Research Program National Cancer Institute SEER*Stat software. (version 8.0.4) ( www.seer.cancer.gov/seerstat)
- 49.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute [accessed November 6, 2013];Joinpoint Regression Program. Available from URL: http://surveillance.cancer.gov/joinpoint/
- 51.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 52.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–82. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–45. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 54.Prentice RL, Kalbfleisch JD, Peterson AV, Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54. [PubMed] [Google Scholar]
- 55. [accessed August 1, 2013];SEER cause-specific death classification. Available from URL: http://seer.cancer.gov/causespecific/
- 56.National Cancer Institute [accessed November 6, 2013];SEER Coding and Staging Manuals. Available from URL: http://seer.cancer.gov/tools/codingmanuals/
- 57.National Cancer Institute [accessed November 6, 2013];SEER Summary Staging Manual -- 2000. Available from URL: http://seer.cancer.gov/tools/ssm/
- 58.Howard DH, Tangka FK, Guy GP, Ekwueme DU, Lipscomb J. Prostate cancer screening in men ages 75 and older fell by 8 percentage points after Task Force recommendation. Health Affairs. 2013;32(3):596–602. doi: 10.1377/hlthaff.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calonge N, Petitti DB, DeWitt TG, et al. Screening for prostate cancer: US preventive services task force recommendation statement. Ann Intern Med. 2008;149(3):185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 60.Howard DH. Declines in prostate cancer incidence after changes in screening recommendations. Arch Intern Med. 2012;172(16):1267–68. doi: 10.1001/archinternmed.2012.2768. [DOI] [PubMed] [Google Scholar]
- 61.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 62.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9(3):R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colditz G, Baer H, Tamimi R. Breast cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. Oxford; New York, NY: 2006. pp. 959–74. [Google Scholar]
- 64.Kalager M, Adami HO, Bretthauer M, Tamimi RM. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Intern Med. 2012;156(7):491–9. doi: 10.7326/0003-4819-156-7-201204030-00005. [DOI] [PubMed] [Google Scholar]
- 65.Breen N, Gentleman JF, Schiller JS. Update on mammography trends: comparisons of rates in 2000, 2005, and 2008. Cancer. 2011;117(10):2209–18. doi: 10.1002/cncr.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins EN. The Collaborative Stage Version 2 Data Validation Project, 2010. J Registry Manag. 2011;38(1):39–60. [PubMed] [Google Scholar]
- 67.National Cancer Institute [accessed November 6, 2013];Cancer Statistics Review: Technical Notes. Available from URL: http://seer.cancer.gov/csr/1975_2010/results_figure/sect_01_intro2_24pgs.pdf.
- 68.Sarfati D. Review of methods used to measure comorbidity in cancer populations: No gold standard exists. J Clin Epidemiol. 2012;65(9):924–33. doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 69.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109–18. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 70.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases - Implications for pay for performance. Journal of the American Medical Association. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 71.Daskivich TJ, Chamie K, Kwan L, Dash A, Greenfield S, Litwin MS. Matching tumor risk with aggressiveness of treatment in men with multiple comorbidities and early-stage prostate cancer. Cancer. 2013 doi: 10.1002/cncr.28226. [DOI] [PubMed] [Google Scholar]
- 72.Multiple chronic conditions--a strategic framework: Optimum health and quality of life for individuals with multiple chronic conditions. U.S. Department of Health and Human Services; Washington, DC: 2010. [Google Scholar]
- 73.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- 74.Crossing the quality chasm: A new health system for the 21st century. Institute of Medicine; Washington, D.C.: 2001. [PubMed] [Google Scholar]
- 75.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–5. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson G. Chronic care: Making the case for ongoing care. Robert Wood Johnsonson Foundation; Princeton: 2010. [Google Scholar]
- 77.Spinks T, Albright HW, Feeley TW, et al. Ensuring quality cancer care: a follow-up review of the Institute of Medicine’s 10 recommendations for improving the quality of cancer care in America. Cancer. 2012;118(10):2571–82. doi: 10.1002/cncr.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Living well with chronic illness: A call for public health action. Institute of Medicine (IOM); Washington, DC: 2012. [DOI] [PubMed] [Google Scholar]
- 79.Soerjomataram I, Lortet-Tieulent J, Ferlay J, et al. Estimating and validating disability-adjusted life years at the global level: a methodological framework for cancer. BMC Med Res Methodol. 2012;12 doi: 10.1186/1471-2288-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380(9856):1840–50. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 81.Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012;380(9856):1797–99. doi: 10.1016/S0140-6736(12)61688-2. [DOI] [PubMed] [Google Scholar]
- 82.National Cancer Institute [accessed November 6, 2013];Patterns of Care/Quality of Care Studies. Available from URL: http://appliedresearch.cancer.gov/surveys/poc/
- 83.Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro-Oncology. 2012;14(3):351–59. doi: 10.1093/neuonc/nor218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention [accessed 11/6/13, 2013];Comparative effectiveness research data collection enhancement project. Available from URL: http://www.cdc.gov/cancer/npcr/cer_data_collection.htm.
- 85.O’Malley CD, Shema SJ, Cress RD, et al. The implications of age and comorbidity on survival following epithelial ovarian cancer: Summary and results from a Centers for Disease Control and Prevention Study. Journal of Womens Health. 2012;21(9):887–94. doi: 10.1089/jwh.2012.3781. [DOI] [PubMed] [Google Scholar]
- 86.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients Age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. Journal of Clinical Oncology. 2009;27(22):3627–33. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 87.Boscoe FP, Schrag D, Chen K, Roohan PJ, Schymura MJ. Building capacity to assess cancer care in the Medicaid population in New York state. Health Serv Res. 2011;46(3):805–20. doi: 10.1111/j.1475-6773.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boscoe FP, Miller BA. Year 2000 population estimation error and its impact on 1991-1999 cancer rates. The Professional Geographer. 2004;56(4):516–29. [Google Scholar]
- 89.Cohn D. State population estimates and Census 2010 counts: Did they match? Pew Research Social & Demographic Trends [serial online] 2011. Available from URL: http://www.pewsocialtrends.org/2011/01/12/state-population-estimates-and-census-2010-counts-did-they-match/
- 90.Humes KR, Jones NA, Ramirez RR. 2010 Census Briefs. U. S. Dept. of Commerce, U. S. Census Bureau; Washington, DC: 2011. Overview of race and Hispanic origin: 2010. [Google Scholar]
- 91.Ennis SR, Rios-Vargas M, Albert NG. 2010 Census Briefs. U. S. Dept. of Commerce/U. S. Census Bureau; Washington, D.C.: 2011. The Hispanic population: 2010. [Google Scholar]
- 92.Norris T, Vines PL, Hoeffel EM. 2010 Census Briefs. U.S. Department of Commerce/U.S. Census Bureau; Washington, DC: 2012. The American Indian and Alaska Native population: 2010. [Google Scholar]
- 93.Hoeffel EM, Rastogi S, Kim MO, Shahid H. 2010 Census Briefs. U.S. Department of Commerce/U.S. Census Bureau; Washington, DC: 2012. The Asian population: 2010. [Google Scholar]
- 94.Hixson L, Hepler BB, Kim MO. 2010 Census Briefs. U.S. Department of Commerce/U.S. Census Bureau; Washington, DC: 2012. The Native Hawaiian and other Pacific Islander population: 2010. [Google Scholar]
- 95.Werner CA. 2010 Census Briefs. U.S. Department of Commerce/U.S. Census Bureau; Washington, DC: 2011. The older population: 2010. [Google Scholar]
- 96.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat. 2008;2(148):1–23. [PubMed] [Google Scholar]
- 97.Rowland JH, Yancik R. Cancer survivorship: the interface of aging, comorbidity, and quality care. J Natl Cancer Inst. 2006;98(8):504–5. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 98.Chubak J, Tuzzio L, Hsu C, et al. Providing care for cancer survivors in integrated health care delivery systems: practices, challenges, and research opportunities. J Oncol Pract. 2012;8(3):184–9. doi: 10.1200/JOP.2011.000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Forsythe LP, Parry C, Alfano CM, et al. Use of survivorship care plans in the United States: associations with survivorship care. Journal of the National Cancer Institute. 2013;105(20):1579–87. doi: 10.1093/jnci/djt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sussman J, Baldwin LM. The interface of primary and oncology specialty care: from diagnosis through primary treatment. J Natl Cancer Inst Monogr. 2010;2010(40):18–24. doi: 10.1093/jncimonographs/lgq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010(40):25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? Journal of Cancer Survivorship-Research and Practice. 2013;7(2):253–61. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–19. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 105.Snyder CF, Frick KD, Herbert RJ, et al. Quality of care for comorbid conditions during the transition to survivorship: Differences between cancer survivors and noncancer controls. Journal of Clinical Oncology. 2013;31(9):1140–48. doi: 10.1200/JCO.2012.43.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.National Cancer Institute [accessed November 6, 2013];Estimated US cancer prevalence counts: Method. Available from URL: http://cancercontrol.cancer.gov/ocs/prevalence/prevalence.html.
- 107.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 108.The state of aging and health in America 2013. Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; Atlanta: 2013. p. 60. [Google Scholar]
- 109.National prevention strategy. U.S. Department of Health and Human Services; Washington, DC: 2011. [PubMed] [Google Scholar]
- 110.Dept. of Health and Human Services [accessed 11/6/13, 2013];Behavioral interventions to address multiple chronic health conditions in primary care. Available from URL: http://grants.nih.gov/grants/guide/pa-files/PA-12-024.html.
- 111.Dept. of Health and Human Services [accessed 11/6/2013, 2013];Examination of Survivorship Care Planning Efficacy and Impact (R01) Available from URL: http://grants.nih.gov/grants/guide/pa-files/PA-12-024.html.
- 112.Levit L, Balogh E, Nass S, Ganz PA. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Institute of Medicine of the National Academies; Washington, D.C.: 2013. p. 360. [PubMed] [Google Scholar]
- 113.Fineberg HV. Foreword: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):1. doi: 10.1093/jncimonographs/lgs009. [DOI] [PMC free article] [PubMed] [Google Scholar]