Abstract
Background
Symptom-based tuberculosis screening identifies less than one-third of eligible HIV-infected patients as candidates for isoniazid preventive therapy (IPT). We evaluated whether testing for C-reactive protein (CRP) improves patient selection for IPT.
Methods
We measured CRP levels (normal < 10mg/L) using a point-of-care (POC) assay on stored serum samples from HIV-infected Ugandan adults initiating antiretroviral therapy. We assessed diagnostic accuracy in reference to baseline tuberculosis status adjudicated by an expert committee and calculated net reclassification improvement (NRI) to quantify the incremental discriminatory benefit of POC-CRP in determining IPT-eligibility compared to the WHO symptom screen.
Results
Of 201 patients (median CD4 cell-count 137 cells/μL, IQR 83-206), five (2.5%) had tuberculosis. Compared to the WHO symptom screen, POC-CRP had similar sensitivity (100% vs. 80%, p=0.30) but greater specificity (21% vs. 87%, p<0.0001) for tuberculosis. If based on the WHO symptom screen, no patients with tuberculosis but only 42/196 patients without tuberculosis would have been considered IPT-eligible. If POC-CRP were used instead, one patient with tuberculosis (reclassification of cases -20%, p=0.32) and 129 patients without tuberculosis (reclassification of non-cases +66%, p<0.001) would have been reclassified as IPT-eligible, an NRI of 46% (p=0.03). In addition, POC-CRP testing would have reduced the proportion of patients without active tuberculosis requiring confirmatory tuberculosis testing (87% vs. 21%, p<0.0001).
Conclusions
POC-CRP testing increased more than four-fold the proportion of HIV-infected adults immediately identified as IPT-eligible and decreased the proportion of patients requiring referral for further tuberculosis diagnostic testing. POC-CRP testing could substantially improve implementation of tuberculosis screening guidelines.
Introduction
Isoniazid preventive therapy (IPT) has been shown in multiple randomized controlled trials to reduce tuberculosis (TB) incidence and mortality among people living with HIV (PLHIV) [1]. Despite strong evidence of its efficacy, the challenges in ruling-out active TB and the fear of developing drug-resistant TB from isoniazid mono-therapy have contributed to its underutilization; in 2009, IPT was provided to only 0.2% of all eligible PLHIV worldwide [2]. To promote its uptake in TB endemic regions, the World Health Organization (WHO) recommends that IPT should be provided to all HIV-infected individuals in whom active TB is deemed unlikely [3] and that a simplified four-part symptom screen (WHO symptom screen) with high sensitivity and high negative predictive value (NPV) for active TB should be used to determine IPT eligibility [4].
Recent studies have questioned the utility of the WHO symptom screen. In prospective studies from South Africa, 69-86% of PLHIV initiating antiretroviral therapy (ART) were symptom-screen positive (presence of any one of the four symptoms: current cough; fever, night sweats, or weight loss in the past 30 days), even though only 6-17% had culture-positive TB [5-8]. Thus, use of the symptom screen requires the vast majority of PLHIV to undergo more costly TB evaluation, which is not routinely accessible in many high TB burden settings. Therefore, a screening algorithm is urgently needed that has higher specificity for active TB than the WHO symptom screen but retains high negative predictive value (NPV) and operational characteristics practical for use in resource-constrained settings.
C-reactive protein (CRP) is an acute phase reactant whose levels rise in the setting of IL-6-mediated pyogenic infections such as active TB. CRP has been consistently shown to have high sensitivity (range: 85-100%) [8,9,11-18] and high NPV [8,9,17] for active pulmonary TB in both HIV-positive and -negative patients, regardless of symptoms. Although elevations in CRP (≥ 10 mg/L) are not limited to active TB, recent studies in patients presenting with TB symptoms suggest that CRP has higher specificity for active TB than symptom-based screening algorithms [8]. Moreover, CRP can be measured using a simple, inexpensive (< $2 per test), and rapid (quantified CRP level result in 3 minutes) point-of-care (POC) assay. These features make POC-CRP testing an ideal candidate as a rapid rule-out test for active TB that can be used by front-line health care providers to improve selection of PLHIV for rapid initiation of ART and IPT and to improve efficiency of intensified case-finding (ICF) activities. Therefore, using clinical data and stored serum obtained from HIV-infected adults (regardless of symptoms) prior to initiating ART at a prototypical HIV/AIDS clinic in sub-Saharan Africa, we evaluated whether testing for POC-CRP improves patient selection for IPT beyond that of the currently recommended WHO symptom screen.
Methods
Participants
The Uganda AIDS Rural Treatment Outcomes (UARTO) study is an ongoing longitudinal cohort study examining consecutive HIV-infected adults initiating ART at the Mbarara University of Science and Technology (MUST) Immune Suppression Syndrome (ISS) Clinic. The ISS Clinic in Mbarara (located in southwestern Uganda, 270 kilometers from Kampala) serves a predominantly rural population. Patients, regardless of symptoms, were eligible for participation if they were > 18 years-old and ART-naïve. We excluded patients with missing baseline symptom data and patients with a known diagnosis of TB and/or receiving TB treatment at the time of study enrollment. For the present analysis, we selected a consecutive sample of patients who began in the cohort between May 2007 and April 2009.
All patients gave written informed consent and the UARTO study was approved by the Institutional Review Boards of the University of California, San Francisco, MUST, and the Uganda National Council of Science and Technology. This study conforms to the Standards for the Reporting of Diagnostic Accuracy Studies (STARD) initiative guidelines [19].
Patient evaluation
Patient evaluation and follow-up for the UARTO study have been described previously [20,21]. Briefly, baseline data collection included measurement of CD4 cell-count, TB history, current medication use, and WHO symptom screen assessment. Study visits occurred every three months after the baseline visit and patients were asked to report any changes to their clinical and medication history since their last study visit.
Index tests
We retrospectively assessed IPT eligibility at the time of study enrollment using both the WHO symptom screen and a POC-CRP assay. In accordance with WHO guidelines, we considered patients to be symptom screen-negative (i.e., eligible for IPT) if they reported none of the four symptoms: cough (any duration), fever, night sweats or weight loss in the previous 30 days.
Using serum collected at the time of study enrollment (just prior to ART initiation [median days pre-ART = 1 day, IQR: 0-2 days]) and stored at -80°C, we measured CRP levels using a standard sensitivity POC assay (iCHROMA POC-CRP Reader, BodiTech Med Inc., South Korea) in accordance with the manufacturer's protocol. The iCHROMA POC-CRP Reader is a United States Food and Drug Administration-approved, fully-quantitative (range: 2.5–300 mg/L; normal < 10 mg/L), lateral flow-based fluorescence sandwich immunoassay that inexpensively (< $2 per test) provides CRP measurements within 3 minutes from whole blood, serum, or plasma. We considered patients with normal POC-CRP levels (< 10 mg/L) [22,23], to be TB screen-negative (i.e., eligible for IPT).
Outcome measurements
Active TB
Although microbiologic testing for TB was not part of the UARTO study protocol (patients suspected of disease were referred by clinicians for further evaluation using available TB diagnostics), baseline TB status was independently adjudicated by a committee of infectious disease specialists [PH, HB, CS] from Uganda and the United States using all available data from baseline and follow-up visits. In addition, the committee reviewed hospital charts and TB clinic, TB laboratory and TB treatment registers. The committee, blinded to POC-CRP levels, assigned study participants a diagnosis of: 1) definitive TB (smear- and/or culture-confirmed TB); 2) probable TB (TB treatment without microbiologic confirmation but documented improvement with treatment); 3) possible TB (TB treatment without microbiologic confirmation and no documented response to treatment); 4) unlikely TB (no signs/symptoms of TB or confirmed alternate diagnosis); or 5) insufficient evidence to judge.
IPT eligibility
We considered patients to be eligible for IPT at study enrollment if there was no evidence of definite or probable TB within six months of study entry.
Statistical Analysis
We calculated point estimates and 95% confidence intervals (CI) for the sensitivity, specificity, NPV, and positive predictive value (PPV) of the WHO symptom screen and POC-CRP using definite or probable TB diagnosed within six months of study enrollment as a proxy reference standard for active TB present at study enrollment. The use of interval diagnosis of TB within six months as a proxy for TB at baseline is supported by data suggesting that up to 87% of HIV/TB cases diagnosed within six months of ART initiation represent prevalent rather than incident TB cases [24,25] and that patients with active TB and advanced HIV disease are unlikely to survive beyond six months without TB treatment [21]. We used McNemar's paired-test of proportions to compare differences between the WHO screen and POC-CRP in sensitivity and specificity. We used the kappa-statistic (κ) to measure agreement between the two screening tests. To quantify the incremental discriminatory benefit of POC-CRP testing for selection of patients for IPT relative to the WHO symptom screen, we calculated net reclassification improvement (NRI) [26]. The NRI reflects the net proportion of non-cases reclassified by POC-CRP testing as being eligible for IPT plus the net proportion of cases reclassified by POC-CRP testing as being ineligible for IPT. We performed all statistical analyses using STATA 11 (STATA Corporation, College Station, TX, USA).
Results
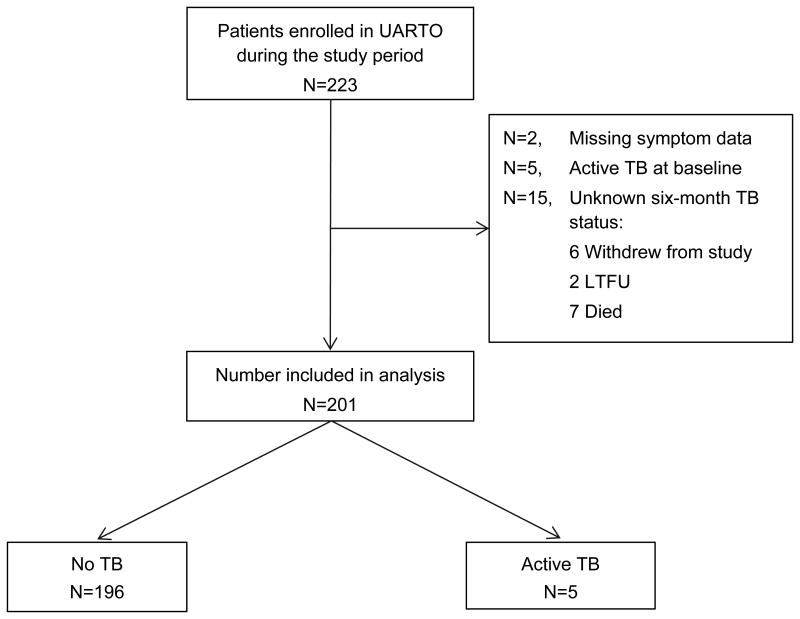
Overall, 223 patients were consecutively enrolled in the UARTO cohort during the study period, all of whom had stored baseline serum available for this analysis (Figure 1). We excluded two patients missing baseline symptom data, five patients receiving TB treatment at the time of study enrollment, and 15 patients with unknown six-month TB status (six withdrew study consent, seven died, and two were lost to follow-up). Causes of death were ascertained by the UARTO study using a standardized death form and included review of all available baseline and follow-up data and telephone interview of family members. Causes of death in this study included: zidovudine-induced hemolytic anemia (n=1), anemia during third trimester of pregnancy (n=1), acute respiratory tract infection (n=1), possible disseminated Kaposis sarcoma (KS; n=1), possible disseminated TB (n=1), and unknown (n=2). Baseline POC-CRP levels were elevated in only two of the patients who died: possible disseminated KS (POC-CRP = 35.9 mg/L) and possible disseminated TB (POC-CRP = 21.4 mg/L). Although one of the two patients lost to follow-up had an elevated baseline POC-CRP level (24.0 mg/L), no additional information beyond the baseline visit was available for either patient.
Figure 1. Patient flow diagram.
Overall, 223 HIV-infected adults initiating ART were enrolled in the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort from May 2007 and April 2009, all of whom had stored baseline serum available for this analysis. We excluded two patients missing baseline symptom data, five patients receiving TB treatment at the time of study enrollment, and 15 patients with unknown six-month TB status (six withdrew study consent, two were lost to follow-up [LTFU] and seven died). Thus, 201 patients were included in this analysis.
Of 201 patients included in the analysis, 140 (70%) were female and the median age was 34 years (interquartile range [IQR] 28-40; Table 1). Patients had advanced HIV disease with a median CD4 cell-count of 138 cells/μL (IQR 84-207). Five patients (2.5%) were assigned a definite (n=3) or probable (n=2) diagnosis of active TB within six months of study enrollment.
Table 1. Demographic and clinical characteristics.
| Characteristic, N (%) | Total | TB* (N=5) |
No TB (N=196) |
p-value |
|---|---|---|---|---|
| Age (years)† | 34 (28-40) | 33 (32-47) | 34 (28-40) | 0.45 |
| Female | 140 (70%) | 2 (40%) | 138 (70%) | 0.15 |
| CD4 count (cells/μL)† | 138 (84-207) | 175 (154-183) | 137 (81-207) | 0.34 |
| Prior TB | 12 (6%) | 1 (20%) | 11 (6%) | 0.19 |
| Cough | 105 (52%) | 3 (60%) | 102 (52%) | 0.73 |
| Fever | 64 (32%) | 4 (80%) | 60 (31%) | 0.02 |
| Night sweats | 64 (33%) | 2 (40%) | 62 (32%) | 0.69 |
| Weight loss | 68 (34%) | 3 (60%) | 65 (33%) | 0.21 |
| WHO symptom screen positive | 159 (79%) | 5 (100%) | 154 (79%) | 0.25 |
| Elevated POC-CRP | 29 (14%) | 4 (80%) | 25 (13%) | <0.001 |
| POC-CRP (mg/L)† | 2.5 (2.5-4.2) | 32.0 (15.0-45.5) | 2.5 (2.5-3.9) | 0.003 |
Abbreviations: TB (tuberculosis); WHO (World Health Organization); POC-CRP (point-of-care C-reactive protein).
Legend:
TB defined as definite (smear- and/or culture-confirmed TB) or probable cases (TB treatment without microbiologic confirmation but documented improvement with treatment).
Continuous variables presented as medians (interquartile range).
Screening for active TB
Despite the low TB prevalence observed in this cohort, 159 (79%) patients screened positive by the WHO symptoms screen. In contrast, only 29 (14%) had an elevated POC-CRP level, a 65% (95% CI: 50-79, p<0.0001) absolute reduction in the proportion who would require further TB testing. Agreement between the WHO symptom screen and POC-CRP for TB screening was poor, κ statistic = 0.09, 95% CI: 0.05- 0.12, p=0.001). Median POC-CRP levels were significantly higher in TB patients compared to those without active TB (32.0 mg/L [IQR 15.0-45.5] vs. 2.5 mg/L [IQR 2.5-3.9], p=0.003; Supporting Figure 1).
The WHO symptom screen was positive in all five patients with active TB (sensitivity 100%, 95% CI: 48-100) and POC-CRP levels were elevated in four of five patients with active TB (sensitivity 80%, 95% CI: 28-100; Table 2). There was no significant difference in sensitivity between the WHO symptom screen and POC-CRP testing (difference 20%; 95% CI: -19 to +59, p=0.30) and both tests had high NPV (WHO symptom screen: 100%, 95% CI: 92-100; POC-CRP: 99%, 95% CI: 97-100).
Table 2. Diagnostic accuracy of TB screening strategies.
| WHO symptom screen | POC-CRP | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| % Sensitivity (95% CI) | 100% (48-100) | 80% (28-100) | -20% (-59 to +19) | 0.30 |
| % Specificity (95% CI) | 21% (16-28) | 87% (82-92) | +66% (+52 to +79) | <0.0001 |
| NPV (95% CI) | 100% (92-100) | 99% (97-100) | -- | -- |
| PPV (95% CI) | 3% (1-7) | 14% (4-32) | -- | -- |
Abbreviations: WHO (World Health Organization); POC-CRP (point-of-care C-reactive protein); TB (tuberculosis); CI (confidence interval); PPV (positive predictive value); NPV (negative predictive value).
Among the 196 patients without active TB, the WHO symptom screen was negative in 42 patients (specificity 21%, 95% CI: 16-28) and POC-CRP levels were normal in 171 patients (specificity 87%, 95% CI: 82-92; Table 2). The specificity of the WHO symptom screen was significantly lower than that of POC-CRP testing (difference 66%, 95% CI: 52-79, p<0.0001).
Selection of patients for IPT
The WHO symptom screen would have classified 42 of 196 (21%, 95% CI: 16-28) patients without active TB and 0 of 5 (0%, 95% CI: 100) patients with active TB as eligible for IPT (Figure 2). If POC-CRP were used instead, one patient with active TB would have been incorrectly reclassified as eligible for IPT (reclassification of cases = -20%, p=0.32). In contrast, among patients without active TB, POC-CRP testing would have correctly reclassified 129 patients as immediately eligible for IPT (reclassification of non-cases = +66%, p=<0.001). Thus, POC-CRP significantly improved selection of patients for IPT (NRI 46%, 95% CI: 2-87, p=0.03).
Figure 2. Reclassification of patients eligible for isoniazid preventive therapy.
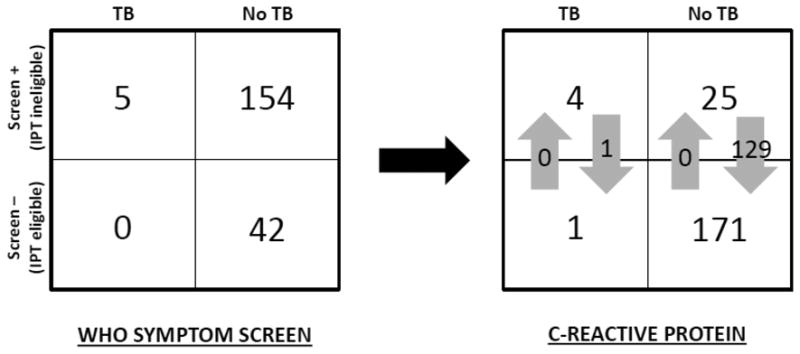
If isoniazid preventive therapy (IPT) eligibility were determined by the WHO symptom screen, no patients with active TB but only 42 of 196 patients without active TB would have been considered eligible for IPT. If point-of-care C-reactive protein (POC-CRP) were used instead, one patient with active TB (reclassification of cases = -20%, p=0.32) and 129 patients without active TB (reclassification of non-cases = +66%, p<0.001) would have been reclassified as being eligible for IPT. Thus, TB screening with POC-CRP would have resulted in a net reclassification improvement (NRI) of 46% (95% CI: 2-87, p=0.03).
Impact of TB screening strategies
In order to estimate the impact of a POC-CRP based screening strategy relative to the WHO symptom screen in settings with higher TB prevalence, we applied the point estimates for sensitivity and specificity of both screening strategies to a hypothetical population of 1,000 patients with varying TB prevalence: 5%, 10% and 15% (Table 3). Both screening strategies had high NPV (≥ 96%) for active TB, irrespective of TB prevalence. POC-CRP incorrectly classified between ten (5% TB prevalence) and 30 (15% TB prevalence) TB cases as IPT-eligible (i.e., false negatives) However, relative to the WHO symptom screen, POC-CRP correctly identified between 561 (from 179 to 740 patients; 15% TB prevalence) and 627 (from 200 to 827 patients; 5% TB prevalence) more PLHIV as immediately eligible for IPT (i.e., true negatives). Furthermore, POC-CRP testing reduced the number of PLHIV requiring additional TB testing because of a positive screen by 591 (from 821 to 230 patients; 15% TB prevalence) to 637 (from 800 to 163 patients; 5% TB prevalence).
Table 3. Impact of TB screening strategies at varying TB prevalence (n=1000).
| 5% TB prevalence | 10% TB prevalence | 15% TB prevalence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Screening strategy | TPa | FNb | FPc | TNd | TPa | FNb | FPc | TNd | TPa | FNb | FPc | TNd |
|
|
|
|
|
|||||||||
| *WHO symptom screen | 50 | 0 | 750 | 200 | 100 | 0 | 711 | 189 | 150 | 0 | 671 | 179 |
| †POC-CRP testing | 40 | 10 | 123 | 827 | 80 | 20 | 117 | 783 | 120 | 30 | 110 | 740 |
Abbreviations: TB (tuberculosis); WHO (World Health Organization); POC-CRP (point-of-care C-reactive protein).
TP, True Positives – number of patients with TB classified as ineligible for isoniazid preventive therapy (IPT)
FN, False Negatives – number of patients with TB classified as eligible for IPT
FP, False Positives – number of patients without TB classified as ineligible for IPT
TN, True Negatives – number of patients without TB classified as eligible for IPT
Legend:
Assume WHO symptom screen sensitivity 100% and specificity 21% (study estimates).
Assume POC-CRP sensitivity 80% and specificity 87% (study estimates).
Discussion
To reduce the incidence of TB among PLHIV, the WHO has recently recommended that all PLHIV who screen-negative by the WHO symptom screen (absence of cough, fever, night sweats and weight loss) be started on IPT [3]. However, this symptom-based screen is falsely positive in most PLHIV without active TB [5-8]. In this study, we found that POC-CRP testing has a significantly lower false-positive rate. The impact of this finding was a greater than four-fold increase (from 21% to 87%) in the proportion of patients immediately eligible for IPT and a corresponding decrease in the proportion of patients who would have required referral for more costly TB testing. These findings strongly suggest that a TB screening algorithm inclusive of POC-CRP could facilitate rapid initiation of IPT in the vast majority of PLHIV without active TB, and target ICF activities to a much smaller group of patients.
The recent identification of a highly-sensitive, symptom-based screen to determine IPT eligibility has been considered a major advancement for both HIV and TB care in resource-limited settings [4]. Reliable exclusion of TB among PLHIV allows for safe initiation of TB preventive therapies (i.e., IPT and ART) and, ultimately leads to substantial reductions in TB incidence and disease transmission. While most studies have confirmed the high sensitivity of the WHO symptom screen, specificity has been lower than the 50% reported in the meta-analysis by Getahun et al that informed WHO guidelines [4], particularly in recent studies conducted in populations with advanced HIV/AIDS [5-8]. Indeed, even in the Getahun et al meta-analysis, the specificity of the symptom screen decreased from 50% to 23% when the population was restricted to patients with CD4 cell-counts <200 cells/μL (personal communication, H. Getahun). Thus, the WHO symptom screen unnecessarily denies IPT to the vast majority of patients who are at greatest risk of developing active TB.
Our findings suggest that a TB screening algorithm inclusive of POC-CRP testing could have enormous public health impact. Even in settings with high (15%) TB prevalence, POC-CRP testing retained high NPV and identified considerably more patients as being eligible for IPT than the WHO symptom screen. Furthermore, POC-CRP substantially reduced the number of patients who screen-positive and who would therefore require additional TB diagnostic testing. By limiting such testing to those at highest risk of active TB, POC-CRP could improve the efficiency and lower the cost of ICF, another WHO-endorsed strategy to reduce the burden of TB among PLHIV [3].
The number of TB cases in our study was too small to adequately assess the sensitivity of POC-CRP for active TB. However, studies evaluating CRP for pulmonary TB diagnosis among symptomatic patients have consistently demonstrated high sensitivity, although different cut-points were used to classify test results. In addition, Lawn et al recently evaluated CRP-based TB screening among HIV-infected patients initiating ART, regardless of symptoms, in Cape Town, South Africa [8]. Although the authors concluded CRP was not useful in their setting, CRP (at a cut-point of 10 mg/L) had similar sensitivity (85% [IQR 75-92] vs. 83% [IQR 73-90]) and greater specificity (58% [IQR 53-62] vs. 33% [IQR 29-38]) than the WHO symptom screen [8]. Moreover, the sensitivity of CRP for active TB was higher than that previously reported in the same setting for POC microbiologic tests such as Xpert MTB/RIF and urine lipoarabinomannan (LAM) [5,27]; these data suggest that if used as an initial screening test, POC-CRP would miss fewer cases of active TB than Xpert MTB/RIF and urine LAM. Ultimately, because all current tests for TB including culture will miss some TB cases, the potential costs of TB screening (generation of isoniazid-resistant TB cases) should be weighed against the benefits (number of TB cases averted through IPT and the number of TB cases diagnosed through more focused ICF activities). Our data and those of Lawn et al suggest that the cost-benefit ratio is far better with POC-CRP than with the WHO symptom screen, the currently recommended TB screening strategy.
This study has several potential limitations. First, microbiologic testing for TB at the time of enrollment was not part of the protocol for the UARTO study; in the absence of microbiologic data, TB status was adjudicated by an expert committee of infectious disease specialists. However, it is unlikely that TB cases were missed and sensitivity estimates inflated because patients with advanced HIV/AIDS co-infected with TB are unlikely to survive beyond six months without TB treatment [24]. Second, we excluded patients for whom six-month TB status was unknown. Although exclusion of patients who died or were lost to follow-up may have resulted in a ‘healthier’ cohort for our analysis, causes of death were ascertained for most of those patients who died – a significant strength of this study – and further support the utility of POC-CRP testing to identify patients with likely TB. Third, we chose to study patients initiating ART (i.e., patients with CD4 cell-counts <200 cells/μL) as the risk for TB (and the need for IPT) is greatest among this sub-population. Thus, our results may not be applicable to other HIV sub-groups such as PLHIV ineligible for ART by CD4 cell-count and those already on ART. Finally, because CRP was measured using a POC assay on stored serum from patients enrolled in the UARTO study, the operational feasibility of a POC-CRP assay at HIV or TB clinics in low-income countries should be further studied in the context of implementation.
In summary, POC-CRP testing is a promising tool to improve implementation of IPT and ICF, two components of the WHO's 3 I's strategy for reducing the burden of TB among PLHIV [3]. In light of the substantial difference in test specificity observed in our study and prior data on the high sensitivity of CRP for pulmonary TB, HIV programs should begin utilizing TB screening algorithms inclusive of POC-CRP testing. Multi-center studies that report on programmatic experience would further strengthen the evidence-base behind POC-CRP testing.
Supplementary Material
Supporting Figure 1. Distribution of point-of-care C-reactive protein by tuberculosis status. Among patients without active TB, the median point-of-care C-reactive protein (POC-CRP) level was 2.5 mg/L (IQR: 2.5-3.9 mg/L) with range: 2.5-185.6 mg/L. Among patients with active TB, the median POC-CRP was 32.0 mg/L (IQR: 15.0-45.5 mg/L) with range: 2.5-126.4 mg/L. Compared to non-TB patients, the median POC-CRP level was significantly higher among TB patients (p= 0.003).
Acknowledgments
The authors would like to thank the patients, staff, and administration of the Mbarara University of Science and Technology Immune Suppression Syndrome Clinic for supporting and/or participating in this study. The authors would also like to thank BodiTech Med Inc., South Korea for providing access to the iCHROMA POC-CRP Reader and test materials.
Source of Funding: This research was supported by a grant from the National Institutes of Health, University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (P30 AI027763 to CY) and the National Heart, Lung and Blood Institutes at the National Institutes of Health [T32 HL007185 to CY]. The iCHROMA POC-CRP Reader and POC-CRP assays used in this study were donated by the manufacturer, BodiTech Med Inc., South Korea. Neither the funders nor BodiTech Med Inc. had any role in study design, data collection/analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization and Stop TB Partnership. WHO; 2011. The Global Plan to Stop TB 2011-2015. Available at http://www.stoptb.org/global/plan/ [Google Scholar]
- 3.World Health Organization. World Health Organization; 2011. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. Available at http://www.who.int/hiv/pub/tb/9789241500708/en/index.html. [Google Scholar]
- 4.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: A prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Kerkhoff AD, Vogt M, et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54(8):1071–1079. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufa T, Mngomezulu V, Charalambous S, et al. Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr. 2012;60(2):e22–e28. doi: 10.1097/QAI.0b013e318251ae0b. [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Kerkhoff AD, Vogt M, et al. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17(5):636–643. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One. 2011;6(1):e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez GG, Sabri E, Ling D, et al. A model to rule out smear-negative tuberculosis among symptomatic HIV patients using C-reactive protein. Int J Tuberc Lung Dis. 2012;16(9):1247–1251. doi: 10.5588/ijtld.11.0743. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Obeng J, Acheampong JW, et al. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(4):340–344. [PubMed] [Google Scholar]
- 12.Schleicher GK, Herbert V, Brink A, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;25(4):688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Wiktor S, Coulibaly D, et al. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95(1):41–42. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- 14.Breen RA, Leonard O, Perrin FM, et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? Int J Tuberc Lung Dis. 2008;12(1):44–49. [PubMed] [Google Scholar]
- 15.Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J. 2003;21:939–943. doi: 10.1183/09031936.03.00055103. [DOI] [PubMed] [Google Scholar]
- 16.Sage EK, Noursadeghi M, Evans HE, et al. Prognostic value of C-reactive protein in HIV-infected patients with Pneumocystis jirovecii pneumonia. Int J STD AIDS. 2010;21(4):288–292. doi: 10.1258/ijsa.2010.009551. [DOI] [PubMed] [Google Scholar]
- 17.Kang YA, Kwon SY, Yoon HI, et al. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med. 2009;24(4):337–342. doi: 10.3904/kjim.2009.24.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi CM, Kang CI, Jeung WK, et al. Role of the C-reactive protein for the diagnosis of TB among military personnel in South Korea. Int J Tuberc Lung Dis. 2007;11(2):233–236. [PubMed] [Google Scholar]
- 19.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 20.Weiser SD, Tsai AC, Gupta R, et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS. 2012;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiser SD, Gupta R, Tsai AC, et al. Changes in food insecurity, nutritional status, and physical health status after antiretroviral therapy initiation in rural Uganda. J Acquir Immune Defic Syndr. 2012;61(2):179–186. doi: 10.1097/QAI.0b013e318261f064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shine B, de Beer FC, Pepys MB. Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta. 1981;117(1):13–23. doi: 10.1016/0009-8981(81)90005-x. [DOI] [PubMed] [Google Scholar]
- 23.Claus DR, Osmand AP, Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976;87:120–128. [PubMed] [Google Scholar]
- 24.Lawn SD, Kranzer K, Edwards DJ, et al. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24(9):1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajasekaran S, Raja K, Jeyaseelan L, et al. Post-HAART tuberculosis in adults and adolescents with HIV in India: incidence, clinical and immunological profile. Indian J Tuberc. 2009;56(2):69–76. [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-212. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Kerkhoff AD, Vogt M, et al. Diagnostic accuracy of a low-cost urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–209. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1. Distribution of point-of-care C-reactive protein by tuberculosis status. Among patients without active TB, the median point-of-care C-reactive protein (POC-CRP) level was 2.5 mg/L (IQR: 2.5-3.9 mg/L) with range: 2.5-185.6 mg/L. Among patients with active TB, the median POC-CRP was 32.0 mg/L (IQR: 15.0-45.5 mg/L) with range: 2.5-126.4 mg/L. Compared to non-TB patients, the median POC-CRP level was significantly higher among TB patients (p= 0.003).