Abstract
Background
HIV-infected individuals have a higher incidence of head and neck cancer.
Methods
Case series of 94 HIV-infected head and neck cancer patients (HIV-HNC) at six tertiary care referral centers in the US between 1991–2011. Clinical and risk factor data were abstracted from the medical record. Risk factors for survival were analyzed using Cox proportional hazard models. Human papillomavirus (HPV) and p16 testing was performed in 46 tumors. Findings were compared with SEER HNC (US-HNC) data.
Results
This study represents the largest HIV-HNC series reported to date. HIV-HNC cases were more likely than US-HNC to be male (91% vs. 68%), younger (median 50 vs. 62 years), non-White (49% vs.18%), and current smokers (61% vs. 18%). Median HIV-HNC survival was not appreciably lower than US-HNC survival (63 vs. 61 months). At diagnosis, most cases were currently on HAART (77%), but had detectable HIV viremia (99%) and median CD4 was 300 cells/μL (IQR=167–500). HPV was detected in 30% of HIV-HNC and 64% of HIV-oropharyngeal cases. Median survival was significantly lower among those with CD4 counts ≤200 than >200 cells/μL at diagnosis (16.1 vs. 72.8 months, p<0.001). In multivariate analysis, poorer survival was associated with CD4 <100 cells/μL (aHR=3.09, 95%CI=1.15–8.30), larynx/hypopharynx site (aHR=3.54, 95%CI=1.34–9.35), and current tobacco use (aHR=2.54, 95%CI=0.96–6.76).
Conclusion
Risk factors for the development of HNC in patients with HIV infection are similar to the general population, including both HPV-related and tobacco/alcohol-related HNC.
Keywords: HIV, HNC, epidemiology, case series, survival, HPV, immunosuppression, OPSCC
Introduction
Recent research has shown that HIV-infected individuals have elevated rates of several cancers, and that an increasing proportion of these cancers are now non-AIDS related malignancies.1–5 HIV-infected individuals have a higher incidence of both virus-related cancers, and tobacco/alcohol-related cancers, due to higher prevalence of HIV-induced inflammation, immunodeficiency, and tobacco use among HIV-infected compared with HIV-uninfected individuals (40–60% vs. 17%).2,4,6–9 Malignancies with an infectious origin are also elevated among HIV-infected individuals, including malignancies related to Epstein Barr Virus (lymphoma, nasopharyngeal cancer), Hepatitis B and C (hepatocellular carcinoma), Human Herpesvirus 8 (Kaposi Sarcoma), and Human Papillomavirus (oropharyngeal, cervical and anal cancer).2–4
Human papillomavirus (HPV) infection causes a subset of oropharyngeal squamous cell cancers.10,11 Incidence of HPV-positive oropharyngeal cancers have increased over the past several decades, in contrast to the decreasing incidence of other head and neck cancer sites over the same time period.10,12 The basic biology and prognosis associated with HPV-related oropharyngeal cancers appears distinct from tobacco related head and neck cancers (HNC)13,14 but these characteristics are unknown for HIV-infected individuals.
HIV-infected individuals are known to have an increased prevalence of oral HPV infection (~2-fold)15–18, and a higher incidence of HNC (~2.3-fold).19–21 An increased risk of oropharyngeal cancer has been observed in AIDS-cancer registry studies3,21,22, as well as two HIV-infected cohort studies which compared HIV cases to population controls20,23, although one other cohort study found a more modest and not statistically significant increased risk2. The AIDS-cancer registry studies suggested that HIV-infected individuals are at 1.6–6.0-fold increased risk of developing oropharyngeal cancer, and 1.7–4.0 fold increased risk of developing head and neck cancer overall compared to the general population3,21,22.
Prevalence of oral HPV16 infection, the HPV type which accounts for the majority of HPV-related oropharynx cancers, is 2–7% among HIV-infected individuals compared to ~1.0% among healthy U.S. adults.15,18,24,25 Since many treated HIV-infected individuals now have a near normal life span because of the effectiveness of highly active antiretroviral therapy (HAART), they now have the “opportunity” to develop HPV-related cancers at higher rates than were observed in the pre-HAART era.20 Given the higher levels of both tobacco use and sexual risk factors among HIV-infected individuals, it is unclear how much of the increased cancer risk is explained by: 1) increased tobacco use, 2) higher number of oral sexual partners (oral HPV exposure), 3) different oral health condition in HIV-infected individuals or 4) the effect of immunosuppression on the natural history of oral HPV infection.
In this study, we compare the epidemiology of HNC among HIV-infected individuals with available data on HNC cases in the U.S. This is one of the first studies to characterize HNC and the role of HPV among HIV-infected HNC cases.
Methods
Study Population
This study is a case series of HIV-infected patients seen at six tertiary care referral centers between 1991 and 2011 across the United States. Cases were contributed from five HNC-SPORE sites including MD Anderson Cancer Center (n=34), Emory University (n=26), University of Pittsburgh (n=12), University of Michigan (n=5), and Johns Hopkins University (n=5), as well as a sixth clinical site at Mount Sinai Medical Center (n=12). HIV-infected patients diagnosed with incident HNC were retrospectively identified through medical record review at each center. To identify cases, pathology records were searched using terms HIV and HNC to identify archived material, then cross-referenced with pathology databases to requisition appropriate tumor material. A second query was performed using the electronic HIV and oncology clinic databases at each institution, to identify those with a history of HIV and HNC. Demographic, risk factor and clinical-pathologic data were abstracted from the medical record and a sample of tumor tissue was obtained, when available, for biomarker testing. IRB approval or exemption to share de-identified data was obtained at each study site.
Medical Record Abstraction
Each site abstracted demographic, biologic, and behavioral information from the medical record of each patient using a standardized survey form. De-identified information was then submitted to the study datacenter. Quality assurance was performed on abstracted data including range and logic checks, with re-verification or correction of all atypical data reported by the sites, through a second medical record review. Cases which had documentation of HIV-infection and had clinical cancer data were included in the study even if the clinical medical record at the cancer center did not include other HIV-related data (CD4, HIV viral load, HAART use) in the medical record; 25% of cases did not have these data.
Tumor HPV Testing
There were 39 cases with available tumor sample for centralized HPV testing including 22 oral cavity, 9 oropharyngeal, 6 laryngeal, 1 hypopharyngeal, and one multi-site (base of tongue/hypopharynx) cancers. One of these cases only had p16 testing, due to insufficient material. In addition to these 39 cases there were 6 cases from Mount Sinai (4 oropharyngeal, 1 supraglottis [laryngeal], and one multi-site pharyngeal wall/laryngeal case) which did not have tumor available for centralized testing but had been tested locally for p16; four of these had been tested locally for HPV. There was also 1 oropharyngeal case from the Pittsburgh which had HPV but not p16 data in the medical record. The abstracted tumor test results for these seven cases were included in this analysis, resulting in 46 total HIV-HNC cases with tumor HPV status: 45 with p16 and 43 with HPV testing. A composite HPV measure was made (using p16 to infer HPV status in those without HPV data). The 46 tumors with HPV data represented a similar distribution of calendar time as the dataset overall, with 48% of tested tumors diagnosed 2006–2011, 35% diagnosed 2001–2005, and 17% diagnosed 1991–2000.
For the 39 tumors centrally tested, formalin fixed paraffin embedded tumor specimens were collected from each center, given a barcode, and tested in a single centralized laboratory. Tumors were tested for: 1) oncogenic HPV by in situ-hybridization (ISH), 2) oncogenic HPV DNA using PCR-MassArray, and 3) p16 expression by immunohistochemistry (IHC). 26,27 Tissue microarrays (TMAs) were created from the tumor tissue when sufficient material was available, or tests were conducted on individual slides when the biopsy was too small. DNA was isolated from a single tumor tissue core or microdissected from tissue sections for small samples. Formalin fixed paraffin embedded blocks were evaluated by a board certified head and neck pathologist in the University of Michigan Pathology Laboratory for the presence of sufficient tumor for construction of a TMA and for DNA isolation. Cases that were p16 positive but HPV negative were re-assessed with L1 consensus primers and sequenced to detect and identify other HPV types. ISH was also performed for HPV DNA using the INFORM HPV III assay (Ventana, AZ) to detect any of 12 high risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 66).. Expression of p16ink4a was assessed by IHC using the CINtec p16INK4a Histology kit and protocol (mtm laboratories, Westborough, MA). These tests were carried out on the TMA or on individual slides from tissue samples too small to be arrayed. The p16 staining was scored for proportion of stained tumor cells and for staining intensity; each on a four-point scale: 1=no staining, 2=low, 3=moderate, and 4=high; proportion of tumor cells staining; and 1: <5%, 2: 5–20%, 3: 21–50%, 4: 51–100%. IHC scores (proportion times intensity) from each 0.6 mm diameter core or tissue section were averaged for each patient and IHC scores of 12–16 were considered to be positive for p16. Both assays were scored at 400× magnification by another pathologist who was blinded to the origin of the individual samples.
Sample with adequate DNA that was positive by p16ink4a but negative by HPV16 ISH were re-examined by consensus PCR targeting the L1 region of the viral genome using PGMY primers28 and sequencing of the PCR product to identify the unknown type. All specimens that were found to contain an identifiable high risk HPV type were scored as HPV positive. High risk oncogenic HPV types were assessed in tumor DNA using PCR-MassArray designed to detect and identify 15 high-risk HPV subtypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 73), using type-specific, multiplex, competitive PCR and single base extension followed by MALDI-TOF mass spectrometry analysis, as previously described.27
Analytic Methods
HIV-infected HNC (HIV-HNC) cases in this study were stratified by tumor site. Sites were classified a priori as oropharynx (ICDO C01.9, C02.4, C09.0–10.9), oral cavity (ICDO C00.3–00.9, C02.0–02.3, C02.8–C6.9), larynx (ICDO C32.0–32.9, C10.1), and hypopharynx (ICDO C12.9, C13.0–13.9).
Characteristics of HIV-HNC cases were described using medians and interquartile range (IQR) for continuous variables and proportions for categorical variables. Demographic and behavioral characteristics were compared by tumor site using Fisher’s exact test for categorical and test of medians for continuous data. Characteristics of HIV-oropharynx cases were similarly compared by tumor HPV status. Survival was analyzed using Kaplan-Meier curves, with log-rank testing to assess for statistically significant differences between groups. Cox proportional hazards regression analysis was used to test the effect of multiple covariates on overall survival. Final models retained risk factors which were marginally or statistically significant (p<0.10) and those variables known to be relevant from previous research.
Characteristics of HNC cases in this study, stratified by tumor site and HPV status, are also presented alongside national HNC data from the Surveillance Epidemiology and End Results (SEER), referred to as US-HNC, using SEER cases between 1991–2009 (analysis performed using SEER 18, from SEER-STAT 8.02).
Results
HIV-infected individuals diagnosed with incident HNC between 1991–2011 (n=94) were identified across six participating U.S. sites. Demographic information was available for all 94 cases, HIV-related clinical data were available for 71 (76%) cases, and tumor samples were available for centralized testing or had local testing result for 46 (49%) cases.
HIV-HNC cases included 38 oral cavity and 31 oropharyngeal cancers, as well as 17 laryngeal, 5 hypopharyngeal, and 3 multi-site cancers with either a laryngeal or hypopharyngeal site of involvement (which were grouped together). As described in Supplemental Digital Content Table 1, oropharynx and oral cavity cases were more likely than larynx/hypopharynx cases to be male (97% vs. 72%, p=0.002). Median age was 50 years, and most cases were current tobacco (59%) and alcohol (55%) users, and half were diagnosed at Stage IV (49%). Only 11% percent of cases were never-smokers, and 15% had never used alcohol. While the majority of oral cavity cases were white (61%), a significantly smaller proportion of oropharynx and larynx/hypopharynx cases were white (36%, p=0.014). Age, gender, and CD4 cell count of cases at diagnosis were similar across study sites. However, tumor site differed by study site, with a higher proportion of oral cavity cases at MDA and a higher proportion of oropharyngeal cases at Mt Sinai and JHU (Supplemental Digital Content Table 1).
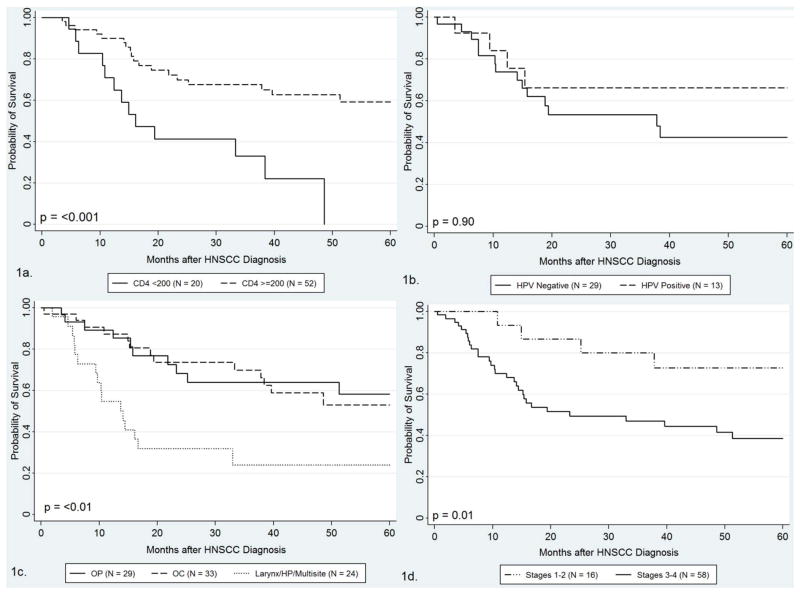
Survival information was available for 86 cases (median=49 months). Survival varied considerably by tumor site, with higher survival among oropharyngeal (82 months) and oral cavity (63 months) cases than the larynx/hypopharynx (14 months) cases, p=0.001 (Supplemental Digital Content Table 1 and Figure 1a). Survival was especially poor among those with CD4<100 at diagnosis (median 15 months IQR=10–38), and increased among those with CD4 of 100–199 (33 months) 200–349 (63 months) and ≥350 (73 months) cells/μL at diagnosis. Survival was significantly poorer among those with CD4 counts<200 vs ≥200 cells/μL at diagnosis (median 16.1 vs. 72.8 months, p<0.001).
Of the 46 HIV-HNC cases with tumor HPV testing, 7 (17%) contained HPV16 DNA, including 6 of 11 (55%) oropharyngeal cancers (Supplemental Digital Content Table 1, p<0.001). Four other HPV types were identified, all in oral cavity tumors, including: HPV26 (hard palate), HPV33 (tongue), HPV69 (alveolar ridge), and HPV82 (mandible). HPV16 was also detected in one pyriform sinus (hypopharyngeal) case. Using our composite HPV-positive status (which included any oncogenic HPV and p16 status for those without HPV data), 30% of HIV-HNC and 64% of HIV-oropharyngeal cancers were considered HPV-related. High p16 expression was detected in 42% of the 45 HIV-HNC cases tested, including 93% of HPV-positive cases but only 23% of HPV-negative cases. Considering only oropharyngeal cases, a marginally higher proportion of white than black cases were HPV16-positive (100% [5/5] vs. 38% [3/8]; p=0.051). Cases with and without HPV tumor data were similar with regards to gender, race, age, subsite and CD4 at diagnosis, but varied by study site including the following proportion of cases with known tumor status at each site: UP (11/12), Emory (20/27), JHU (3/5), Mt Sinai (6/12), Mich (2/6)m and MDA (4/34).
Table 1 describes HIV-related variables of interest among the 71 cases (76% of all HNC) for whom HIV clinical information could be found in the medical record. Cases with and without HIV-related variables were similar with regards to gender, race, age, and subsite, but were more likely to come from MDA, Mich and UP (data not shown). Most patients were on HAART at cancer diagnosis (77%). Interestingly, only one case had an undetectable HIV viral load at cancer diagnosis, suggesting HIV might be inadequately controlled in many of these cases (Table 1). Median time from HIV diagnosis to cancer was 6 years (IQR=2–10 years). Median CD4 counts at cancer diagnosis varied by tumor site (Table 1), with a higher proportion of immunosuppressed (CD4<200 cells/μL) patients among laryngeal/hypopharyngeal (53%) than oropharyngeal (12%, p=0.004) or oral cavity (26%, p=0.07) cases. Among the 39 cases with available nadir CD4 cell counts, 28 (72%) had nadir CD4 cell counts <200 cells/μL, including 13 individuals with CD4 counts <50 cells/μL.
Table 1.
HIV-related characteristics of cases at cancer diagnosis, among the 71 HIV-HNC cases with HIV-related clinical data available
| Characteristics at cancer diagnosis | Total (All HNC) (N=71) | Oropharyngeal (N=26) | Oral cavity (N=27) | Larynx/HP (N=18) | p-value # |
|---|---|---|---|---|---|
| Currently on antiretroviral therapy (ART) | 0.098 | ||||
| No | 16 (23%) | 6 (23%) | 3 (11%) | 7 (39%) | |
| Yes | 55 (77%) | 20 (77%) | 24 (89%) | 11 (61%) | |
| Ever on antiretroviral therapy (ART) | 1.00 | ||||
| No | 5 ( 7%) | 2 ( 8%) | 2 ( 7%) | 1 ( 6%) | |
| Yes | 57 (80%) | 21 (81%) | 24 (89%) | 12 (67%) | |
| Unknown | 9 (13%) | 3 (12%) | 1 ( 4%) | 5 (28%) | |
| HIV Viral Load | |||||
| Median (IQR) | 26388 (65,>million) | >million (58,>million) | 5300 (50,>million) | 63,235 (244,>million) | 0.20 |
| Undetectable | 1 ( 1%) | 0 (0%) | 1 ( 4%) | 0 | 0.35 |
| 40–1000 copies/ul | 28 (39%) | 9 (35%) | 12 (44%) | 7 (39%) | |
| 1001–100,000 copies/ul | 10 (14%) | 2 (8%) | 6 (22%) | 2 (11%) | |
| > 100,000 copies/ul | 32 (45%) | 15 (58%) | 8 (30%) | 9 (50%) | |
| CD4 cell count | |||||
| Median (IQR) | 300 (167–500) | 442 (307, 715) | 290 (147,500) | 205 (108,342) | 0.002 |
| <200 | 19 (26%) | 3 (12%) | 7 (26%) | 8 (53%) | 0.029 |
| 200–349 | 24 (33%) | 7 (27%) | 11 (41%) | 5 (33%) | |
| ≥ 350 | 29 (40%) | 16 (62%) | 9 (33%) | 2 (13%) | |
| Nadir CD4 cell count | |||||
| Median (IQR) | 110 (25, 236) | 148 (46, 263) | 104 (3, 258) | 64 (25, 170) | 0.50 |
| <50 | 13 (18%) | 5 (19%) | 3 (11%) | 4 (27%) | 0.24 |
| 50–199 | 15 (21%) | 7 (27%) | 3 (11%) | 4 (27%) | |
| ≥ 200 | 11 (15%) | 6 (23%) | 3 (11%) | 1 ( 7%) | |
| Unknown | 33 (46%) | 8 (31 %) | 18 (67%) | 6 (40%) | |
| Year of HIV Diagnosis | 0.27 | ||||
| 1991–2000 | 15 (21%) | 9 (35%) | 3 (11%) | 3 ( 17%) | |
| 2001–2011 | 10 (14%) | 3 (12%) | 2 ( 7%) | 5 (28%) | |
| Unknown | 46 (65%) | 14 (54%) | 22 (81%) | 10 (56%) | |
p-value were calculated with Fisher’s exact test for categorical and test of medians for continuous variables. P-values were calculated excluding subjects with missing data
Among 42 individuals with tumor HPV status and follow up, median survival was longer, but not statistically different among HPV-positive than HPV-negative (62.9 vs. 37.8 months, p=0.90, Figure 1b) and p16 positive compared to p16 negative (62.9 vs. 37.8 months, p=0.84) HIV-HNC cases. When restricted to oropharyngeal cancers, there was also a longer median survival of 9 HPV-positive compared to 5 HPV-negative cases, although this was not statistically significant (81 vs. 16 months, p=0.74).
Figure 1.
Survival among the subset of 86 HIV-infected HNC cases with survival data, by CD4 at diagnosis (1a), HPV composite status (1b~), tumor site (1c), and cancer stage (1d).
Suscript for Figure one that should appear ~ Of the 46 subjects with tumor HPV data, 42 had follow-up, therefore survival figure for composite HPV status (Figure 1b) is based on 42 HIV-HNC subjects.
Multivariate risk factors for survival are shown in Table 2. Risk factors associated with poorer overall survival included CD4 <100 cells/μL (aHR=2.52, 95%CI=1.06–6.0), larynx/hypopharynx site (aHR=3.12, 95%CI=1.35–7.23), current tobacco use (aHR=1.92, 95%CI=0.92–4.03), and minority race (aHR=2.73, 95%CI=1.30–5.76). Age, gender, tumor HPV status and tumor stage were not significant predictors of survival
Table 2.
Multivariate risk factors for survival among 84 HIV-HNC patients with data on survival and tumor stage (33 of whom also had data on tumor HPV status).
| Including tumor stage and HPV Status | Final Model | |||||
|---|---|---|---|---|---|---|
| Variable at cancer diagnosis | Hazard Ratio | P value | 95% CI | Hazard Ratio | P value | 95% CI |
| CD4 cell count | ||||||
| ≥200 | 1.00 | 1.00 | ||||
| 100–199 | 1.18 | 0.802 | (0.32–4.42) | 1.22 | 0.742 | (0.38,3.90) |
| <100 | 3.09 | 0.025 | (1.15–8.30) | 2.52 | 0.036 | (1.06,6.0) |
| Unknown | 3.29 | 0.019 | (1.22,8.87) | 1.68 | 0.225 | (0.73,3.88) |
| Tumor Site | ||||||
| Oropharynx | 1.00 | 1.00 | ||||
| Oral Cavity | 1.35 | 0.582 | (0.46,3.91) | 1.03 | 0.949 | (0.44,2.40) |
| Larynx/HP/HNC multisite | 3.54 | 0.011 | (1.34,9.35) | 3.12 | 0.008 | (1.35,7.23) |
| Race/Ethnicity | ||||||
| White non-Hispanic | 1.00 | 1.00 | ||||
| Black NH or Hispanic any race | 2.02 | 0.093 | (0.89,4.58) | 2.73 | 0.008 | (1.30,5.76) |
| Current Tobacco Use | ||||||
| Current smoking status | 2.54 | 0.062 | (0.96,6.76) | 1.92 | 0.083 | (0.92,4.03) |
| Cancer Stage | ||||||
| 1–2 | 1.00 | |||||
| 3–4 | 1.46 | 0.571 | (0.40,5.32) | |||
| Tumor HPV Status | ||||||
| Negative | 1.00 | |||||
| Positive | 0.87 | 0.832 | (0.23,3.25) | |||
| Unknown Status (not tested) | 1.18 | 0.696 | (0.51,2.74) | |||
Among the subset of oropharyngeal cases with tumor available for testing, characteristics of HIV-infected oropharyngeal cases (HIV-OP) with HPV-positive (n=9) and HPV-negative (n=5) tumors were contrasted (Supplemental Digital Content Table 2). Compared with HPV-negative cases, HPV-positive OP cases appear to have higher CD4 cell counts at diagnosis (668 vs. 266, p=0.54) and to be more likely to be younger (52 vs. 58 years, p=0.27) and white (56% vs. 0%, p=0.054), although the number of these cases was limited and none of these differences were statistically significant (Supplemental Digital Content Table 2). When compared to results from two recent large U.S. general population OP studies (US-OP), HIV-OP cases in this study were more likely than US-OP cases to be non-white, to be current smokers, and to be detected at a later stage. HIV-OP cases appeared to have poorer survival than US-OP: (81 vs. 131 months for HPV-positive OP; 16 vs. 20–58 months for HPV-negative); Supplemental Supplemental Digital Content Table 2.10,14
Next we compared characteristics of all HIV-HNC to national HNC cases (US-HNC). Since laryngeal cases are excluded from these US-HNC numbers, laryngeal cases were similarly excluded from the HIV-HNC results in this table, leaving 77 cases (Table 3). HIV-HNC cases were more likely than US-HNC to be male (91% vs. 68%), younger (median age 50 vs. 62 years), non-white (49% vs.18%), and current smokers (61% vs. 18%29), as expected given the different distribution in age, race, and tobacco use in the HIV-infected US population than the general US population. HIV-HNC cases were also more likely than US-HNC to be an advanced stage (60% vs. 20%). Survival was not notably different between HIV-HNC and US-HNC (Table 3).
Table 3.
Comparison of HIV-HNC in this study with general U.S. HNC patient data from Surveillance Epidemiology and End Results (SEER) data from 1991–2009: stratified into all non-laryngeal HNC, oropharyngeal cancer (OPC) only, and oral cavity (OC) cancer only.
| Non-larynx HNC^ | OPC | OC | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HIV+ (N=77) | SEER* | HIV+ (N=31) | SEER* | HIV+ (N=38) | SEER* | |
| Stage~ | ||||||
| T1–2 | 25% | 66% | 11% | 63% | 46% | 76% |
| T3 | 14% | 13% | 19% | 13% | 7% | 9% |
| T4 | 60% | 20% | 70% | 24% | 46% | 15% |
| Gender | ||||||
| Male | 91% | 68% | 97% | 78% | 97% | 62% |
| Female | 9% | 32% | 3% | 22% | 3% | 38% |
| Race | ||||||
| White NH | 51% | 82% | 35% | 85% | 68% | 85% |
| Non-White | 49% | 18% | 65% | 15% | 32% | 15% |
| Age: Median (IQR) | 50 (46, 57) | 62 (53–72) | 52 (46–58) | 58 (52–66) | 49 (46–57) | 65 (53–76) |
| P16 positive tumor | 45% | 62% | 66%14 | 36% | ||
| HPV positive tumor | 31% | 55% | 64%14 | 18% | ||
| Tobacco Smoke | ||||||
| never smoker | 14% | 10% | 23%14 | 18% | ||
| former smoker | 25% | 20% | 33% | |||
| current smoker | 61% | 18%27 | 70% | 17%14 | 48% | |
| Survival in months: median (IQR) | 63 (15,162) | 61 | 82 (22,162) | 82 | 63 (19, not reached) | 69 |
| 12 months | 85% | 80% | 89% | 82% | 87% | 82% |
| 24 months | 65% | 66% | 68% | 71% | 74% | 68% |
| 36 months | 62% | 59% | 64% | 64% | 70% | 61% |
Data from Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.0.2 1991–2009
HNC included C000–C148 (larynx not included in the SEER data, therefore larynx not included in the HIV-HNC column in this table for consistency). Oropharynx coding included C090–C109, C019, C024. Oral Cavity coding included C000–C009, C020–C023, C028–C069.
Data for cancer stage among our HIV-infected cases was limited to cases with known data on stage, including 63 HNC, 27 oropharyngeal, and 28 oral cavity cases.
Discussion
This study is, to our knowledge, the largest case series of HIV-infected HNC to date and these findings help to characterize the epidemiology of HIV-infected individuals with head and neck cancer. HIV-infected individuals have increased exposure to tobacco, alcohol, and HPV infection, the three primary HNC risk factors. This study suggests that a subset of HIV-HNC are HPV-positive, and that as in the general population, these HPV-HNC are primarily oropharyngeal cancers. Tobacco use was common in this population, however HNC were detected in some HIV-infected individuals without the traditional HNC risk factors of tobacco and alcohol use. Thus, this research suggests that head and neck cancer cases among HIV-infected individuals include both HPV-related and tobacco/alcohol related HNC.
While oral HPV prevalence has been associated with HIV-infection and current CD4 cell count in several studies, this is one of the first studies to test for HPV in HIV-HNC tumors. Half of the HIV-oropharyngeal cancers and one quarter of all HIV-HNC were HPV-positive, consistent with some recent population studies,30 but lower than others.11,14 These results are also consistent with one smaller case series of HIV-HNC which reported HPV tumor detection in 6 of 25 (24%) cases.31 As 42% of oropharyngeal cases and 72% of HNC overall were HPV-negative, it suggests that while HPV is a causal factor in a notable number of cases, tobacco use and other factors remain important contributors in the majority of HIV-HNC.
Survival was not notably different between HPV-positive and HPV-negative HNC in this study, however there were a limited number of cases with tumor HPV data and differences in cancer therapy or cause of death were not accounted for. Current tobacco use was associated with poorer survival, consistent with the poorer prognosis seen among HNC smokers in the general population.14
Although almost all of HIV-HNC cases had been on HAART therapy at some point in time, and a large proportion were on HAART at diagnosis, current CD4 cell count was <350 cells/μL in 60% of cases and many participants had a history of low nadir CD4 cell counts. This is consistent with the possibility that immunosuppression may increase oral HPV persistence and/or progression to cancer, similar to that observed for cervical HPV infection and immunosuppression.32 However, the higher median CD4 of HPV16-positive than HPV16-negative oropharyngeal cases argues against a strong role of current CD4 on the final stages of HPV-transformation. Cases with lower CD4 cell count at diagnosis had significantly poorer survival, which might be due to poorer cancer-related outcomes and/or to mortality from other HIV-related comorbidities,. The relatively high current CD4 count among some HPV-positive oropharyngeal cases in our study contrasts with several previous studies, where lower current CD4 count was associated with increased risk of HNC, as well as other HPV-related cancers.2,3,23,33 However, many of the HIV-positive oropharyngeal cases in this study had a history of low nadir CD4 cell counts, suggesting that immunosuppression, if it had an effect on HPV progression, may have acted earlier in the cancer process. Although median CD4 was 300 cells/μL among HIV-HNC cases at diagnosis, all but one case had detectable HIV viremia.
HIV-HNC cases in this study differed from HNC cases in the general US population in terms of demographic (gender, age, race) and behavioral (smoking, drinking) characteristics, although it is unclear whether these differences are entirely explained by differences in the characteristics of the HIV-infected population. Indeed, the median age of HIV-infected individuals in the US is younger than the general US population (~30 years vs. ~ 37years)34. Similarly, a greater proportion of HIV-infected individuals in the US are smokers (50–70%) compared to less than 20% of individuals in the general population.35 Therefore it is of note, that there were some cases in our study that were non-smokers/non-drinkers.
This study has several limitations as well as strengths. Behavioral data were retrospectively abstracted from the medical record and not available for all cases. Because clinical information was not collected prospectively, data on sexual behavior were not available, although we did have data on tobacco and alcohol use for most cases. Tumor tissue was not available for all cases; however, the tumor HPV results that are presented are one of the first explorations of HPV in HIV-HNC tumors. Cases were not systematically sampled but represent a convenience sample of all HIV-HNC cases identified at participating centers. Treatment of cases across sites was not standardized and likely varied, impacting overall survival. Finally, while this is the largest non-registry study of HIV-HNC to date, sample sizes remained limited when stratified by tumor site. Strengths of this study included centralized validated tumor testing, analysis stratification by tumor site and tumor HPV status, inclusion of several U.S. clinical centers, restriction to cases diagnosed between 1991–2011, inclusion of survival data, and a larger sample size than any previous non-registry study.
This multiple institution study describes the epidemiology of head and neck cancer among a case series of HIV-infected individuals in the HAART era in the U.S. It suggests that a subset of HIV-HNC are HPV-positive, and many of these cancers occurred among individuals who were not currently immunosuppressed, but had been immunosuppressed in the past. Many of the HIV-HNC cases were on HAART therapy, but had detectable viremia indicating they were either poorly adherent to HAART, were failing their regimen, or had only recently started therapy. As this study included cases occurring over the past 20 years, the current role of HPV might currently be larger than that captured in this study. As HIV-infected individuals live longer due to HAART, they now have the “opportunity” to develop non-AIDS related cancers including HPV-HNC. Given the higher prevalence of oral HPV16 among HIV-infected individuals, and the suggestion that HPV persistence is increased among immunosuppressed individuals, HIV-infected individuals remain at increased risk for developing HPV-HNC. Earlier diagnosis and treatment of HIV infection with HAART leading to full suppression of viremia and immune reconstitution may reduce cancer risk and improve outcomes overall.
Supplementary Material
Acknowledgments
Source of Funding:
Supported by Head and Neck SPORE Consortium NCI supplement: U. Michigan: P50 CA097248, PI Gregory T. Wolf; U of M Cancer Center Core Grant P30 CA46592; M.D. Anderson: 5P50CA097007, PI: Jeffrey Myers; U. Pittsburgh: P50 CA097190, PI Jennifer Grandis; Johns Hopkins, P50 DE019032, PI: David Sidransky; Emory University: P50CA128613, PI Dong M. Shin; Emory University Center for AIDS research P30 AI050409, PI: James Curran and R01DE021395, PI Gypsyamber DSouza.
Footnotes
Conflicts of Interest: No conflicts of interest.
Author Contributions: The SPORE HNC network contributed collectively to this study. Biospecimens were provided by the sites and processed by the centralized testing laboratory. In addition to the leading contributions of the writing team authors listed above, other important contributions were made by the following:
Tumor Testing and Pathology Contributors
Martin P. Graham, Christine M. Komarck, Lisa A. Peterson, Jonathan B. McHugh (Univ. of Michigan)
Raja Seethala, Simion Chiosea (Univ. of Pittsburgh)
Marina Mosunjac Emory University)
Adel K. Adel El-Naggar (MD Anderson Cancer Center)
William H. Westra (Johns Hopkins University )
Data Coordinating Center
Jeff Lewis (M.D. Anderson Cancer Center)
Nicole Kluz, Alicia Wentz (Johns Hopkins School of Public Health)
Additional Clinical Contributors
Belinda Akpeng (Johns Hopkins University)
Marshall Posner (Mount Sinai Medical Center)
Li Mao (Univ. of Maryland Dental School)
Sharon Riddler (Univ. of Pittsburgh)
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. Journal of the National Cancer Institute. 2011 May 4;103(9):753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiology, Biomarkers & Prevention. 2011 Dec;20(12):2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005 Mar 16;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 4.Franzetti M, Adorni F, Parravicini C, et al. Trends and Predictors of Non Aids-Defining Cancers in Men and Women with Hiv-Infection. A Single-Institution Retrospective Study before and after the Introduction of Haart. J Acquir Immune Defic Syndr. 2012 Dec 28; doi: 10.1097/QAI.0b013e318282a189. [DOI] [PubMed] [Google Scholar]
- 5.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. The lancet oncology. 2009 Dec;10(12):1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 6.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010 Nov 15;116(22):5306–5315. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 7.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007 Oct 1;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Lise M, Franceschi S, et al. Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer. 2012 Jan 31;106(3):447–452. doi: 10.1038/bjc.2011.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking Among HIV Positive New Yorkers: Prevalence, Frequency, and Opportunities for Cessation. AIDS and behavior. 2008 Sep 7; doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi A, Engels E, Pfeiffer R, et al. Human papillomavirus (HPV) and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 12.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer. 2009 Feb 6; doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 13.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008 Mar 19;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 14.Ang K, Harris J, Wheeler R, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiology, Biomarkers & Prevention. 2012 Jan;21(1):122–133. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza G, Fakhry C, Sugar EA, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007 Jul 1;121(1):143–150. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 17.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004 Feb 15;189(4):686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 18.Read TR, Hocking JS, Vodstrcil LA, et al. Oral human papillomavirus in men having sex with men: risk-factors and sampling. PloS one. 2012;7(11):e49324. doi: 10.1371/journal.pone.0049324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 20.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 21.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi AK, Madeleine MM, RJB, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engsig FN, Gerstoft J, Kronborg G, et al. Head and neck cancer in HIV patients and their parents: a Danish cohort study. Clinical epidemiology. 2011;3:217–227. doi: 10.2147/CLEP.S19875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. Jama. 2012 Feb 15;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron JE, Mercante D, O’Brien M, et al. The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus-seropositive adults. Sex Transm Dis. 2005 Nov;32(11):703–709. doi: 10.1097/01.olq.0000175398.34610.2e. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head & neck. 2010 May;32(5):562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang AL, Hauff SJ, Owen JH, et al. UM-SCC-104: a new human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2012 Oct;34(10):1480–1491. doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutlee F, Gravitt P, Kornegay J, et al. Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol. 2002 Mar;40(3):902–907. doi: 10.1128/JCM.40.3.902-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008 Feb 1;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 30.Shiels PG. CDKN2A might be better than telomere length in determining individual health status. BMJ. 2012;344:e1415. doi: 10.1136/bmj.e1415. [DOI] [PubMed] [Google Scholar]
- 31.McLemore MS, Haigentz M, Jr, Smith RV, et al. Head and neck squamous cell carcinomas in HIV-positive patients: a preliminary investigation of viral associations. Head and neck pathology. 2010 Jun;4(2):97–105. doi: 10.1007/s12105-010-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005 Apr 20;97(8):577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 33.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008 Jul 1;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen N, Holodniy M. HIV infection in the elderly. Clinical interventions in aging. 2008;3(3):453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benard A, Bonnet F, Tessier JF, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS. 2007 Jul;21(7):458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.