Abstract
It is now accepted that the anion nitrite, once considered an inert oxidation product of nitric oxide (NO), contributes to hypoxic vasodilation, physiological blood pressure control, and redox signaling. As such, its application in therapeutics is being actively testing in pre-clinical models and in human phase I–II clinical trials. Major pathways for nitrite bioactivation involve its reduction to NO by members of the hemoglobin or molybdopterin family of proteins, or catalyzed dysproportionation. These conversions occur preferentially under hypoxic and acidic conditions. A number of enzymatic systems reduce nitrite to NO and their activity and importance are defined by oxygen tension, specific organ system and allosteric and redox effectors. In this work, we review different proposed mechanisms of nitrite bioactivation, focusing on analysis of kinetics and experimental evidence for the relevance of each mechanism under different conditions.
Keywords: nitrite, nitric oxide, heme proteins, xanthine oxidase, aldehyde oxidase, signal transduction
Introduction
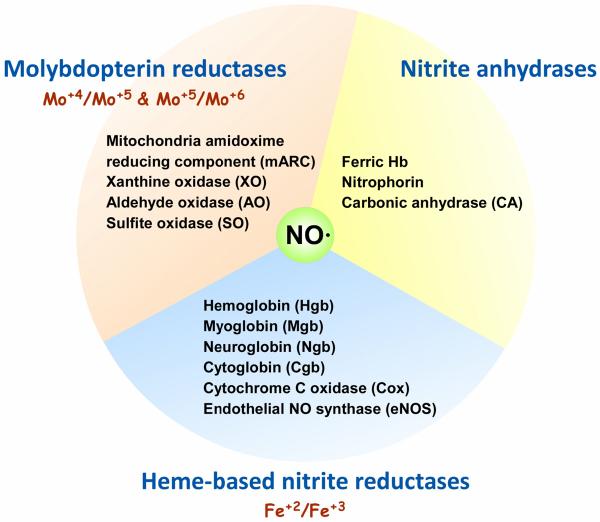
Even recently nitrite was considered inert and incapable of functioning as a vasodilator in the human circulation (NO) [1]. However, bioactivation of nitrite through formation of NO and other nitrogen oxides is now widely recognized and the importance of this pathway in the physiological control of blood flow and pressure accepted [2, 3]. Despite agreement that nitrite is bioactive, the mechanisms responsible for bioactivation remain to be clearly defined and remain a source of controversy. In this review, we evaluate the studied mechanisms for reduction or bioactivation of nitrite (illustrated in Figure 1) and discuss strengths and weaknesses related to their relevance in nitrite bioactivation.
Figure 1.
Proposed protein or enzyme-based systems for nitrite reduction. The mechanisms shown here are based on those that are heme-based, molybdopterin-based, and those that function as nitrite anhydrases including nitrophorin [131, 132].
There are now many pieces of evidence demonstrating bioactivity of nitrite. Nitrite administered to rats via intragastric route lowered blood pressure [4]. Infusions of slightly supraphysiological levels of nitrite cause increased blood flow that are potentiated by hypoxia and low pH in humans [5, 6]. Salivary nitrite has been show to increase gastric mucosal blood flow and mucous thickness [7]. Physiologically relevant levels of nitrite have been shown to counter ischemic-reperfusion injury [8–10] that is likely mediated by effects on mitochondrial respiration [10]. In addition, nitrite has been shown to decrease platelet activation [11, 12]. All of these effects are consistent with nitrite being reduced to NO and mediating NO-dependent signal transduction.
Numerous other examples of nitrite bioactivation have been demonstrated after initial conversion from nitrate by oral bacteria [13, 14]. Physiological effects of increasing dietary nitrate include improving intestinal health [7], enhanced exercise performance [15–21], and acute reduction in blood pressure[22–26]. These effects are eliminated and nitrite levels decreased when volunteers either spit or use mouthwash [22, 23], thereby implicating the importance of nitrate reduction to nitrite by oral bacteria. The fact that dietary nitrate is capable of raising plasma nitrite levels to a sufficient extent to elicit substantial physiological effects supports the notion that nitrite bioactivation plays a role in modulating normal biological function.
In this review we discuss the major pathways for nitrite reduction to NO and the evidence to support a role in physiology and disease. The major reductase enzyme pathways considered include the heme-based nitrite reductases, the molybdopterin enzyme nitrite reductases, and the nitrite anhydrase enzymes (Figure 1).
History
In 1952 Furchgott and Bhadrakhom demonstrated the ability of nitrite to effect relaxation of pre-constricted aortic vessels, but the concentrations used where substantially higher than that found in physiological conditions (100 μM compared to normal levels of tens to hundreds of nanomolar) [27]. In humans, nitrite reduction to NO was discovered in expelled air and attributed to non-enzymatic reduction in the stomach [28]. This conversion of nitrite to NO in the stomach was suggested to play a role in controlling gut pathogens [29]. In 1995, nitrite was also shown to be reduced to nitric oxide in saliva [30]. In that same year, Zweier and colleagues demonstrated reduction of nitrite to NO in an ischemic heart model and attributed the reduction to non-enzymatic processes [31]. In 2000 Gladwin, Cannon and others observed an arterial-venous gradient in nitrite along with a substantial consumption occurring during exercise when nitric oxide synthase (NOS) is blocked in humans [32]. The authors suggested that nitrite may function as source of NO that is activated in hypoxia and acidic conditions to increase blood flow [32]. This suggestion would explain the observation by Classen and coworkers where intragastric administration of nitrite lowered blood pressure in a rodent model [4]. In 2001, Lundberg, Modine and colleagues showed that physiological levels of nitrite could effect vasorelaxation in rat aorta under acidic conditions of ischemia [33]. Then, in 2003, we showed that infusion of as little of a few micromolar nitrite increased blood flow in the human blood flow in a NOS independent manner and this was potentiated when subjects performed exercise (Figure 2) [5]. In a subsequent study, Gladwin and coworkers showed that infusion of physiological concentrations of nitrite caused increased forearm blood flow [6].
Figure 2.
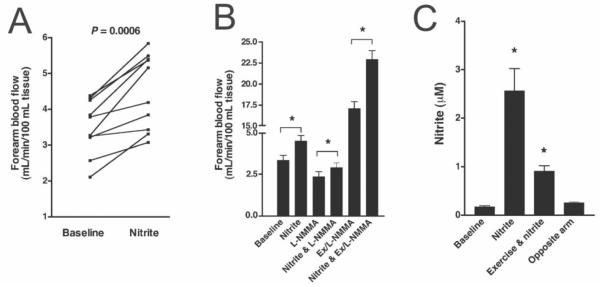
Nitrite mediated increase in forearm blood flow (Reproduced with permission from [5]). (A) Slightly supraphysiological levels of infused nitrite (2.5 μM) lead increased human forearm blood flow as measured by strain gauge plethysmography (n = 10). (B) Nitrite increases forearm blood flow in the absence and presence of the nitric oxide synthase (NOS) inhibitor NG-monoethyl-L arginine (L-NMMA), indicating the effect is independent of NOS. In addition, the increase in nitrite-mediated blood flow is enhanced during exercise, suggesting potentiation in hypoxia. (C) Exercise lowers plasma nitrite in the infused arm suggesting that its consumption is increased under acidic, hypoxic conditions.
Part 1 Nitrite reduction by Heme Proteins
Mechanism of Hemoglobin mediated bioactivation
In our 2003 paper demonstrating nitrite's ability to increase blood flow we suggested that the mechanism may involve reduction to NO by deoxygenated Hb (deoxyHb) [5]. The chemistry for this reaction was first described by Brooks [34] and later elaborated upon by Doyle and colleagues [35] as well as others [36, 37],
| (1) |
| (2) |
where HbFe2+ refers to deoxyHb (with the iron state in the reduced, +2, state), HbFe3+ refers to methemoglobin (where the heme iron is oxidized), and HbFe2+-NO refers to the NO adduct of hemoglobin, iron nitrosyl hemoglobin. The reduction of nitrite occurs as described in Equation 1 and the dependence on low oxygen saturation and pH is manifest in the requirements for deoxygenated hemes and protons.
Interestingly, the rate of reduction has been found to depend on the allosteric state of the protein, with vacant hemes in an R-state Hb tetramer reacting about 100 times faster than those in the T-state [36–38]. The rate of NO production is thus maximal at partial oxygen saturations near the p50 of Hb (Figure 3) [36, 38]. The maximum rate of NO production at this oxygen pressure, taking a bimolecular rate constant of 1 M−1s−1 [36], 0.01 M heme, and 100 nM nitrite would be 1 nanomolar/sec. This rate would increase linearly with a decrease in pH so that at a pH of 6.5 it would be about 8 nM/s. With higher plasma nitrate (such as that achievable after high oral nitrate intake as occurs when drinking beet juice), one could envision an NO production rate of 80 nM/s. It is interesting to compare this rate with that of NO production by NOS. Unfortunately, the reports for the maximum rate of NO production by NOS vary from 1 nM/s to 70 μM/s [39–41]. These and other estimated rates are summarized in Table 1. Given that NOS function decreases in hypoxia (while nitrite reductase activity increases), it is reasonable to consider NO production rates that are on the order of 1 nM/s or more may contribute to NO bioactivity while ones that are substantially lower would not.
Figure 3.
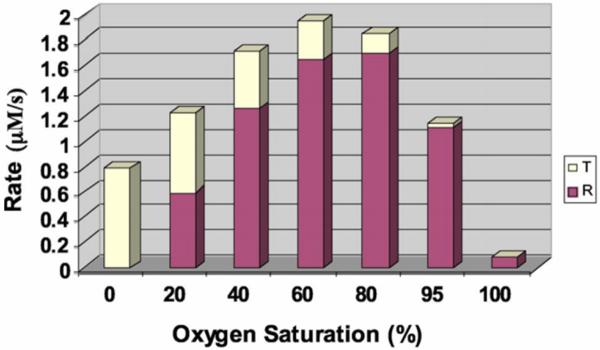
Oxygen dependence of the rate of hemoglobin-mediated nitrite reduction (This research was originally published in Blood. D. B. Kim-Shapiro and M. T. Gladwin. The functional nitrite reductase activity of the heme-globins. Blood. 2008; 112: 2636-2647. © The American Society of Hematology [38]). Using a two-state model of hemoglobin allostery which predicts how much R-state and T-state Hb there is at each oxygen pressure, the rate of nitrite reduction was calculated. The contribution at each oxygen pressure from R-state and T-state Hb is illustrated. The plot is for a situation where the product of the nitrite and Hb concentrations are 10−6 M2 (so, for example, 10 mM Hb and 100 μM nitrite). The rate of nitrite reduction for R-state Hb was taken as 100 times greater than that for T-state Hb.
Table 1.
| Protein | Estimated in vivo rate of NO production (nM/s)a | References used in estimation |
|---|---|---|
| Hemoglobin | 8–80 | [36, 38] |
| Myoglobin | 10 | [54, 57] |
| Neuroglobin | 0.5 | [58, 63, 64] |
| Cytochrome c | 14 | [73] |
| Cytoglobin | 0.35 | [74, 75] |
| Cytochrome c oxidase | 1.5 | [79, 80] |
| Nitric Oxide Synthase | 0.18 | [41, 83] |
| Xanthine oxidoreductase | 1 | [39] |
| Aldehyde oxidase | 1.5 | [93] |
These estimates are based on favorable conditions and further discussed in the text.
The biggest conceptual challenge to the notion that deoxyHb plays a major role in bioactivation of nitrite is that NO produced in the red blood cell would be rapidly scavenged by the dioxygenation reaction where NO reacts with oxygenated Hb (OxyHb) to form MetHb and nitrate,
| (3) |
This reaction is governed by a bimolecular rate constant of 6–8 × 107 M−1s−1 [42–44]. . With 20 mM Hb in the red cell (in heme), the lifetime of NO would only be about τ = 1 microsecond. Thus, NO could only diffuse about 0.1 micrometers or less (taking a diffusion constant D = 1000 μm2/s with distance going as the square root of D τ). Thus, very little NO that is made in the red blood cell can get out. Several proposed mechanisms have been constructed to explain how nitrite-derived bioactivity can be exported from the red blood cell and these generally involve either a compartment model with submembrane localization of the reaction allowing for inefficient free NO escape, or the formation of another nitrogen oxide such as a nitrosothiol or N2O3 that can get out of the red blood cell and carry NO activity [45–53].
Mechanism of Myoglobin mediated bioactivation
The mechanism by which myoglobin (Mb) would lead to nitrite bioactivation is the same as for Hb (Equation 1), but the reaction was found to be about substantially faster for Mb than Hb due to its lower heme redox potential [54]. The bimolecular rate constant was measured to be 12 M−1s−1 at pH 7.4 and 37 °C [54]. As predicted by Equation 1, the rate increases as the pH decreases so that it will be substantially faster under ischemic conditions. At pH 6.4 it would be 120 M−1s−1.
Mb-mediated nitrite reduction has been reported to be important in both cardiomyocytes [55] and smooth muscle [56]. Cardiomyocytes contain about 300 μM Mb [57]. Nitrite has been found to be about 1 μM in heart tissue. Thus, in acidic conditions, NO could be produced here at a rate of about 36 nM/s, if all of the Mb is deoxygenated. Given the high affinity of Mb for oxygen (p50 about 3 Torr), 100% deoxygenation is unlikely even under extreme hypoxia. At 1% oxygen (7.6 torr), about 30% of the Mb hemes would be deoxygenated so the rate of NO production would be about 10 nM/s, which is still considerable. The rate of NO production via smooth muscle Mb depends on its concentration which has not been determined, but is likely to be substantially less than that of cardiomyocytes.
NO scavenging by oxyMb is less of a problem than in the case if Hb in red blood cells due to the lower concentration of Mb. With 300 μM Mb, the lifetime of NO would be about 70 μs in a cardiomyocyte and it could diffuse about 0.3 μm. The diffusion distance in the smooth muscle is likely greater. Moreover, the targets for the produced NO by Mb are likely to be closer than in the case of NO produced by red cell Hb.
As discussed below, a functional role for myoglobin in nitrite reduction and signaling is strongly supported by multiple studies in myoglobin knockout mice showing a reduction in nitrite mediated effects on mitochondrial respiration, cardiac function, ischemia reperfusion cytoprotection, and systemic hypoxic vasodilation [54–56].
Mechanism of Neuroglobin mediated bioactivation
The basic mechanism of reduction of nitrite to NO by Neuroglobin (Ngb) is also similar to that of Mb and Hb (Equation 1), except that it is complicated by the fact that the heme iron of Ngb is predominately hexacoordinate [58]. Whereas the heme iron of deoxyHb is pentacoordinate as it only binds to the proximal histidine, that of Ngb binds to both the pentacoordinate and hexacoordinate heme. In order to better describe Ngb reduction of nitrite, we need to expand discussion of the mechanism involving Hb. The dependence on pH is likely due to the heme actually predominately reacting with nitrous acid, HONO, rather than the nitrite anion. Thus, following Doyle and colleagues [35], the rate of nitrite reduction by Hb can be written
| (4) |
where k0 is the bimolecular rate constant for the reaction of deoxyHb and HONO, k' is the bimolecular rate constant for the reaction of deoxyHb with nitrite anion, the subscript T indicating all nitrite (acid and anion), and Ka is 10−pK for nitrite with pK = 3.15. At neutral pH and below, the k'can be ignored. Doyle and colleagues found k0 to be 12.3 × 103 M−1s−1 [35]. Equation 4 can be adapted to describe the rate that nitrite is reduced by Ngb as follows
| (5) |
where KHis is the ratio of hexacoordinate to pentacoordinate Ngb [58]. With reported values of being about 3000 for KHis [59], the binding of the iron plays a major role in limiting the rate of nitrite reduction by Ngb, giving an overall bimolecular rate constant of about 0.1 M−1s−1 at neutral pH [58].
Interestingly, the affinity of the heme iron for the distal histidine can be increased through protein modification, thereby increasing the rate of nitrite reduction [58, 60]. Formation of a disulfide bond between cysteines 46 and 55 the pentacoordinate Ngb form [58]. In addition, Ngb phosphorylation has been shown to increase Ngb reductase activity about four-fold [60]. The fact that Ngb can reduce nitrite so efficiently, approaching the rate for Hb, despite being predominately in the hexcoordinate form (generally less than 1%) suggests that the intrinsic rate constant for the reaction of Ngb with HONO, k0, is very large. Indeed, work with histidine mutants has shown that k0for Ngb (Equation 5) is about 5 ×106 M−1s−1 (Figure 4). This is 500 fold faster than for Hb and approaching a rate constant characteristic of that which rate-limited by how fast nitrite can get to the heme pocket [61, 62]. Physiological levels of Ngb have been reported to be between 1 μM in the brain and up to 100 μM in the retina [63, 64]. The rate of reduction of nitrite by Ngb in physiology depends on oxygenation state (with Ngb having an oxygen affinity similar to Mb [64]), pentacoordination, and pH. If nitrite concentrations are taken as 1 μM then in extreme hypoxia capable of making the Ngb completely deoxygenated, the rate of NO production would be about 0.005 nM/s in brain tissue and 0.5 nM/s in retina, taking a bimolecular rate constant of 5 M−1s−1. These rates would increase at lower pH and could be much higher under conditions where the pentacoordinate form of the protein is achieved. Of course, heme oxygenation or oxidation would reduce the rates.
Figure 4.
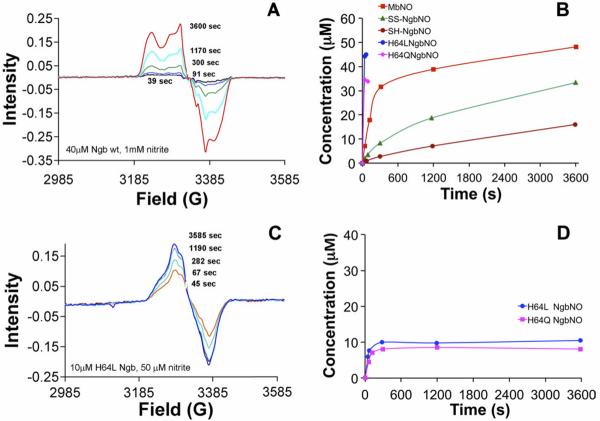
Nitrite reduction by wild type and mutant (H64L and H64Q) neuroglobin (Reproduced with permission from [58] © <2011> The American Society for Biochemistry and Molecular Biology.). Nitrite reduction was measured by assessing formation of nitrosyl (NO-bound) using electron paramagnetic resonance (EPR) spectroscopy. (A) Raw EPR spectra from mixing 1 mM nitrite with 40 μM wild type Ngb. (B) Nitrosyl Ngb formation from mixing from mixing 1 mM nitrite with 40 μM Ngb is shown for thiol reduced (SH) and oxidized (SS) wild type Ngb and for the pentacoordinate mutants. Nitrosyl Mb formation is also shown for reference. (C) Raw nitrosyl Ngb spectra obtained using lower concentrations of Ngb H64L employed to better assess the fast kinetics of nitrite reduction (10 μM H64L Ngb and 50 μM nitrite). (D) Kinetics of NO formation from 50 μM nitrite with either 10 μM H64L or H64Q Ngb.
Mechanism of bioactivation by other heme proteins: Cytochrome c, cytoglobin, cytochrome c oxidase, and endothelial NOS
Similar to Ngb, the heme iron of cytochrome c is predominately hexacoordinate, but can become pentacoordinate when the iron-methionine bond is ruptured which occurs when the methionine is oxidized [65, 66], tyrosines are nitrated [67, 68], or the cytochrome interacts with anionic phospholipids such as those present in the inner membrane of the mitochondria [69–71]. Zweier, Mason and colleagues demonstrated cytochrome-c mediated nitrite reduction secondary to protein oxidation by hypochlorite [72]. We subsequently demonstrated that cytochrome-c gained ability to reduce nitrite in the presence of anionic phospholipids [73]. The mechanism of nitrite reduction would follow Equation 4. Under conditions where pentacoordination is favored, we previously calculated that cytochrome-c mediated NO production from nitrite could reach 14 nM/s [73].
Cytoglobin is another hexacoordinate hemoprotein that can reduce nitrite following a similar mechanism as Ngb [74]. Cytoglobin is found in micromolar concentrations but can be increased about 10-fold under hypoxic conditions [75].The rate constant was measured to be about 0.14 M−1s−1 (pH 7, 25°C and NO production rates were estimated to be capable of reaching 0.35 nM/s under physiologically-relevant hypoxia and acidosis [74]. Although the mechanism of nitrite reduction by these hexacoordinate heme proteins, as well as others [76], seems to depend on transient formation of a pentacoordinate heme, the absolute necessity of pentacoordination has been questioned [77].
Cytochrome c oxidase has been proposed to reduce nitrite following a mechanism similar to Hb [78, 79]. The rate of NO production increases at lower oxygen concentration and depends on the subunit 5 isozyme [79]. Based on the amount of NO required to inhibit cytochrome c oxidase, there is likely a few hundred nanomolar cytochrome c in cells [80]. Using maximum measured rates of NO production with 1 mM nitrite [79]and assuming the rate of production is roughly linear down to a nitrite concentration of 10 μM, the rate of NO production from cytochrome c oxidase could be roughly estimated to be 1.5 nM/s in anoxia and less at higher oxygen concentrations.
Endothelial NOS has also been shown to be capable of reducing nitrite to NO [81–83]. Slama-Schwok and colleagues showed that endothelial NOS is the only NOS isoform that is capable of effectively reducing nitrite and it does so at a rate of 3.5 nM/sec per micromolar protein [83]. Nitrite reduction is most effective in the presence of calcium/calmodulin, tetrahydrobiopterin, and arginine, and is suggested to involve an additional active site besides the heme [81]. With a concentration of 0.05 μM in an arteriole [41], the rate of nitrite reduction to NO by eNOS in anoxia would be 0.18 nM/sec. This calculated value is not consistent with the reported observation that the rate NO production from endothelial cells in the presence of 10 mM nitrite actually increases 4-fold in hypoxia and 8-fold in anoxia [83].
Part 2 Nitrite reduction by Molybdopterin-containing Proteins
Mechanism of Xanthine oxidoreductase mediated bioactivation
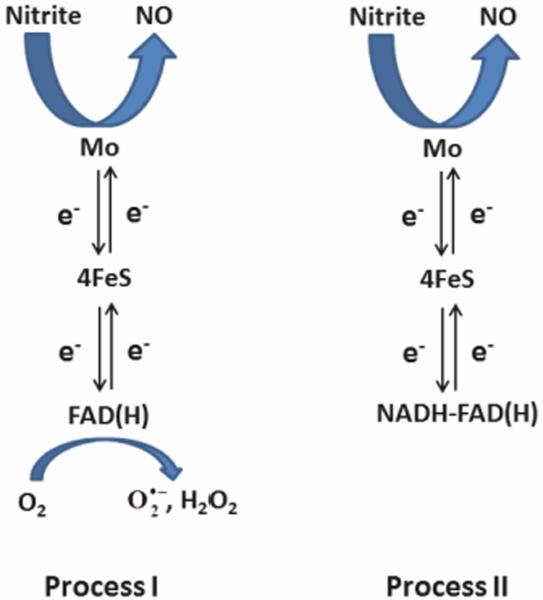
Xanthine oxidoreductase (XOR) is a flavoprotein that reduces xanthine to hypoxanthine and can also reduce hypoxanthine to uric acid. It has an FAD binding site, iron-sulfur centers and a molybdenum center. Several groups have studied its ability to reduce nitrite to NO [8, 39, 84–91]. Nitrite is reduced by the molybdenum which can itself be reduced by one of two pathways so that overall nitrite reduction has been described as occurring by two separate processes [87]. Process I involves molybdenum reduction by xanthine or 2,3-dihydroxybenz-aldehyde [87]. In this process, the FAD site is open and oxygen competes with nitrite for electrons so that process 1 is favored in hypoxia and anoxia (Figure 5). In Process II, NADH occupies the FAD site so oxygen no longer competes with nitrite for electrons [87]. Process II is then independent of oxygen tension (Figure 5).
Figure 5.
Mechanisms of Xanthine Oxidoreductase mediated nitrite reduction (Based on scheme from [87]). In Process I, reduced XOR can donate electrons to either nitrite or oxygen so that Process I occurs preferentially under hypoxic or anoxic conditions. In Process II, NADH binds to the FAD site so that oxygen cannot accept electrons. Process II is independent of oxygen pressure.
The relatively low affinity of XOR for nitrite limits the rate of NO production. The Michaelis constant Km has been reported to be 2.5 mM under some conditions [86]. and 12 mM to 59 mM with oxygen tensions of 2% to 21% [87]. The Vmax is constant over different oxygen tensions and equal to about 10 nM/mg protein/s [87]. Levels of XOR vary by tissue and have been reported to be about 7g/g tissue in the heart and 80 g/g tissue in the liver but these levels can increase dramatically along with levels of NADPH in ischemia [39]. Tissue generation from nitrite was found to increase greatly as the pH was reduced from 7 to 6 and approached 1 nM/s [39]. This is consistent with estimates of XOR mediated NO generation of nitrite reaching 1 nM/s.
In addition to XOR mediated NO generation from nitrite occurring in heart, liver, and smooth muscle tissue, XOR has been proposed to be associated with endothelial cells and RBCs and reduce nitrite there [88]. RBC XOR has recently been proposed to play a particularly important role in producing NO from nitrite in patients with hypertension where XOR is suggested to be upregulated [25]. However, XOR produces negligible NO from nitrite in normal volunteer red blood cells at physiological pH values. Other studies have reported negligible XOR in blood [39]. The extent to which RBC XOR can contribute to NO generation from nitrite will depend on the concentrations of XOR that accumulate from circulating levels [88]. In addition, NO scavenging by Hb would also limit the effectiveness of NO generated by RBC XOR, but XOR localization to the RBC membrane could limit NO scavenging to some extent.
The importance of XOR in mediating nitrite bioactivation has been studied using specific XOR inhibitors and this is discussed further below.
Mechanism of Aldehyde oxidase mediated bioactivation
Aldehyde oxidase (AO) catalyzes oxidation of aldehyde to carboxylic acid and functions in metabolizing xenobiotics. Like XOR, it has an FAD binding site, iron-sulfur centers and a molybdenum center [92]. It is in the same family of molybdenum –containing enzymes as XOR [92]. Use of an AO inhibitor raloxifene substantially reduced NO production from rat liver and heart tissue in the presence of nitrite [39]. In addition, NO generation from nitrite was directly measured when AO was present along with NADH [39]. The mechanism of nitrite reduction by AO is similar to that of XOR where nitrite is reduced at the molybdenum site and either an aldehyde can supply electrons at that same site, or NADH can supply electrons from the flavin site [93]. Under aerobic conditions, the NADH-dependent process is favored as oxygen acts as a competitive inhibitor of nitrite reduction in the absence of NADH [93]. Since AO has a higher affinity for NADH than XOR, it is more likely to contribute to aerobic nitrite reduction than XOR with physiological levels of NADH [93]. Like other nitrite reduction pathways, lowering pH increases the rate of AO-mediated reduction, but not as much as other pathways; about 35% in going from pH 7.4 to pH 6.0 [93]. Again, the low Km of AO for nitrite (about 3 mM) limits its NO production rate, but it has been estimated that AO mediated NO production from nitrite can reach 1.5 nM/s at low pH in the heart [93].
Mechanism of bioactivation by other molybdenum-containing enzymes
Xanthine oxidoreductase and aldehyde oxidase are members of the molybdenum oxotransferase enzyme family which also includes nitrate reductases [94]. Thus, studies have investigated if other members of this family can function as mammalian nitrite reductases. Preliminary work presented at the Fifth International Meeting on the Role of Nitrite and Nitrate in Physiology, Pathophysiology, and Therapeutics suggests that sulfite oxidase, which catalyzes the oxidation of sulfite to sulfate, functions as a nitrite reductase both as an isolated enzyme and in cultured fibroblasts [95]. Another mammalian molybdenum-containing enzyme that has identified is mitochondrial amidoxime reducing component (mARC) [96]. Preliminary work with this enzyme presented at the meeting by the Gladwin group has demonstrated that it too can reduce nitrite to NO and is capable of using NADH or cytochrome b5 and cytochrome b5 reductase as electron sources [95].
Part 3. Nitrite bioactivation by anhydrase activity
Nitrite anhydrase activity of heme proteins
Several suggested pathways for nitrite bioactivation involve the formation of N2O3, as mentioned above in the case of potential Hb-mediated export of NO bioactivity from the red blood cell. N2O3 can carry NO bioactivity through subsequent nitrosation or through NO formation
| (6) |
In the case of Hb, nitrite anhydrase has been proposed whereby
| (7) |
In one proposed mechanism for nitrite anhydrase, an intermediate ferric nitrosyl species is formed (which is equivalent to a ferrous nitrosonium) and this then reacts with a second nitrite molecule [49, 97, 98].
| (8) |
| (9) |
Another possibility is that nitrite binds to MetHb which subsequently reacts with NO to make N2O3 [47]. Here, subsequent to the initial reaction of nitrite and deoxyHb (Equation 1), nitrite binds,
| (10) |
where the metHb-nitrite species is proposed to have some ferrous nitrogen dioxide character. This species reacts with NO to make N2O3 [47],
| (11) |
Formation of N2O3 from nitrite would facilitate export of NO activity from the red blood cell and evidence for the formation of this species has been reported [45–47, 99]. However, others have questioned whether this species can and does form [100, 101]. The major challenge to the pathway to N2O3 formation via the pathway involving the ferric nitrosyl intermediate (Equation 8 and 9) is that the ferric nitrosyl species releases NO on the order of one second [102] so that it's reactivity with nitrite is limited. The biggest challenges for the pathway involving a metHb-nitrite intermediate are that metHb has a low to moderate affinity for nitrite and the reaction with NO must compete with the fast reactions of NO and oxygenated and deoxygenated Hb.
Nitrite anhydrase activity of Carbonic Anhydrase
Carbonic anhydrase (CA) is a zinc-containing enzyme that catalyzes the formation of carbonic acid from carbon dioxide and water,
| (12) |
Formation of NO from nitrite through action of CA has been demonstrated using isolated enzyme [103]. It was suggested that CA catalyzes formation of N2O3 similar to the reverse of the reaction shown in Equation 12,
| (7) |
With subsequent formation of NO and nitrogen dioxide via Equation 6 [103]. The reaction was found to be faster at higher pH (7.2) but produce more total NO at lower pH (5.9) [103]. These results were suggested to be due to the involvement of the nitrite anion in the anhydrase reaction (rather than nitrous acid) balanced by a requirement for protons (Equation 7) [103]. The fact that the percentage of nitrite that is in the anion form only changes a tiny bit when going from pH 5.9 to pH 7.2 (99.82% to 99.99% assuming a pK of 3.15), suggests that additional phenomena are involved that account for the observed pH dependence of CA reactivity with nitrite.
The affinities of CA for carbon dioxide and bicarbonate are low enough that they are not likely to inhibit reactivity of CA with nitrite [103–105]. The affinity of nitrite itself for CA is also likely to be low based on its measured inhibition constant (KI of about 40 mM [106]) [103]. However, recent work in the Gladwin lab has shown that the Km of CA for nitrite is about 40 μM and the Kcat is very low at about 2 × 10−4 s−1 at pH 7.2 (unpublished results). A concentration of 400 nmol/g Hb [107] of erythrocytic CA would be about 40 μM CA in blood. This would yield N2O3 at a rate of about 0.2 μM/s in the presence of 1 μM nitrite.
Part 4. Potential physiological and therapeutic relevance of different mechanisms
Mechanisms involved in modulating blood flow, blood pressure, and hemostasis/thrombosis
Infusion of just slightly supraphysiological levels of nitrite leads to increased blood flow that is potentiated in hypoxia [5, 108]. Several studies have shown that oral nitrate decreases blood pressure and that this effect is dependent on reduction of nitrate to nitrite by commensal bacteria [22–26]. Nitrite has also been shown to decrease platelet activation and aggregation [11, 12]. All of these effects of nitrite can be explained by formation of NO bioactivity in the blood or blood vessels, but the mechanisms for bioactivation in these tissues has been the subject of considerable debate and active study.
Evidence that increased blood flow is due to reduction by deoxyHb is provided by the temporal relationship between vasodilation and NO formation in the red blood cell. Additionally, blood flow effects of nitrite are not inhibited by infusions of inhibitors of NO synthase or XOR [5, 6]. Additional evidence derives from ex-vivo experiments using aortic ring bioassays [5, 109, 110]. The combination of red blood cells and nitrite (500 nM) was shown to relax these aortic vessels more efficiently than when nitrite or red cells alone were present [5]. At oxygen tensions of 15 and 25 mmHg as little as 200 nM nitrite was subsequently shown to effect relaxation in aortic rings in the presence of red blood cells and relaxation was abrogated by the NO scavenger CPTIO [109]. Relaxation by nitrite and red blood cells was associated with cGMP formation and dependent on Hb oxygen affinity, with maximal effect occurring near the Hb p50, consistent with the notion that R-state Hb reduces nitrite more effectively than T-state Hb [109]. Another study by Dalagaard and coworkers using aortic rings has questioned whether Hb and red cells actually contribute to nitrite-mediated vasodilation [111]. Dalsgaard and coworkers found no additional effect of deoxygenated Hb on vasodilation compared to control [111]. The discrepancy in effects of deoxyHb on nitrite mediated vasodilation might be explained in terms of the fact that Hb both produces NO from nitrite and scavenges NO via the dioxygenation reaction or NO binding [110]. Thus, one must consider the balance of NO scavenging and NO production from nitrite in the presence of Hb to assess the overall effect on NO bioavailability. NO scavenging by Hb is similar under oxygenated and deoxygenated conditions (with the scavenging by oxygenated Hb being slightly faster [112]). On the other hand, NO production increases as Hb oxygenation is decreased from 100%, so that the balance tips towards relative NO production upon deoxygenation.
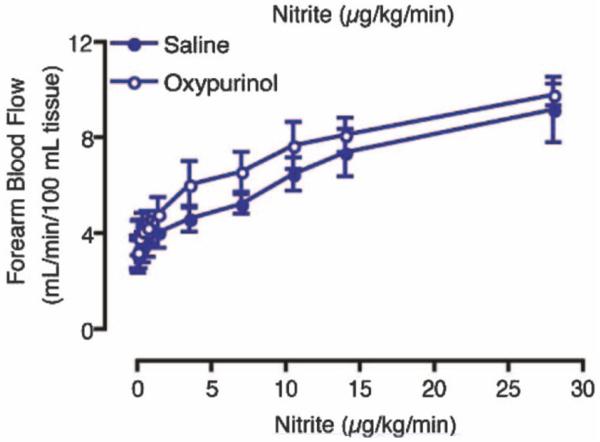
Several studies have emphasized the importance of XOR in mediating nitrite-dependent increases in blood flow either due to XOR in the blood vessels [39, 88] or erythrocytic XOR [25, 88]. Li and coworkers found that NO production rates are much higher from tissue, including rat aorta, than from blood [39]. Webb and colleagues showed that nitrite-mediated NO production from human left internal mammary artery was substantially (but not completely) reduced by allopurinol, an XOR inhibitor [88]. XOR was also demonstrated to be active on the erythrocytic membrane [25, 88]. They also suggested that eNOS, both in the RBC and endothelial cells, played a role in vascular NO production from nitrite using a NOS blocker [88]. The importance of XOR-mediated nitrite reduction was emphasized in blood pressure reduction in hypertensive rat models and based on NO formation from human red cells from patients with hypertension [25]. Erythrocytic XOR was found to increase in human patients with mild hypertension and in a rodent spontaneous hypertension model [25]. The increase in protein content was associated with increased ability to reduce nitrite to NO in erythrocytes but not in rodent arterial vessels and this erythrocytic ability to reduce nitrite was substantially reduced upon administration of the XOR inhibitor allopurinol in the rodent model and in erythrocytes form hypertensive patients [25]. An important study countering evidence that suggests XOR is a major player in vascular NO generation is one in which oxypurinol (another XOR inhibitor) was found to actually increase blood flow when co-infused with nitrite into the human forearm (Figure 6) [6].
Figure 6.
Nitrite mediated increased blood flow is not affected by XOR inhibitor oxypurinol (Adapted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: [CIRCULATION] [6] copyright 2007). Forearm blood flow increased as the rate of infused increases and oxypurinol tended to increase blood flow.
Smooth muscle myoglobin has also been implicated in modulating nitrite-dependent increases in blood flow [56, 113]. Using murine aortic rings under anoxic conditions, Ormerod and coworkers showed that nitrite-mediated vasodilation is reduced by CO (presumably due to blockage of the heme) for wild type but not myoglobin knockout mice [113]. The remainder of the nitrite mediated effects were attributed to XOR and SO based on use of inhibitors [113]. Totzeck and coworkers conducted in vitro and in vivo experiments supporting a role for smooth muscle Mb in nitrite mediated vasodilation using wild type and Mb knockout mice [56]. NO production from nitrite in aorta were greatly attenuated in the knockout compared to wild type and inhibition of XOR did not affect NO production in the wild type [56] Blood pressure from wild type mice was substantially more reduced compared to Mb knockout mice when exposed to hypoxia in vivo [56]. In addition, NO formation from administered nitrite and associated vasorelaxation were much higher in wild type mice compared to knockouts .[56] A role for nitrite reduction by NOS was not supported by experiments using NOS knockout mice [56].
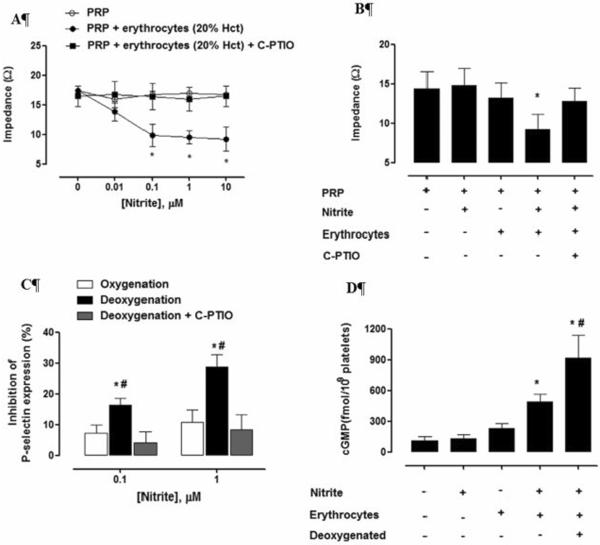
In addition to modulating blood flow, NO has important function in maintain preventing proper platelet function. NO is known to reduce platelet activation, probably through binding to soluble guanylyl cyclase within platelets Platelet aggregation was reduced in individuals who consumed potassium nitrate compared to potassium chloride [114]. Platelet aggregation induced by ADP or collagen measured in blood taken from individuals who consumed a single dose of beetroot juice (containing high nitrate) was reduced compared to a control group and the reduction in platelet aggregation was reduced when volunteers spit, which interrupts the conversion of nitrate to nitrite [23]. Dietary restriction of nitrite and nitrate in a murine model led to reduced platelet aggregation and activity while supplementation with nitrite or nitrate inhibited platelet aggregation [12]. Importantly, in contrast to the effects observed in vivo, the in vitro treatment of platelet rich plasma with nitrite has no effect on platelet activation, but reduces platelet activation in the presence of erythrocytes (Figure 7) [115]. This effect was potentiated upon red cell deoxygenation and abrogated in the presence of the NO scavenger C-PTIO (Figure 7) [115]. These data support the notion that erythrocytes can export NO bioactivity from nitrite.
Figure 7.
Inhibition of platelet activation demonstrates nitrite-mediated export of NO bioactivity from red blood cells (Reproduced with permission from [11] Plos One, 2012. 7(1): pe3080. (A) Platelet aggregation was measured using impedance as a function of nitrite concentrations. Nitrite reduced collagen-induced platelet aggregation only when erythrocytes were present. This action was abrogated when the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazole-1-oxyl 3-oxide (C-PTIO) was employed. (B) Aggregation induced by U46619 (which acts as an agonist of the thromboxane A2 receptor) was inhibited by 100 nM nitrite in the presence, but not absence, of erythrocytes and the effect was abolished with C-PTIO. (C) Inhibition of platelet activation induced by ADP measured by reduction in expression of P-selectin is enhanced when red blood cells are deoxygenated. (D) The mechanism of inhibition of platelet activation appears to involve the platelet NO-soluble guanylyl cyclase pathway as evidenced by increased platelet cCMP when red blood cells and nitrite (100 nM) are present and this is potentiated upon deoxygenation.
It is important to remember in evaluating sets of data that some discrepancies may be explained by the different models (animal vs human) employed. It is quite possible that more than one mechanism plays a role and that the dominant one depends on the physiological environment such as pH and oxygen tension.
Mechanisms involved in cytoprotection
Ischemia and ischemia/reperfusion (IR) injury are major causes of mortality that, to a large extent, involves mitochondrial dysfunction [116]. Ischemic preconditioning is an effective method of protecting against IR injury and NO production plays an important role in the late phase of protection [117]. Nitrite could be an effective source of NO in cytoprotection due to its role an ample source of NO bioactivity and ability to be bioactivated in ischemic conditions [116]. The efficacy of low concentrations of nitrite in mediating cytoprotection has been demonstrated in a variety of tissues in animal models including heart [8, 9, 55, 118–122] liver [9, 120, 123], brain [124–126], kidney [127, 128], hindlimb [129], and human forearm [23].
Numerous studies have suggested that nitrite-mediated cytoprotection is mediated by NO production from the molybdenum containing enzyme XOR [8, 39, 86, 118, 127] and to some extent SO [39] as well. The Zweier group demonstrated the sufficiency of XOR mediated NO generation from nitrite in conditions mimicking those of the ischemic heart [86]. They subsequently found that between about 60% to 80% of NO generation from 100 μM nitrite is abrogated when inhibitors of XOR and AO are administered [39]. The importance of XOR in cytoprotection was also demonstrated by Webb and coworkers using an isolated, perfused heart wherein allopurinol blocked NO production in ischemia [8]. Nitrite was shown to be protective in a rat in vivo model of myocardial IR and also showed that XOR inhibition abolished the protective effect of nitrite in perfused rate heart models [118]. In addition, topical administration of nitrite was found to be protective against renal IR injury in an in vivo rat model and NO production from nitrite in kidney homogenates was largely attenuated by allopurinol [127]. In a slightly different context, inhaled nitrite was demonstrated to inhibit hypoxia and inflammation-induced pulmonary arterial hypertension in a murine model and these effects were shown to be dependent on NO generation which was greatly attenuated upon administration of allopurinol [130]. Consistent with these effects, NO production from nitrite in lung tissue was found to be inhibited by allopurinol [54].
In contrast to some studies emphasizing the dependence of nitrite-mediated myocardial cytoprotection on bioactivation by XOR, allopurinol was found to have no effect in a study of nitrite-mediated NO generation from heart homogenates [54]. However, addition of ferricyanide greatly attenuated NO generation, consistent with a role for deoxygenated Mb [54]. The notion that Mb is primarily responsible for myocardial cytoprotection was examined in crucial tests using Mb knockout mice [55]. Perfused hearts from wild type mice produced about twice as much NO from nitrite as knockouts ex vivo when subject to ischemia [55]. In addition, whereas administration of nitrite decreased infarct size in wild type hearts by 36% when subject to IR, nitrite had no effect on infarct size on heats from knockouts [55]. This Mb-dependent effect was also demonstrated in vivo where nitrite administration reduced infract size from myocardial infarction by 61% in wild type mice and nitrite had no effect in knockout mice [55]. These data strongly support the role of Mb in myocardial cytoprotection.
Conclusions
Several enzymes appear capable of producing NO at a sufficient rate as to influence NO bioavailability and hence NO signaling. Under favorable conditions NO production rates from XOR, AO, cytochrome oxidase, Hb and Mb could be one to tens of nanomolar per second under physiological conditions. Cytochrome c can produce NO this fast when in the pentacoordinate heme state. Neuroglobin falls short of this production rate but may be much faster if factors also favor the heme pentacoordinate state. Signaling NO production rates would depend on competing reactions and NO scavenging. The scavenging of NO presents a major barrier to red cell mediated nitrite bioactivation but several lines of evidence, particularly recent studies in vitro, in mice and in humans that show inhibition of platelet activation via red cell mediated NO production from nitrite [11], suggest that red cells do indeed export NO bioactivity from nitrite.
Different mechanisms of action are likely to predominate in different tissues. Lack of an effect of oxypurinol (which inhibits XOR) on nitrite-mediated increased blood flow suggests that XOR is not a major player in human nitrite-mediated vasodilation [6], suggesting a more important role for hemoglobin and myoglobin. In contrast, nitrite reduction in lung and liver is likely mediated mostly by XOR and perhaps AO. In the heart, both XOR/AO and myoglobin are the largest contributors. To the extent that these pathways act synergistically is currently unknown. The biological significance of these and other pathways involving SO, carbonic anhydrase, NOS, and Marc require further study.
Highlights
Several mechanisms are fast enough to account for nitrite reduction in vivo.
Different mechanisms are likely to predominate in different tissues and conditions.
We suggest, deoxyhemoglobin plays a major role in nitrite-mediated vasodilation.
Myoglobin, xanthine oxidase and aldehyde oxidase predominate in other tissues.
Acknowledgments
This work was supported by NIH grants HL058091 and HL098032
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lauer T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladwin MT, et al. The emerging biology of the nitrite anion. Nature Chemical Biology. 2005;1(6):308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg JO, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nature Chemical Biology. 2009;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Classen HG, Steinhammer C, Thoni H. Hypothesis - the Effect of Oral Nitrite on Blood-Pressure in the Spontaneously Hypertensive Rat - Does Dietary Nitrate Mitigate Hypertension after Conversion to Nitrite. Journal of the American College of Nutrition. 1990;9(5):500–502. doi: 10.1080/07315724.1990.10720407. [DOI] [PubMed] [Google Scholar]
- 5.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 6.Dejam A, et al. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 7.Bjorne H, et al. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. Journal of Clinical Investigation. 2004;113(1):106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. PNAS. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemiareperfusion of the heart and liver. Journal of Clinical Investigation. 2005;115(5):1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiva S, Gladwin M. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Research in Cardiology. 2009;104(2):113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 11.Srihirun S, et al. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7(1):e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JW, et al. Effect of Blood Nitrite and Nitrate Levels on Murine Platelet Function. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kevil CG, et al. Inorganic nitrite therapy: historical perspective and future directions. Free Radical Biol. Med. 2011;51:576–593. doi: 10.1016/j.freeradbiomed.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 15.Bailey SJ, et al. Dietary nitrate supplementation reduces the O-2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of Applied Physiology. 2009;107(4):1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 16.Larsen FJ, et al. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radical Biology and Medicine. 2010;48(2):342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Cermak NM, Gibala MJ, van Loon LJC. Nitrate Supplementation's Improvement of 10-km Time-Trial Performance in Trained Cyclists. International Journal of Sport Nutrition and Exercise Metabolism. 2012;22(1):64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 18.Lansley KE, et al. Dietary nitrate supplementation reduces the O-2 cost of walking and running: a placebo-controlled study. Journal of Applied Physiology. 2011;110(3):591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 19.Lansley KE, et al. Acute Dietary Nitrate Supplementation Improves Cycling Time Trial Performance. Medicine and Science in Sports and Exercise. 2011;43(6):1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 20.Kenjale AA, et al. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. Journal of Applied Physiology. 2011;110(6):1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen FJ, et al. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica. 2007;191(1):59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 22.Govoni M, et al. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide-Biology and Chemistry. 2008;19(4):333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Webb AJ, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51(3):784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapil V, et al. Inorganic Nitrate Supplementation Lowers Blood Pressure in Humans. Role for Nitrite-Derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh SM, et al. Enhanced Vasodilator Activity of Nitrite in Hypertension Critical Role for Erythrocytic Xanthine Oxidoreductase and Translational Potential. Hypertension. 2013;61(5):1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 26.Larsen FJ, et al. Effects of dietary nitrate on blood pressure in healthy volunteers. New England Journal of Medicine. 2006;355(26):2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 27.Furchgott RF, Bhadrakom S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. Journal of Pharmacological and Experimental Therapeutics. 1953;108:129–43. [PubMed] [Google Scholar]
- 28.Lundberg JON, et al. Intragastric Nitric-Oxide Production in Humans - Measurements in Expelled Air. Gut. 1994;35(11):1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamin N, et al. Stomach No Synthesis. Nature. 1994;368(6471):502–502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 30.Duncan C, et al. Chemical Generation of Nitric-Oxide in the Mouth from the Enterosalivary Circulation of Dietary Nitrate. Nature Medicine. 1995;1(6):546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 31.Zweier JL, et al. Enzyme-Independent Formation of Nitric-Oxide in Biological Tissues. Nature Medicine. 1995;1(8):804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 32.Gladwin MT, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modin A, et al. Nitrite-derived nitric oxide: a possible mediator of `acidic-metabolic' vasodilation. Acta Physiologica Scandinavica. 2001;171(1):9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 34.Brooks J. The action of nitrite on Haemoglobin in the absence of oxygen. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1937;123:368–382. [Google Scholar]
- 35.Doyle MP, et al. Kinetics and Mechanism of the Oxidation of Human Deoxyhemoglobin by Nitrites. Journal of Biological Chemistry. 1981;256(23):12393–12398. [PubMed] [Google Scholar]
- 36.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces Nitric oxide under allosteric control. Journal of Clinical Investigation. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang KT, et al. The Reaction Between Nitrite and Deoxyhemoglobin: Reassment of Reaction Kinetics and Stoichiometry. Journal of Biological Chemistry. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 38.Kim-Shapiro DB, Gladwin MT. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, et al. Nitric Oxide Production from Nitrite Occurs Primarily in Tissues Not in the Blood: CRITICAL ROLE OF XANTHINE OXIDASE AND ALDEHYDE OXIDASE. Journal of Biological Chemistry. 2008;283(26):17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giraldez RR, et al. Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. Journal of Biological Chemistry. 1997;272(34):21420–21426. doi: 10.1074/jbc.272.34.21420. [DOI] [PubMed] [Google Scholar]
- 41.Chen K, Popel AS. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radical Biology and Medicine. 2006;41(4):668–680. doi: 10.1016/j.freeradbiomed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Doyle MP, Hoekstra JW. Oxidation of Nitrogen-Oxides by Bound Dioxygen in Hemoproteins. Journal of Inorganic Biochemistry. 1981;14(4):351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 43.Eich RF, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35(22):6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 44.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40(11):3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 45.Jeffers A, et al. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2005;142(2):130–135. doi: 10.1016/j.cbpb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Robinson JM, Lancaster JR. Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide - Mechanism(s) and physiologic versus pathophysiologic relevance. American Journal of Respiratory Cell and Molecular Biology. 2005;32(4):257–261. doi: 10.1165/rcmb.F292. [DOI] [PubMed] [Google Scholar]
- 47.Basu S, et al. Catalytic generation of N2O3 by a concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 48.Nagababu E, Ramasamy S, Rifkind JM. S-Nitrosohemoglobin: A mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15(1):20–29. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Salgado MT, Nagababu E, Rifkind JM. Quantification of Intermediates Formed during the Reduction of Nitrite by Deoxyhemoglobin. Journal of Biological Chemistry. 2009;284(19):12710–12718. doi: 10.1074/jbc.M808647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navati MS, Friedman JM. Reactivity of Glass-Embedded Met Hemoglobin Derivatives toward External NO: Implications for Nitrite-Mediated Production of Bioactive NO. Journal of the American Chemical Society. 2009;131(34):12273–12279. doi: 10.1021/ja903364h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navati MS, Friedman JM. Glass Matrix-Facilitated Thermal Reduction: A Tool for Probing Reactions of Met Hemoglobin with Nitrite and Nitric Oxide. Journal of Physical Chemistry B. 2010;114(8):2938–2943. doi: 10.1021/jp909425z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel RP, Hogg N, Kim-Shapiro DB. The potential role of the red blood cell in nitrite-dependent regulation of blood flow. Cardiovascular Research. 2011;89(3):507–515. doi: 10.1093/cvr/cvq323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiva S, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100(5):654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 55.Hendgen-Cotta UB, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences. 2008;105(29):10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Totzeck M, et al. Nitrite Regulates Hypoxic Vasodilation via Myoglobin-Dependent Nitric Oxide Generation. Circulation. 2012;126(3):325–+. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee-de Groot MBE, des Tombe AL, van der Laarse WJ. Calibrated histochemistry of myoglobin concentration in cardiomyocytes. Journal of Histochemistry & Cytochemistry. 1998;46(9):1077–1084. doi: 10.1177/002215549804600912. [DOI] [PubMed] [Google Scholar]
- 58.Tiso M, et al. Human Neuroglobin Functions as a Redox-regulated Nitrite Reductase. Journal of Biological Chemistry. 2011;286(20):18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiger L, et al. Neuroglobin ligand binding kinetics. Iubmb Life. 2004;56(11–12):709–719. doi: 10.1080/15216540500037711. [DOI] [PubMed] [Google Scholar]
- 60.Jayaraman T, et al. 14-3-3 Binding and Phosphorylation of Neuroglobin during Hypoxia Modulate Six-to-Five Heme Pocket Coordination and Rate of Nitrite Reduction to Nitric Oxide. Journal of Biological Chemistry. 2011;286(49):42679–42689. doi: 10.1074/jbc.M111.271973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson JS, Rohlfs RJ, Gibson QH. Ligand Recombination to the Alpha-Subunits and Beta-Subunits of Human-Hemoglobin. Journal of Biological Chemistry. 1987;262(27):12930–12938. [PubMed] [Google Scholar]
- 62.Olson JS, Phillips GN. Kinetic pathways and barriers for ligand binding to myoglobin. Journal of Biological Chemistry. 1996;271(30):17593–17596. [PubMed] [Google Scholar]
- 63.Schmidt M, et al. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. Journal of Biological Chemistry. 2003;278(3):1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- 64.Burmester T, Hankeln T. What is the function of neuroglobin? Journal of Experimental Biology. 2009;212(10):1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 65.Chen YR, et al. Protein oxidation of cytochrome c by reactive halogen species enhances its peroxidase activity. Journal of Biological Chemistry. 2002;277(33):29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 66.Estevam ML, et al. Changes in the spin state and reactivity of cytochrome c induced by photochemically generated singlet oxygen and free radicals. Journal of Biological Chemistry. 2004;279(38):39214–39222. doi: 10.1074/jbc.M402093200. [DOI] [PubMed] [Google Scholar]
- 67.Batthyany C, et al. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44(22):8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 68.Cassina AM, et al. Cytochrome c nitration by peroxynitrite. Journal of Biological Chemistry. 2000;275(28):21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 69.Spooner PJR, Watts A. Cytochrome-C Interactions with Cardiolipin in Bilayers - a Multinuclear Magic-Angle Spinning Nmr-Study. Biochemistry. 1992;31(41):10129–10138. doi: 10.1021/bi00156a037. [DOI] [PubMed] [Google Scholar]
- 70.Belikova NA, et al. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45(15):4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuominen EKJ, Wallace CJA, Kinnunen PKJ. Phospholipid-cytochrome c interaction - Evidence for the extended lipid anchorage. Journal of Biological Chemistry. 2002;277(11):8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- 72.Chen YR, et al. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the Hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radical Biol. Med. 2004;37:1591–1603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Basu S, et al. Nitrite Reductase Activity of Cytochrome c. Journal of Biological Chemistry. 2008;283(47):32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, et al. Characterization of the Mechanism and Magnitude of Cytoglobin-mediated Nitrite Reduction and Nitric Oxide Generation under Anaerobic Conditions. Journal of Biological Chemistry. 2012;287(43):36623–36633. doi: 10.1074/jbc.M112.342378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt M, et al. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. Journal of Biological Chemistry. 2004;279(9):8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 76.Tiso M, et al. Nitrite Reductase Activity of Nonsymbiotic Hemoglobins from Arabidopsis thaliana. Biochemistry. 2012;51(26):5285–5292. doi: 10.1021/bi300570v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kundu S, Trent JT, Hargrove MS. Plants, humans and hemoglobins. Trends in Plant Science. 2003;8(8):387–393. doi: 10.1016/S1360-1385(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 78.Castello PR, et al. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3(4):277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Castello PR, et al. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proceedings of the National Academy of Sciences. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper CE. Competitive, reversible, physiological? Inhibition of mitochondrial cytochrome oxidase by nitric oxide. Iubmb Life. 2003;55(10–11):591–597. doi: 10.1080/15216540310001628663. [DOI] [PubMed] [Google Scholar]
- 81.Gautier C, et al. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochemical and Biophysical Research Communications. 2006;341(3):816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Vanin AF, et al. Nitric oxide synthase reduces nitrite to NO under anoxia. Cellular and Molecular Life Sciences. 2007;64(1):96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikula I, et al. Isoform-specific differences in the nitrite reductase activity of nitric oxide synthases under hypoxia. Biochemical Journal. 2009;418:673–682. doi: 10.1042/BJ20080987. [DOI] [PubMed] [Google Scholar]
- 84.Millar TM, et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. Febs Letters. 1998;427(2):225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, et al. Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase: A potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochemical and Biophysical Research Communications. 1998;249(3):767–772. doi: 10.1006/bbrc.1998.9226. [DOI] [PubMed] [Google Scholar]
- 86.Li HT, et al. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction - Evaluation of its role in nitric oxide generation in anoxic tissues. Journal of Biological Chemistry. 2001;276(27):24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 87.Li HT, et al. Characterization of the effects of oxygen on xanthine oxidase-mediated 44 nitric oxide formation. Journal of Biological Chemistry. 2004;279(17):16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 88.Webb AJ, et al. Mechanisms Underlying Erythrocyte and Endothelial Nitrite Reduction to Nitric Oxide in Hypoxia Role for Xanthine Oxidoreductase and Endothelial Nitric Oxide Synthase. Circulation Research. 2008;103(9):957–U114. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Golwala NH, et al. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Canadian Journal of Physiology and Pharmacology. 2009;87(12):1095–1101. doi: 10.1139/Y09-101. [DOI] [PubMed] [Google Scholar]
- 90.Li HT, et al. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42(4):1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 91.Webb A, et al. Xanthine oxidase activity provides an alternative source of no during ischaemia from inorganic nitrite in rat blood vessels. British Journal of Clinical Pharmacology. 2005;59(5):631–632. [Google Scholar]
- 92.Calzi ML, et al. Purification, cDNA cloning, and tissue distribution of bovine liver aldehyde oxidase. Journal of Biological Chemistry. 1995;270(52):31037–31045. doi: 10.1074/jbc.270.52.31037. [DOI] [PubMed] [Google Scholar]
- 93.Li HT, Kundu TK, Zweier JL. Characterization of the Magnitude and Mechanism of Aldehyde Oxidase-mediated Nitric Oxide Production from Nitrite. Journal of Biological Chemistry. 2009;284(49):33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hille R. The molybdenum oxotransferases and related enzymes. Dalton Transactions. 2013;42(9):3029–3042. doi: 10.1039/c2dt32376a. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, et al. Sulfite oxidase catalyzes single electron transfer at molybdenum domain to reduce nitrite to NO. Nitric Oxide-Biology and Chemistry. 2013;31:S39–S40. [Google Scholar]
- 96.Havemeyer A, et al. Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. Journal of Biological Chemistry. 2006;281(46):34796–34802. doi: 10.1074/jbc.M607697200. [DOI] [PubMed] [Google Scholar]
- 97.Nagababu E, et al. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. Journal of Biological Chemistry. 2003;278(47):46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 98.Nagababu E, Ramasamy S, Rifkind JM. Intermediates detected by visible Spectroscopy during the reaction of nitrite with deoxyhemoglobin: The effect of nitrite concentration and diphosphoglycerate. Biochemistry. 2007;46:11650–11659. doi: 10.1021/bi700364e. [DOI] [PubMed] [Google Scholar]
- 99.Roche CJ, Friedman JM. NO reactions with sol-gel and solution phase samples of the ferric nitrite derivative of HbA. Nitric Oxide-Biology and Chemistry. 2010;22(2):180–190. doi: 10.1016/j.niox.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tui C, et al. Reactions of nitrite with hemoglobin measured by membrane inlet mass spectrometry. Free Radical Biology and Medicine. 2009;46(1):14–19. doi: 10.1016/j.freeradbiomed.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koppenol WH. Nitrosation, Thiols, and Hemoglobin: Energetics and Kinetics. Inorganic Chemistry. 2012;51(10):5637–5641. doi: 10.1021/ic202561f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooper CE. Nitric oxide and iron proteins. Biochimica Et Biophysica Acta-Bioenergetics. 1999;1411(2–3):290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 103.Aamand R, et al. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297(6):H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 104.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacology & Therapeutics. 1997;74(1):1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 105.Maren TH, Rayburn CS, Liddell NE. Inhibition by Anions of Human Red-Cell Carbonic Anhydrase-B - Physiological and Biochemical Implications. Science. 1976;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- 106.Innocenti A, et al. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (Cab) with anions. Bioorganic & Medicinal Chemistry Letters. 2004;14(17):4563–4567. doi: 10.1016/j.bmcl.2004.06.073. [DOI] [PubMed] [Google Scholar]
- 107.Kiso Y, et al. Erythrocyte Carbonic Anhydrase-I Concentrations in Patients with Graves-Disease and Subacute Thyroiditis Reflect Integrated Thyroid-Hormone Levels over the Previous Few Months. Journal of Clinical Endocrinology & Metabolism. 1991;72(2):515–518. doi: 10.1210/jcem-72-2-515. [DOI] [PubMed] [Google Scholar]
- 108.Maher AR, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117(5):670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 109.Crawford JH, et al. Hypoxia, red blood cells and nitrite regulate NO-dependent hypoxic vasodilatation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293(4):H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 111.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292(6):H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 112.Huang KT, Huang Z, Kim-Shapiro DB. Nitric Oxide Red Blood Cell Membrane Permeability at high and low Oxygen Tension. Nitric Oxide. 2007;16:209–216. doi: 10.1016/j.niox.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ormerod JOM, et al. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovascular Research. 2011;89(3):560–565. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson G, et al. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide-Biology and Chemistry. 2002;7(1):24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 115.Srihirun S, et al. Platelet Inhibition by Nitrite Is Dependent on Erythrocytes and Deoxygenation. PLOS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murillo D, et al. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide-Biology and Chemistry. 2011;25(2):70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bolli R, et al. The nitric oxide hypothesis of late preconditioning. Basic Research in Cardiology. 1998;93(5):325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker JE, et al. Nitrite confers protection against myocardial infarction: Role of xanthine oxidoreductase, NADPH oxidase and K-ATP channels channels. Journal of Molecular and Cellular Cardiology. 2007;43(4):437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez FM, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shiva S, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. Journal of Experimental Medicine. 2007;204(9):2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bryan NS, et al. Dietary nitrite supplementation protects against myocardial ischemiareperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(48):19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dezfulian C, et al. Nitrite Therapy After Cardiac Arrest Reduces Reactive Oxygen Species Generation, Improves Cardiac and Neurological Function, and Enhances Survival via Reversible Inhibition of Mitochondrial Complex I. Circulation. 2009;120(10):897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raat NJH, et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radical Biology and Medicine. 2009;47(5):510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jung K-H, et al. Effects of long term nitrite therapy on functional recovery in experimental ischemia model. Biochemical and Biophysical Research Communications. 2010;403(1):66–72. doi: 10.1016/j.bbrc.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 125.Jung K-H, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37(11):2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 126.Dezfulian C, et al. Nitrite therapy is neuroprotective and safe in cardiac arrest survivors. Nitric Oxide-Biology and Chemistry. 2012;26(4):241–250. doi: 10.1016/j.niox.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tripatara P, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: Role for xanthine oxidoreductase. Journal of the American Society of Nephrology. 2007;18(2):570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 128.Kelpke SS, et al. Sodium nitrite protects against kidney injury induced by brain death and improves post-transplant function. Kidney International. 2012;82(3):304–313. doi: 10.1038/ki.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar D, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(21):7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zuckerbraun BS, et al. Nitrite Potently Inhibits Hypoxic and Inflammatory Pulmonary Arterial Hypertension and Smooth Muscle Proliferation via Xanthine Oxidoreductase-Dependent Nitric Oxide Generation. Circulation. 2010;121(1):98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 131.He C, Knipp M. Formation of Nitric Oxide from Nitrite by the Ferriheme b Protein Nitrophorin 7. Journal of the American Chemical Society. 2009;131(34):12042–+. doi: 10.1021/ja9040362. [DOI] [PubMed] [Google Scholar]
- 132.Knipp M, He C. Nitrophorins: Nitrite Disproportionation Reaction and Other Novel Functionalities of Insect Heme-based Nitric Oxide Transport Proteins. Iubmb Life. 2011;63(5):304–312. doi: 10.1002/iub.451. [DOI] [PubMed] [Google Scholar]