Abstract
Objective
Despite standardization in care, heterogeneity in outcomes persists for infants with hypoplastic left heart syndrome (HLHS). One potential factor is in utero stressors. Uteroplacental insufficiency (UPI) induces systemic vascular and myocardial adaptations in the absence of structural heart disease. The effect of UPI in HLHS is unknown.
Methods
We retrospectively analyzed infants undergoing Norwood palliation for HLHS from 2007 to 2012. We compared the umbilical artery systolic to diastolic (SD) ratio to growth outcomes and post-operative right ventricular (RV) function.
Results
Forty three infants met our inclusion criteria. Fetuses without a declining SD ratio with advancing gestational age had asymmetric birth biometry, defined as birth weight minus head circumference z scores (−0.9 vs. −0.05, p<0.01). The SD ratio near the end of gestation negatively correlated with asymmetric birth biometry (R=−0.521, p<0.01) and interstage growth (R=−0.49, p=0.04). Males with higher SD ratios had a greater post-operative incidence of abnormal RV function.
Conclusions
A higher umbilical artery SD ratio was associated with asymmetric prenatal growth, poor weight gain and decreased myocardial performance in infants with HLHS. Better understanding UPI's effects on cardiovascular development and metabolism in HLHS will help identify new strategies for targeting morbidity in this high risk population.
INTRODUCTION
Despite improvements in surgical technique and standardization of clinical practice, heterogeneity in outcomes persists for apparently similar infants undergoing staged palliation of hypoplastic left heart syndrome (HLHS). One potential, unidentified risk factor may be explained by the Developmental Origins of Disease Theory which proposes that adaptations to the in utero environment affect post-natal outcomes. Epidemiologic evidence across multiple organ systems supports this theory.1–4 In the cardiovascular system, the in utero insult of uteroplacental insufficiency (UPI), in the absence of structural heart disease, is associated with higher blood pressures in childhood5 and increased aortic stiffness in infancy.6 How these vascular adaptations to placental resistance affect outcomes in the single ventricle physiology of HLHS is unknown.
In severe UPI, regional changes in systemic vascular resistance (SVR) preserve cerebral blood flow in what has been termed head-sparing circulation. An increase in splanchnic and placental resistance with a concomitant decrease in cerebral resistance discrepantly alters the afterload of the two ventricles hypothetically providing increased nutrient delivery to the head thereby preserving brain growth.7, 8 Single ventricle physiology has its own set of regional vascular adaptations that occur in utero. Pulsatility and resistance indices (PI, RI) in the middle cerebral artery (MCA) as well as the cerebral to placental resistance ratio (CPR) are decreased in fetuses with left sided obstructive lesions.9–12 This relatively low cerebral artery resistance may represent a similar head-sparing circulation, but neonates with HLHS have disproportionately small heads suggesting the adaptation is inadequate.10, 13–16 How the vasculature and myocardium in HLHS adapt to the additional insult of UPI may further complicate outcomes in this high risk population.
The umbilical artery systolic to diastolic (SD) ratio is often a reliable marker of uteroplacental health17, 18 but can be altered by regional vascular adaptations to HLHS. The aim of this study was to describe the variability in SD ratio in HLHS and its association with growth and myocardial performance outcomes.
METHODS
Study Design and Population
In this retrospective, cohort study we reviewed all consecutive infants who underwent stage I palliation for hypoplastic left heart syndrome (HLHS) at our institution between January of 2007 and January of 2012. The study was approved by the institutional review board of both the University of Utah and Primary Children’s Medical Center. A waiver for consent was obtained from the review boards. Inclusion criteria were anatomy of HLHS or HLHS variant (including double outlet-right ventricle with mitral atresia and critical aortic stenosis) and a prenatal echocardiogram that included Doppler interrogation of the umbilical artery. Exclusion criteria included anatomy of unbalanced atrioventricular septal defect, known chromosomal abnormality and surgical or catheter intervention prior to stage I palliation.
Echocardiography
All pre- and post-natal imaging was performed on commercially available systems (Acuson Sequoia 512, Siemens Medical Solutions, Mountain View, CA, USA; and Phillips IE33, Royal Phillips Electronics, Amsterdam, The Netherlands). All images were stored digitally and reviewed on our digital archiving system (Syngo, Siemens Medical Solutions, Mountain View, CA, USA). All fetal echocardiograms performed on a given subject were reviewed. The umbilical artery SD ratio that was calculated at the time of the study was utilized. If no SD ratio was calculated, it was retrospectively measured by a single reviewer (TM), while blinded to the patient’s outcome. We analyzed outcomes based on both the SD ratio at the time of the latest fetal echocardiogram as well as the SD ratio trend with advancing gestational age. The trend was based on whether there was a decline or there was no decline between the earliestand the latest fetal echocardiogram. For analysis of dichotomous outcome variables, an SD ratio of >3 at the time of the latest fetal echocardiogram was chosen because it is at the cut-off for the 75th percentile for the average gestational age of our cohort,19 and has been considered abnormal in late gestation in other reports.20 Velocity vector imaging was used to determine fetal right ventricular (RV) strain as previously described.21 The latest fetal echocardiogram with an adequate four chamber view was utilized for this analysis. The postoperative RV performance was determined from the subject’s routine post-Norwood complete study that was performed prior to hospital discharge. The subjective findings of abnormal RV function and greater than mild tricuspid regurgitation were based on the report of the reading echocardiographer at the time of the study.
Biometry and Clinical Data
Estimated fetal weight and fetal head circumference to abdominal circumference ratio were taken from the referring obstetrician’s records. Birth weight, birth length and head circumference were taken from the record of the delivering neonatal intensive care unit. Ponderal index was calculated as (birth weight (kg))/(birth length (m)3). Biometrics-for-age z-score were calculated using the WHO anthropometric calculator. Weight for gestational age was determined from recently published, gender-based normative data.22 Interstage growth was defined as the change in weight-for-age z-score from Norwood discharge to the pre-Glenn evaluation.
Statistical Analysis
Chi-square test was used to compare categorical variables. Pearson correlation coefficients were used to assess the linear association between continuous variables. Comparison of two un-paired groups was performed with a t-test for normally distributed populations and a Mann-Whitney test for non-normally distributed populations. Statistical significance was set at p<0.05. Statistical analysis was performed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and Graph Pad Prism (Graph Pad Software, San Diego, CA, USA).
RESULTS
Study Population
There were 43 infants (24 males) who met our inclusion criteria. The most common anatomical diagnosis was hypoplastic left heart syndrome (HLHS) with aortic and mitral atresia (44%). Hypoplastic left heart variants comprised 17% of our population with 5% of the cohort having critical aortic stenosis and 12% having double outlet right ventricle with mitral atresia. The average gestational age at the time of the latest fetal echocardiogram was 33 5/7 (±2.1) weeks. The mean gestational age at birth was 38.3 (±0.9) weeks and mean birth weight was 3.1 (±0.5) kilograms. The vast majority of patients (93%) underwent Norwood with Sano conduit while the small remainder underwent Norwood with modified Blalock-Taussig shunt (7%). Stage I palliation was performed at a mean age of 4.3 (±1.9) days of life with 145 (±39) minutes of cardiopulmonary bypass and 58 (±17) minutes of cross-clamp time. The average intensive care unit and hospital length of stay was 20 (±17) and 28 (±22) days, respectively. Survival to hospital discharge was 81%.
Umbilical Artery Systolic to Diastolic (SD) Ratio
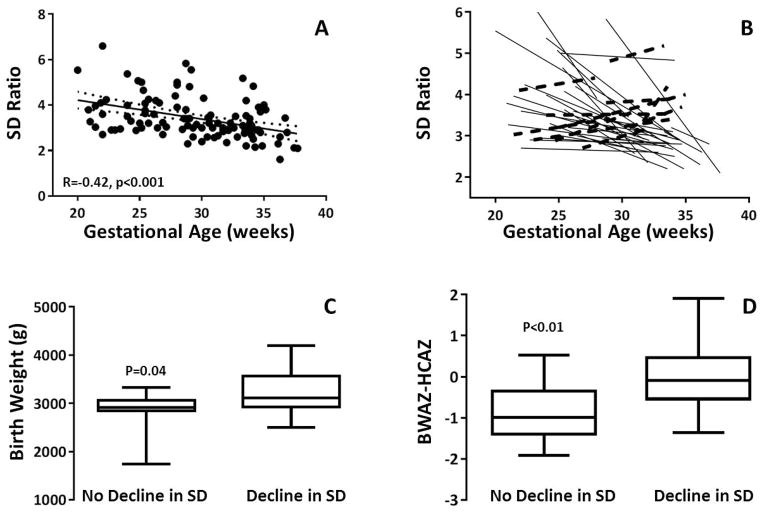
The umbilical artery SD ratio declined with advancing gestational age for the cohort (Figure 1A). When examining this trend for individuals, 11 of the 38 fetuses showed no SD ratio decline over the course of gestation (Figure 1B).The average umbilical artery SD ratio at the time of the latest echocardiogram for the entire cohort was 3.05 (range 1.61 – 4.83). The SD ratio at the time of the latest echocardiogram was >3 in 16 (37%) of the fetuses. There was no significant difference in gestational age at time of evaluation for those with SD ratio >3 and 3 (33.2 ±2 and 34.0 ±2 weeks gestational age, respectively, p=0.2). There was no difference in the average SD ratio in those with aortic atresia (3.03, ±0.65) and those with aortic stenosis (3.39, ±0.92) (p=0.21).
Figure 1.
Umbilical Artery SD Ratio and Biometry
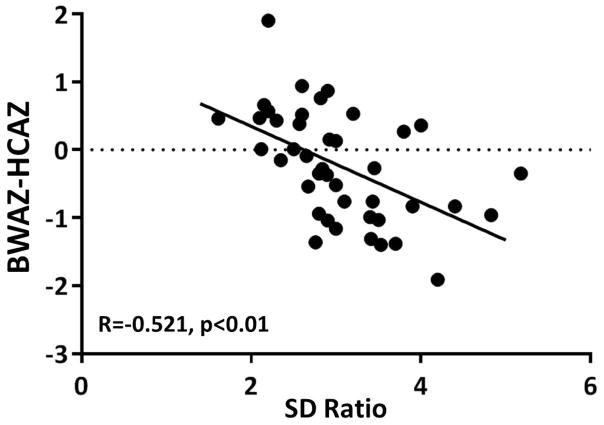
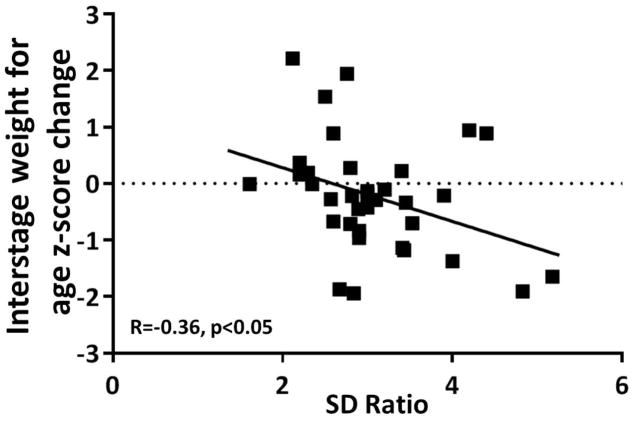
There was no correlation between the umbilical artery SD ratio at the time of the latest fetal echocardiogram and estimated fetal weight, fetal head circumference:abdominal circumference ratio, birth ponderal index, absolute birth weight, birth weight for age z-score or birth weight percentile for gestational age. There was a negative correlation between the umbilical artery SD ratio and asymmetric birth biometry as defined by the birth weight for age z-score minus the birth head circumference for age z-score (Figure 2, R= −0.51, p<0.01). The 11 gestations that did not demonstrate a decline in SD ratio with advancing gestational age had a lower birth weight, birth weight percentile for gestational age and asymmetric birth biometry (Figure 1C, D). The difference in birth weight for age z-score did not reach statistical significance (p=0.06). There was a negative correlation between umbilical artery SD ratio at the latest echocardiogram and interstage growth (R= −0.36, p<0.05) (Figure 3).
Figure 2.
Figure 3.
Umbilical Artery SD Ratio and Myocardial Performance
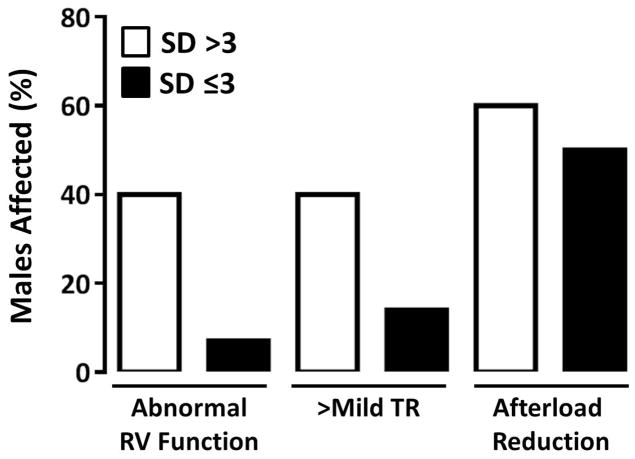
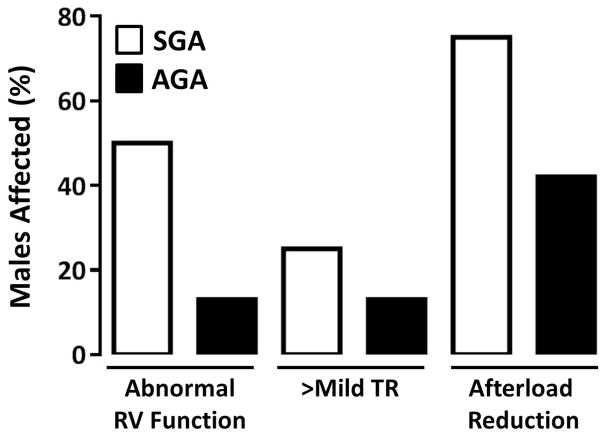
In the study cohort, there were 35 patients who survived to hospital discharge (81%). Their routine discharge echocardiogram was performed on post-operative day 15 (±18). Of those infants, 17% had abnormal right ventricular (RV) systolic function and 23% had greater than mild tricuspid regurgitation (TR) at the time of their routine discharge echocardiogram. Forty-five percent were discharged on afterload reduction. Abnormal ventricular function or TR at some point during the hospital stay was the indication for afterload reduction on all patients. No patients were started on afterload reduction for the indication of hypertension. Using an SD ratio cut-off of 3, a disproportionate percentage of males with the higher SD ratio at the time of their latest fetal echocardiogram had decreased RV systolic function (40%), greater than mild TR (40%) and were discharged on afterload reduction (60%) (Figure 4). This is similar to the association between weight for gestational age and these surrogates for myocardial performance. Small for gestational age males, but not females, had a disproportionate percentage of decreased RV systolic function (50%), greater than mild TR (25%) and likelihood of being discharged on afterload reduction (75%) (Figure 5). These differences were not statistically significant, in either the SD or SGA pairings, with this small sample size. The effect of shunt type on myocardial performance was not evaluated given that all males who survived to hospital discharge had a Sano conduit. While there was no difference in global RV longitudinal strain (at the time of discharge echo) based on weight for gestational age, gender or umbilical artery SD ratio, fetal RV strain was increased in females who had a higher umbilical artery SD ratio (R=−0.702, p=0.005).
Figure 4.
Figure 5.
DISCUSSION
In this small, retrospective cohort, we have found that there is heterogeneity in the umbilical artery SD ratio in fetuses with HLHS. In patients with HLHS, a higher umbilical artery SD ratio was associated with asymmetric prenatal growth and worse weight gain following Norwood palliation. The males with a higher SD ratio had worse myocardial performance following Norwood palliation compared to those with a lower SD ratio. These findings suggest that adaptations in in utero vascular physiology affect post-natal outcomes in HLHS. .
While there is no universally accepted standard cut-off for a normal umbilical artery SD ratio, it is known that the SD ratio decreases with gestational age.17, 18 Abnormal umbilical artery hemodynamics have been seen in both UPI and structural heart disease. In fetuses with HLHS, there is an abnormal cerebral artery to umbilical artery pulsatility index ratio (CPR) implicating a vascular autoregulatory mechanism attempting to preserve brain growth. Hypothetically, an increase in umbilical artery vascular resistance is a major contributor to this CPR balance. However, in a series of fetuses with decreased CPR, only a fraction had evidence of elevated umbilical artery pulsatility index.11 Control of the umbilical artery resistance, therefore is likely multifactorial. One unexplored factor is the contribution of placental pathology or UPI to vascular adaptations in CHD. The effects of UPI may compound the single ventricle vascular adaptations and alter post-natal outcomes. The heterogeneity within our cohort suggests that factors beyond the cardiac lesion contribute to fetal vascular regulation.
In regards to somatic growth, others have speculated that newborns with HLHS have proportionally smaller heads because of decreased cerebral perfusion secondary to a hypoplastic aortic arch.14, 15 Smaller head circumference is present even in the setting of decreased MCA-PI in utero.9, 10 Traditionally, this finding of smaller head circumference has been reported as an average across the cohort compared to controls. In these studies, the degree of individual anthropomorphic asymmetry is not reported. In our HLHS population, many of our patients had proportionally larger heads compared to body weight at birth suggesting that there is heterogeneity in the success of brain sparing fetal circulatory adaptations. Concordant with the Developmental Origins of Disease Theory, however, a successful fetal adaptation may be deleterious post-natally (as evidenced by poor interstage weight gain). Our finding of smaller body weight for head circumference with higher SD ratios is consistent with the pattern seen in asymmetric IUGR. That is to say, increasing placental resistance was associated with preserved head growth and smaller body weights. With severe IUGR this growth pattern is thought to be secondary to an adaptive circulation.8 Whether or not the asymmetric growth in our population is secondary to vascular adaptations requires further investigation. Placental health also affects metabolism 23 and it may be that the head growth potential is relatively fixed in HLHS and the outcomes observed in our population are due to the metabolic effects of UPI that persist post-natally.
The pattern of decreased myocardial performance following Norwood palliation in males with higher SD ratios that mimics the pattern in SGA males, parallels the findings of myocardial recovery in animal models of UPI and ischemia-reperfusion injury.24–26 The long bypass and cross-clamp times needed to perform Norwood palliation represent a form of ischemia-reperfusion injury. We found that males with UPI (as defined by the SD ratio) had impaired tolerance of this insult. Whether the effect is primarily from changes within the myocardium, however, is unknown. UPI also induces sex-specific changes in the renin-angiotensin system in the rat.27 It may be that the myocardial changes are secondary to differences in vascular reactivity between the two groups. Our finding of increased myocardial deformation in female fetuses with increasing SD ratio suggests that the females may perform better post-nataly due to preconditioning adaptations in utero.
This study is limited by its retrospective nature and small size. Given that the outcomes in this study are associative, conclusions regarding causation must be speculative. Despite these limitations, this study is the first that we are aware of which extends findings of the Developmental Origins of Disease Theory into the congenital heart disease population. There is clinical, epidemiologic and experimental evidence that in utero stressors increase the risk of post-natal cardiovascular and metabolic disease in the absence of structural heart disease. It is also known that low birth weight and premature infants with HLHS endure increasing rates of morbidity and mortality.28–30 We propose that more subtle in utero stressors, that may not be severe enough to cause IUGR, also impact the morbidity and mortality of this high risk population.
CONCLUSION
Despite standardization of care, there still exists marked heterogeneity in outcomes for infants with HLHS. In this retrospective study, we have found that a higher umbilical artery SD ratio was associated with asymmetric prenatal growth, poor weight gain and decreased myocardial performance in infants with HLHS. Better understanding of vascular and metabolic adaptations to in utero stressors and their interplay with fetal adaptations to structural heart disease may help us to better understand and treat the heterogeneity in outcomes.
What's already known about the subject
Low birth weight infants, as a cohort, suffer increased rates of morbidity and mortality following surgical palliation for HLHS, but some individuals do very well.
Uteroplacental insufficiency (UPI) is an in utero stressor that increases the risk of cardiovascular disease in the absence of structural heart disease.
What does this study add
While birth weight is multifactorial, UPI may compound morbidity by its effect on growth and myocardial performance in HLHS.
Acknowledgments
The authors would like to thank research coordinators Mason Heywood and Anna Jolley for their assistance with data collection and organization. Study data were collected and managed using REDCap31 electronic data capture tools hosted at University of Utah Center for Clinical and Transitional Sciences. The Center is supported by NIH funding (CTSA 5UL1RR025764-02).
Financial Support: None
Footnotes
Conflicts of Interest and Disclosures: None
References
- 1.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–83. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Brutsaert TD, Tamvada KH, Kiyamu M, et al. Low ponderal index is associated with decreased muscle strength and fatigue resistance in college-aged women. Early Hum Dev. 2011;87:663–9. doi: 10.1016/j.earlhumdev.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells JC. Body composition in infants: evidence for developmental programming and techniques for measurement. Rev Endocr Metab Disord. 2012;13:93–101. doi: 10.1007/s11154-012-9213-9. [DOI] [PubMed] [Google Scholar]
- 4.Lithell HO, McKeigue PM, Berglund L, et al. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312:406–10. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 6.Akira M, Yoshiyuki S. Placental circulation, fetal growth, and stiffness of the abdominal aorta in newborn infants. J Pediatr. 2006;148:49–53. doi: 10.1016/j.jpeds.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Severi FM, Rizzo G, Bocchi C, et al. Intrauterine growth retardation and fetal cardiac function. Fetal Diagn Ther. 2000;15:8–19. doi: 10.1159/000020969. [DOI] [PubMed] [Google Scholar]
- 8.Verburg BO, Jaddoe VW, Wladimiroff JW, et al. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation. 2008;117:649–59. doi: 10.1161/CIRCULATIONAHA.107.709717. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Khoo NS, Brooks PA, et al. Severe Left Heart Obstruction with Retrograde Arch Flow Importantly Influences Fetal Cerebral and Placental Blood Flow. Ultrasound Obstet Gynecol. 2013 doi: 10.1002/uog.12448. [DOI] [PubMed] [Google Scholar]
- 10.Berg C, Gembruch O, Gembruch U, Geipel A. Doppler indices of the middle cerebral artery in fetuses with cardiac defects theoretically associated with impaired cerebral oxygen delivery in utero: is there a brain-sparing effect? Ultrasound Obstet Gynecol. 2009;34:666–72. doi: 10.1002/uog.7474. [DOI] [PubMed] [Google Scholar]
- 11.Szwast A, Tian Z, McCann M, et al. Comparative analysis of cerebrovascular resistance in fetuses with single-ventricle congenital heart disease. Ultrasound Obstet Gynecol. 2012;40:62–7. doi: 10.1002/uog.11147. [DOI] [PubMed] [Google Scholar]
- 12.McElhinney DB, Benson CB, Brown DW, et al. Cerebral blood flow characteristics and biometry in fetuses undergoing prenatal intervention for aortic stenosis with evolving hypoplastic left heart syndrome. Ultrasound Med Biol. 2010;36:29–37. doi: 10.1016/j.ultrasmedbio.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arduini M, Rosati P, Caforio L, et al. Cerebral blood flow autoregulation and congenital heart disease: possible causes of abnormal prenatal neurologic development. J Matern Fetal Neonatal Med. 2011;24:1208–11. doi: 10.3109/14767058.2010.547961. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol. 1996;143:505–13. doi: 10.1093/oxfordjournals.aje.a008771. [DOI] [PubMed] [Google Scholar]
- 15.Shillingford AJ, Ittenbach RF, Marino BS, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17:189–95. doi: 10.1017/S1047951107000248. [DOI] [PubMed] [Google Scholar]
- 16.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–40. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kofinas AD, Espeland MA, Penry M, et al. Uteroplacental Doppler flow velocity waveform indices in normal pregnancy: a statistical exercise and the development of appropriate reference values. Am J Perinatol. 1992;9:94–101. doi: 10.1055/s-2007-994679. [DOI] [PubMed] [Google Scholar]
- 18.Schulman H, Fleischer A, Stern W, et al. Umbilical velocity wave ratios in human pregnancy. Am J Obstet Gynecol. 1984;148:985–90. doi: 10.1016/0002-9378(84)90541-6. [DOI] [PubMed] [Google Scholar]
- 19.Acharya G, Wilsgaard T, Berntsen GK, et al. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192:937–44. doi: 10.1016/j.ajog.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Bracero L, Schulman H, Fleischer A, et al. Umbilical artery velocimetry in diabetes and pregnancy. Obstet Gynecol. 1986;68:654–8. [PubMed] [Google Scholar]
- 21.Miller TA, Puchalski MD, Weng C, Menon SC. Regional and global myocardial deformation of the fetal right ventricle in hypoplastic left heart syndrome. Prenat Diagn. 2012;32:949–53. doi: 10.1002/pd.3939. [DOI] [PubMed] [Google Scholar]
- 22.Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 23.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 24.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011;90:285–94. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 25.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther. 2009;330:624–32. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbasi A, Thamotharan M, Shin BC, et al. Myocardial macronutrient transporter adaptations in the adult pregestational female intrauterine and postnatal growth-restricted offspring. Am J Physiol Endocrinol Metab. 2012;302:E1352–62. doi: 10.1152/ajpendo.00539.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moritz KM, Cuffe JS, Wilson LB, et al. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta. 2010;31 (Suppl):S40–6. doi: 10.1016/j.placenta.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch JC, Copeland G, Donohue JE, et al. Population-based analysis of survival for hypoplastic left heart syndrome. J Pediatr. 2011;159:57–63. doi: 10.1016/j.jpeds.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 29.Hornik CP, He X, Jacobs JP, et al. Complications after the Norwood operation: an analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:1734–40. doi: 10.1016/j.athoracsur.2011.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tweddell JS, Sleeper LA, Ohye RG, et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–9. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]