Abstract
Most systemic autoimmune diseases occur more frequently in females than in males. This is particularly evident in Sjögren’s Syndrome, Systemic Lupus Erythromatosis (SLE) and thyroid autoimmunity, where the ratio of females to males ranges from 20:1 to 8:1. Our understanding of the etiology of SLE implies important roles for genetics, environmental factors and sex hormones, but the relative significance of each remains unknown. Using the New Zealand hybrid mouse model system of SLE we present here a new fetal liver chimera-based system in which we can segregate effects of immune system genes from that of sex hormones in vivo. We show that female hematopoietic cells express an intrinsic capacity to drive lupus-like disease in both male and female recipient mice, suggesting that this capacity is hormone independent. Particularly, only chimeric mice with a female hematopoietic system showed significantly increased numbers of germinal center B cells, memory B cells and plasma cells followed by a spontaneous loss of tolerance to nuclear components and hence elevated serum anti-nuclear autoantibodies. A protective effect of testosterone was noted with regards to disease onset, not disease incidence. Thus, genetic factors encoded within the female hematopoietic system can effectively drive lupus-like disease even in male recipients.
Keywords: Autoimmunity, genetic, sex hormone, hematopoiesis, autoantibody, interferon-alpha
INTRODUCTION
Autoimmune diseases such as systemic lupus erythematosus (SLE) have a strong female bias1. A female predominance is also observed in the New Zealand hybrid mouse model of SLE ((NZB × NZW)F1), where 100% of females, but less than 40% of males develop end-stage renal disease within 1 year of age2,3. Lupus-like disease in (NZB × NZW)F1 mice is characterized by elevated anti-nuclear autoantibodies (ANA), IgG-immune complex (IgG-IC) deposition and complement fixation in the kidney glomeruli, and glomerulonephritis (GN) resembling the human disorder4. The disease is generally believed to be mediated by immune system defects as shown in bone marrow (BM) transfer studies5.
Levels of sex hormones or differences in sex-linked gene expression patterns are proven explanations for the pronounced sex difference observed for (NZB × NZW)F1 lupus-like disease. In this regard, prepubertal hormonal manipulation studies have shown a protective effect of testosterone and exacerbating effect of estrogens3,6–9. In addition, exposure to sex hormones during embryogenesis can affect autoimmune development in adult mice10.
Genetic overexpression of X-linked genes, as seen in mice carrying the Yaa lupus susceptibility locus, has also been strongly associated with disease development11,12. Particularly, a link between copies of Tlr7 and the development of ANA have been demonstrated12–15, although other genes expressed on the X chromosome probably also play a role, as demonstrated in TLR7-deficient male B6.Nba2(Yaa) congenic lupus-prone mice16. Also supporting an effect of the X chromosome are data showing a correlation between pristane-induced lupus-like disease and X chromosome dosage in castrated Sry-transgenic male mice and the accelerated spontaneous development of lupus in XX versus XY− NZM2328 mice17.
Type I interferons (IFNα) play a crucial role in SLE and lupus-like disease development18,19. In (NZB × NZW)F1 mice, elevation of the levels of IFNα increases autoantibody production and accelerates renal disease onset20. IFNα can be produced by many cell subsets, but most noticeably by plasmacytoid dendritic cells (pDCs) in response to a variety of stimuli targeting intracellular toll-like receptors (TLR) 7, 8 and 9 and cytoplasmic DNA sensors such as Aim2, DAI/ZBP1, Lrrfip1 and IFI16 (Ifi204)21–24. IFNα is known to affect T cells, as well as B cells, although whether one or both mechanisms are involved in IFNα-driven lupus-like disease is still unknown25,26.
In this study, we investigated whether female hematopoietic stem- and progenitor cells were capable of driving autoimmunity in the presence of male and/or female sex hormones. Using a mixed sex chimera system, we found that female hematopoietic cells (HCs) could drive the development of lupus-like renal disease, elevated levels of germinal center (GC) B cells, memory B cells and plasma cells, and increased ANA in all recipients regardless of sex hormone levels. In addition, mice receiving female HCs expressed elevated levels of serum IFNα prior to the generation of ANA and the onset of renal disease. Male recipients of female HCs exhibited a delay in the onset of disease as compared with female recipients, suggesting that the protective effect of testosterone affected early events in disease propagation only. Thus, surprisingly the disease-driving capacity of female HCs appears more potent in driving disease than the known protective effect of testosterone.
RESULTS
Female BM cells transfer renal disease into male and female recipients with a higher incidence and faster kinetic than male BM cells
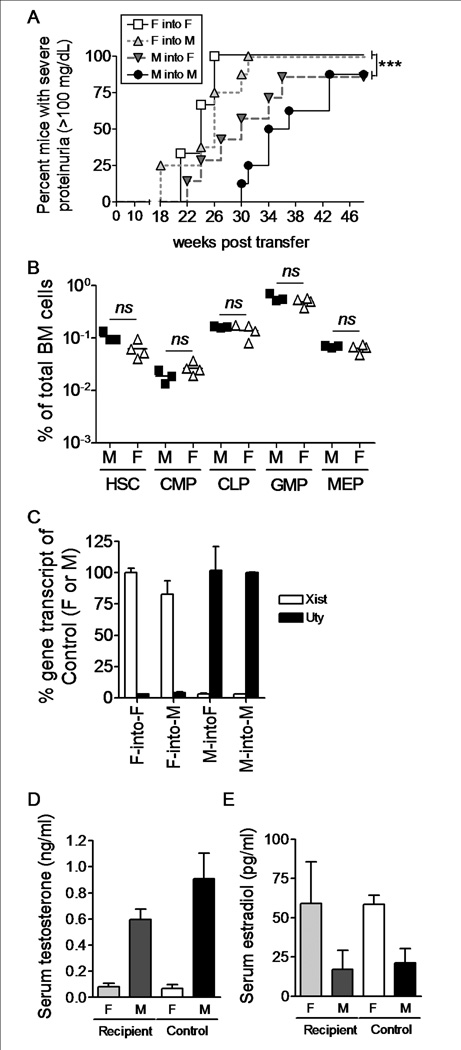
Estrogens are known to promote lupus-like disease development in (NZB × NZW)F1 mice, while testosterone has been found to protect against the disease6,7. In addition, X chromosome dosage has been found to affect disease development in other mouse models of SLE17,27. Since sex hormones affect the immune system28,29, distinguishing the effect of hormones from the effect of genes has been challenging. We asked if female HCs from (NZB × NZW)F1 mice could transfer disease into hormonally intact, lethally irradiated, age-matched male recipients. The major male antigen H-Y is not presented by H2d and H2z, allowing such reconstitution to occur without rejection30. To avoid potential effects of pubertal sex hormones, we performed the experiments using 4 wk old, prepubertal mice. Female HCs were capable of driving lupus-like renal disease in 100% of recipient mice by 31 wks post transfer, regardless of the sex of the recipient mouse (Fig. 1A). In contrast, within the same timeframe, male HCs transferred disease into 57% and 25% of female and male recipients, respectively (Fig. 1A, p < 0.05–0.001). Even when kept until 1 year of age (48 weeks post transfer), only ~85% of recipient mice accepting male hematopoietic cells developed renal disease (p<0.001). Irradiation and reconstitution itself accelerated end-stage lupus-like disease development in all recipient mice regardless of sex, although the characteristic difference between control male-into-male and female-into-female remained statistically significant (p < 0.001). Disease onset was similar in male and female mice receiving female BM cells, while M-into-M BM chimera mice started developing disease slightly later than M-into-F BM chimera mice (Fig. 1A, not statistically significant).
Figure 1.
Female prepubertal BM cells transfer lupus-like disease in a hormone independent fashion. Four wk old BWF1 male and female mice were lethally irradiated and reconstituted with male or female BM cells from age-matched mice. A) Mice were followed for the development of renal disease by detection of proteinuria every two weeks. Mice with severe proteinuria (> 100mg/dL on two consecutive readings) were considered positive. All mice were euthanized 48 weeks post transfer, regardless of disease stage. Female-into-female (open square, n = 6); female-into-male (light grey triangle, n = 8); male-into-female (dark grey triangle, n = 7); male-into-male (filled circle, n = 8). B) BM cells were isolated from 4 wk old unmanipulated BWF1 mice (n = 5 for both males and females) and the proportions of hematopoietic stem cells and progenitor cell subsets were determined by flow cytometry. C) PBMCs were isolated from BM chimera mice 18 weeks post transfer (n = 2 of each). Total RNA was isolated and cDNA generated. The levels of Xist and Uty transcripts were normalized to the levels of beta-2-microglobulin and the % was calculated relative to the levels in control female-into-female (100% Xist) or male-into-male (100% Uty) BM chimera mice. D and E) Serum was isolated from BM chimera mice 18 weeks post transfer and levels of testosterone (D) and estradiol (E) were measured by ELISA. Female recipient (n = 13 (testosterone), n = 6 (estradiol)), male recipient (n = 15 (testosterone), n = 5 (estradiol)), female control (n = 10 (testosterone), n = 10 (estradiol)), male control (n = 6 (testosterone), n = 7 (estradiol)). * p < 0.05; ** p < 0.01; *** p < 0.001.
The differential disease development was not driven by differences within the stem cell and progenitor cell compartment of males and females, as BM samples from 4 wk old unmanipulated male and female (NZB × NZW)F1 mice showed equivalent levels of hematopoietic stem cells (HSC), common myeloid progenitors (CMP), common lymphoid progenitors (CLP), granulocyte-macrophage progenitors (GMP) and megakaryocyte-erythrocyte progenitors (MEP) (Fig. 1B). In addition, all mice analyzed had grafted successfully, as noted by the relative expression of Xist and Uty transcripts in PBMC fractions from mice receiving female or male hematopoietic cells (Fig. 1C). Moreover, recipient mice continued to express sex hormones at levels equivalent to unmanipulated mice as determined by serum levels of estradiol and testosterone (Fig. 1D–E). Thus, female HCs from prepubertal 4 wk old (NZB × NZW)F1 mice transferred accelerated renal disease into both male and female age-matched (NZB × NZW)F1 mice independently of the recipient’s sex hormone environment.
The capacity of female hematopoietic cells to transfer renal disease is present in utero
Sex hormones are produced at high levels starting at puberty. However even in utero and during the postnatal period sex hormones are produced, and hence hematopoietic cells from 4 wk old female (NZB × NZW)F1 mice could have acquired their autoimmune capacities as a result of such exposure. To test for this possibility, we generated fetal liver (FL) mixed chimera mice. Fetal liver cells were isolated from male or female (NZB × NZW)F1 embryos at day E13.5-E14.5 and transferred into lethally irradiated 4 wk old prepubertal male or female (NZB × NZW)F1 mice. Mice were followed for the development of proteinuria until 32 weeks post transfer. Diagnosis of disease was confirmed by discovery of elevated co-localized IgG-IC deposition and complement fixation in kidney glomeruli in chimera mice that had received female FL cells (Fig. 2D).
Figure 2.
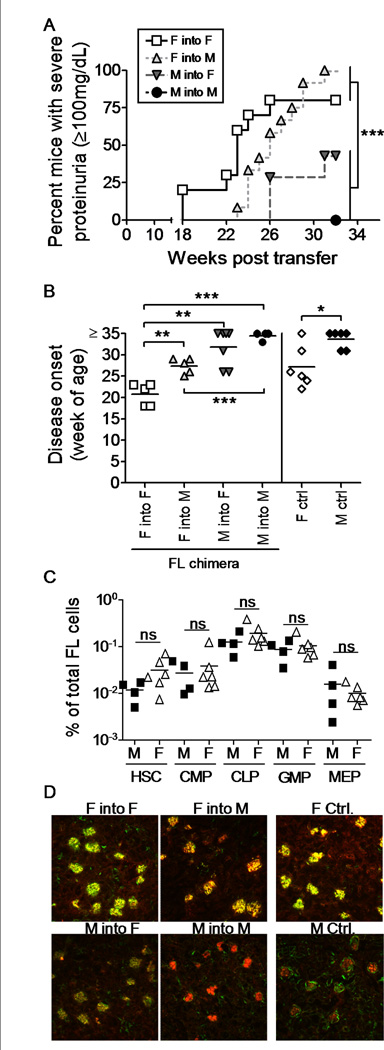
Female FL cells transfer lupus-like disease into both male and female recipients with 100% incidence. Four week old BWF1 male and female mice were lethally irradiated and reconstituted with male or female fetal liver cells from E14.5 male or female BWF1 embryos. A) Two cohorts of FL chimera mice were followed for the development of renal disease by detection of proteinuria every two weeks. Mice with severe proteinuria (> 100mg/dL on two consecutive readings) were considered positive. All mice were euthanized 35 (cohort 1) or 32 (cohort 2) weeks post transfer, regardless of disease stage. Female-into-female (open square, n = 10); Female-into-male (light grey triangle, n = 12); Male-into-female (dark grey triangle, n = 7); Male-into-male (filled circle, n = 4). B) Disease onset up to 35 weeks post transfer (cohort 1) is shown. Female-into-female (open square, n = 5); Female-into-male (light grey triangle, n = 5); Male-into-female (dark grey triangle, n = 7); Male-into-male (filled circle, n = 4). Control unmanipulated mice are included for comparison: females (open diamonds, n = 6); males (filled diamonds, n = 6). C) FL cells were isolated from E14.5 embryos and the ratios of stem cell and progenitor cell subsets were determined in male cells (filled squares, n = 4) and female cells (open triangles, n = 6). ns: not statistically different. D) Upon sacrifice of the mice described in A), kidneys were harvested and analyzed for IgG deposition (red) and C3 fixation (green). Pictures shown represent averages per condition. Each symbol represents an individual mouse. * p < 0.05; ** p < 0.01; *** p < 0.001.
Similarly to the experiments involving BM cell transfer from 4 week old donors, female FL cells induced a rapid onset of disease in 100% of recipient mice, while male FL cells induced less disease and significantly delayed disease onset (Fig. 2A–B, p < 0.001). However, disease occurred somewhat later in male, versus female, recipients of female FL cells (Fig. 2B, p < 0.01). Again, we did not find this to be a result of differences among the transferred HCs, as analyses of FL cells from male and female (NZB × NZW)F1 embryos showed no differences in the distribution of cell subsets (Fig. 2C). Similar to the BM chimeric mice, serum levels of sex hormones in FL chimeric recipient mice were comparable to that of unmanipulated male and female (NZB × NZW)F1 mice (data not shown).
Reconstitution with Female FL cells specifically affects levels of post-activation B cell subsets
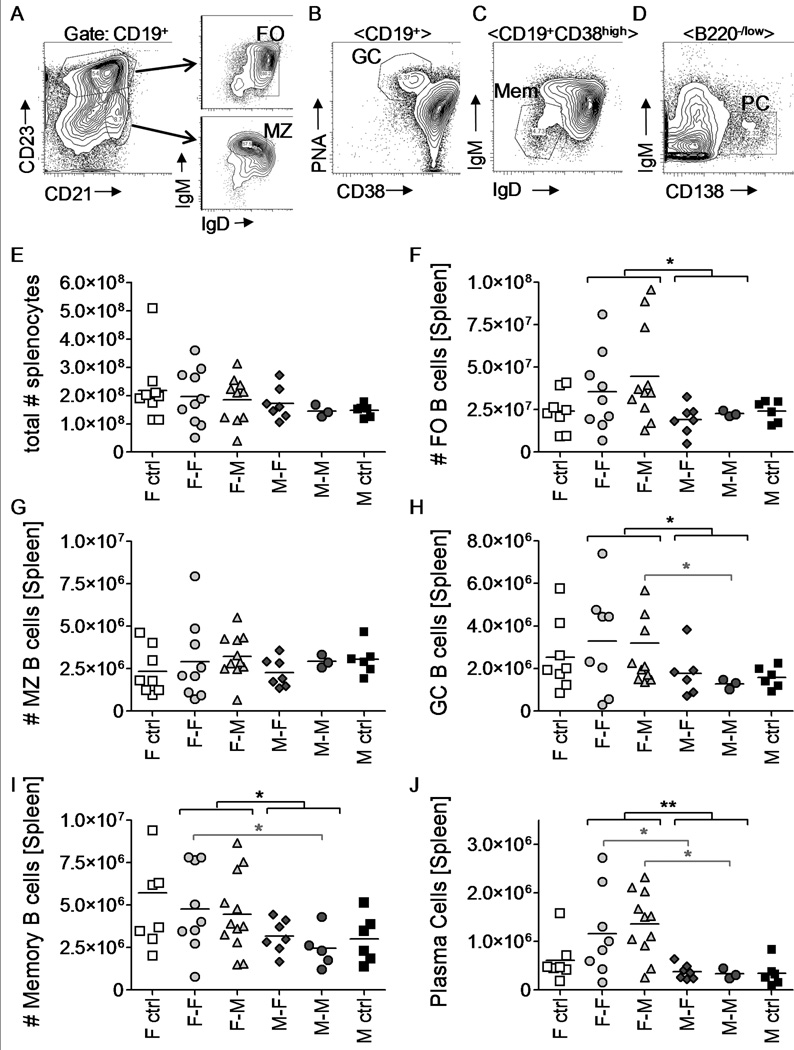
Lupus is a B cell and autoantibody mediated disorder. We tested if B cell numbers and subset distribution were different between the four groups of FL chimera mice. Gating strategies are depicted in Fig. 3A–D. In spleens, neither the total numbers of B cells (CD19+) nor of marginal zone B cells (CD19+CD21highCD23−IgMhighIgDlow) were significantly different between the various FL chimera mice (Fig. 3E,G). However, mice that had received female FL cells displayed overall increased levels of follicular mature B cells (CD19+CD21lowCD23highIgMlowIgDhigh) (Fig. 3F) regardless of the sex of the recipient. Even more strikingly, the numbers of germinal center B cells (CD19+PNA+CD38lowIgMlow), memory B cells (CD19+CD38hiIgMlow) and plasma cells (B220low/negCD138+IgM−) were significantly elevated in chimeric mice that had received female FL cells (Fig. 3H–J, p < 0.05–0.01). Consistent with a female-driven effect driving differentiation of mature B cells in the periphery, we found no differences among the relative levels of pro-B, pre-B and immature B cell subsets in the bone marrow of FL chimera mice (data not shown).
Figure 3.
The female hematopoietic system of (NZB × NZW)F1 mice promotes B cell differentiation. Spleens were harvested from FL chimera mice at the time of sacrifice. Samples were analyzed for the presence of B cell subsets by flow cytometry. A–D) Representative plots show the gating strategy used to determine follicular mature (FO), Marginal Zone (MZ), germinal center (GC), Memory (Mem) B cells and plasma cells (PC). E–J) Total numbers of indicated B cell subsets are presented per FL chimera mouse. Each symbol represents one mouse. * p < 0.05; ** p < 0.01. Brackets in black indicate statistical differences between groups receiving male or female FL cells, while brackets in grey represent statistical differences between individual groups of chimeras. Mice analyzed included female-into-female (n = 9), Female-into-male (n = 11), male-into-female (n = 7), and male-into-male (n = 3) FL chimeric mice.
Autoantibody production is driven by female hematopoietic cells and not affected by the presence of male sex hormone
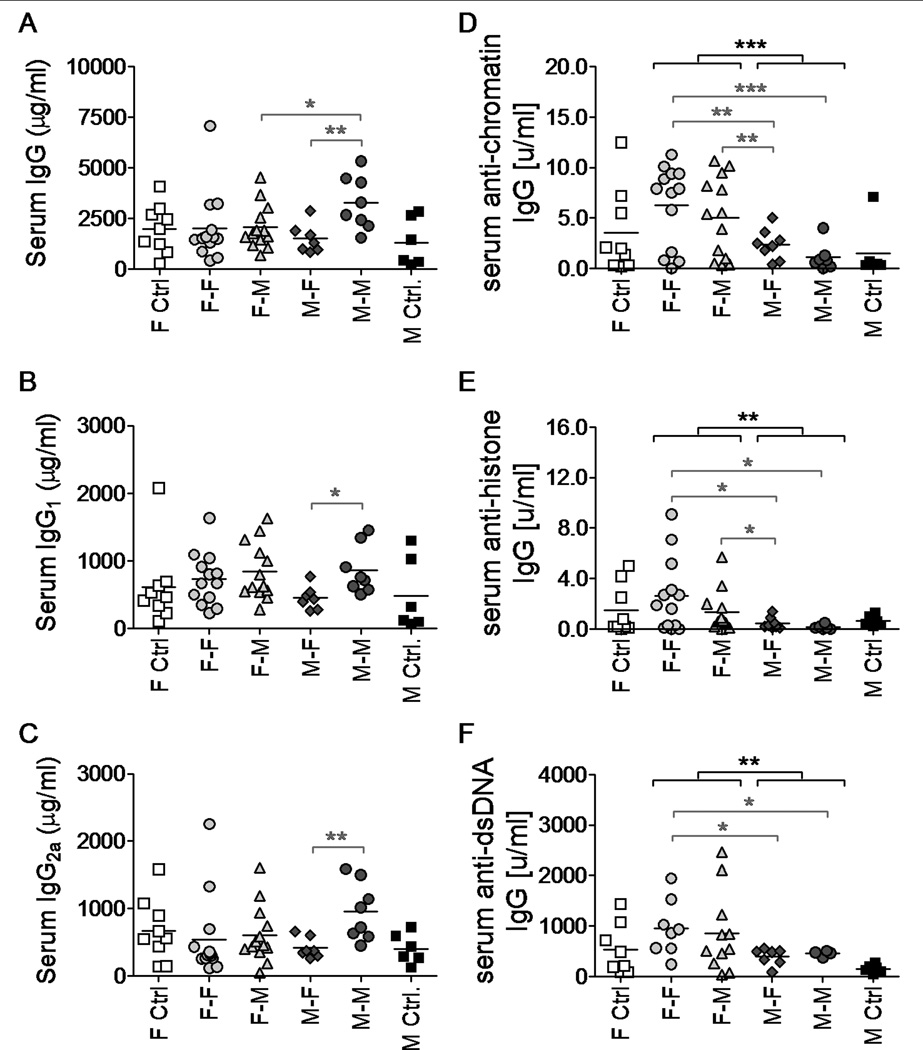
Female (NZB × NZW)F1 mice develop hypergammaglobulinemia at early ages followed by a specific loss of tolerance to nuclear autoantigens31,32. We analyzed whether total immunoglobulin and ANA levels were associated with the sex of the FL donor in our chimera system. Increased levels of total IgG, IgG1 and IgG2A in the serum of FL chimera mice did not correlate with the groups of mice developing renal disease (Fig. 4A–C). In fact, male-into-male FL chimera mice, which did not develop lupus-like disease, had the highest levels of circulating total immunoglobulins. In contrast, the levels of anti-chromatin IgG, anti-histone IgG and anti-dsDNA IgG were all significantly elevated in chimera mice that had received female FL cells as compared with those mice receiving male FL cells (Fig. 4D–F, p < 0.05–0.001), suggesting that mice that had received female HCs displayed a specific loss of tolerance to nuclear antigens.
Figure 4.
Serum anti-nuclear IgG autoantibody levels in FL chimera (NZB × NZW)F1 mice are elevated in mice that received female donor cells. Serum total IgG (A), IgG1 (B), IgG2a (C), anti-chromatin IgG (D), anti-histone IgG (E) and anti-dsDNA IgG (F) levels were measured by ELISA in samples from FL chimera mice at 12 (A–C) or 20 (D–F) weeks post irradiation/reconstitution. * p < 0.05; ** p < 0.01; *** p < 0.001. Brackets in black indicate statistical differences between groups receiving male or female FL cells, while brackets in grey represent statistical differences between individual groups of chimeras. Groups of mice analyzed included female-into-female (n = 8–13), Female-into-male (n = 11–13), male-into-female (n = 7–8), and male-into-male (n = 4–8) FL chimeric mice along with 6–9 female and 6 male unmanipulated age-matched controls.
Levels of serum IFNα, but not BAFF, are elevated in chimera mice receiving female FL cells
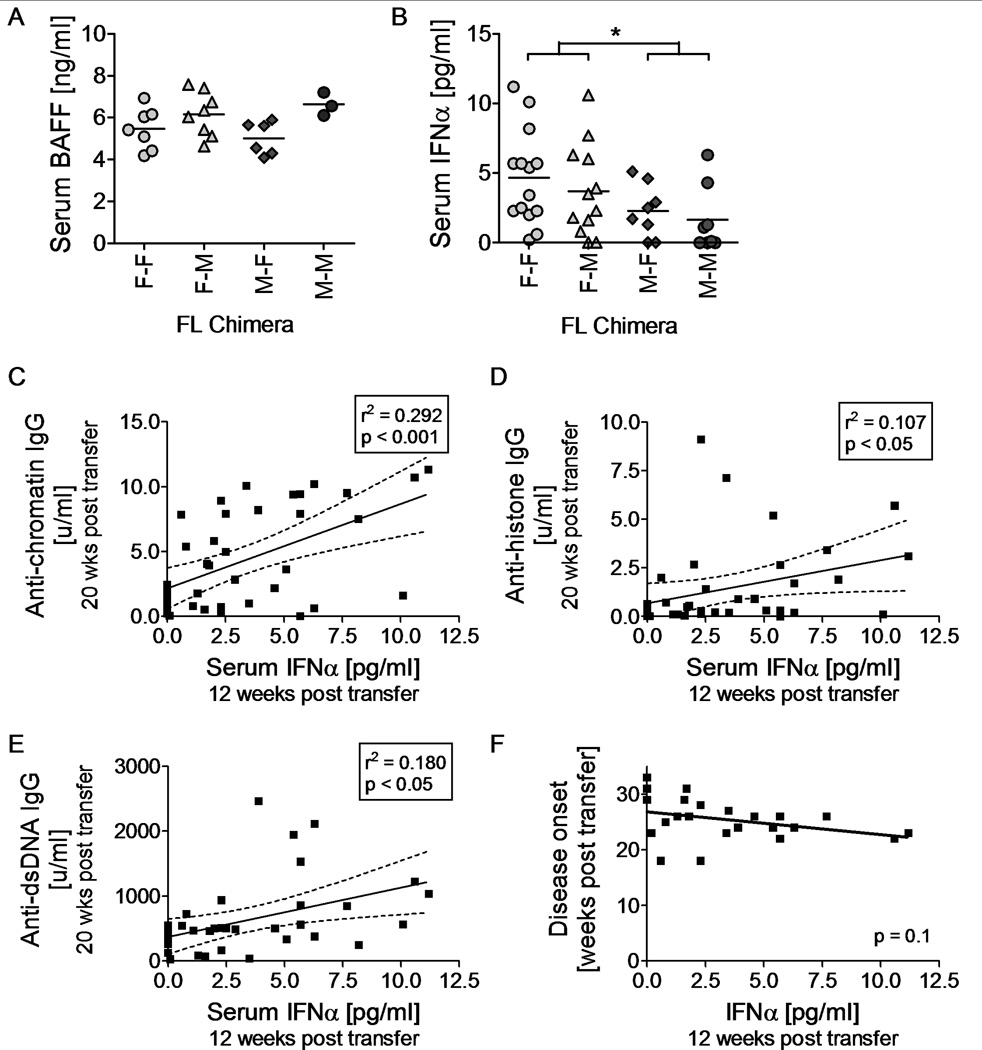
The data above suggest that loss of tolerance to nuclear antigens and renal disease is accelerated by female HCs. Possibly explanations for this observation include an increased capacity of female-derived B cells to differentiate into autoantibody producing cells or increased levels of B cell differentiating signals secreted by female-derived non-B cell HCs. To test the latter idea, we examined FL chimera mice for levels of serum BAFF at 16 weeks post irradiation and reconstitution; before the onset of renal disease. BAFF is known to be involved in B cell survival and differentiation and has previously been found to be associated with lupus in several mouse models33–37. FL chimera mice that had received female HCs did not express elevated levels of BAFF (Fig. 5A). In fact, male FL chimera mice were found to express higher levels of BAFF than female FL chimera mice, regardless of whether these had received male or female FL cells (p < 0.05).
Figure 5.
Elevated serum IFNα levels are driven by the female hematopoietic system and correlates with early disease onset. Serum was obtained from FL chimera mice 12 or 16 week post irradiation and reconstitution. Levels of BAFF were measured 16 weeks post transfer (A), while levels of IFNα were measured 12 weeks post transfer (B). Levels of IFNα correlated positively with autoantibody levels measured 20 weeks post irradiation/reconstitution (C–E) and negatively with onset of disease (F). Disease onset was defined as the time point where any given mouse presented with severe proteinuria (≥100 mg/dL) for the first of two consecutive readings (also see Figure 2). Each symbol represents one FL chimera mouse. * p < 0.05. Groups of mice analyzed included female-into-female (n = 7–14), Female-into-male (n = 8–12), male-into-female (n = 6–8), and male-into-male (n = 3–8) FL chimeric mice.
Interferon α is also known to influence B cell differentiation38 and is capable of driving disease development in (NZB × NZW)F1 mice and related strains20,39–41. We tested serum levels of IFNα in FL chimera mice 12 weeks post irradiation and reconstitution, before the onset of renal disease. Mice reconstituted with female FL cells displayed higher levels of serum IFNα than mice reconstituted with male FL cells (Fig. 5B, female versus male donor: p < 0.05). Furthermore, levels of serum IFNα at 12 weeks post transfer correlated statistically with levels of serum anti-chromatin IgG (p < 0.001), anti-histones IgG (p < 0.05) and anti-dsDNA IgG (p < 0.05) measured 20 weeks post transfer (Fig. 5C–E). Serum IFNα levels measured 12 weeks post reconstitution also trended towards a negative correlation with the onset of renal disease in all FL chimeras (p = 0.1, Fig. 5F).
DISCUSSION
Although tremendous amounts of research have gone into determining the etiology of SLE, the underlying mechanism(s) driving disease initiation and/or progression are still poorly defined. We and others have previously shown that manipulation of sex hormone production from puberty significantly alters the development of renal disease3,6–9. Specifically, castration of male lupus-prone (NZB × NZW)F1 mice was found to remove the protective effect of testosterone resulting in disease development equivalent to that of female unmanipulated mice3,6. Conversely, ovariectomy of female (NZB × NZW)F1 mice prior to puberty fails to alter disease kinetics6,8, suggesting that after the immune system is established in female mice around 2–3 weeks of age, estrogens are not crucial for disease progression. Here we have shown that, female HCs are capable of driving lupus-like disease in hormonally intact, lethally irradiated male (NZB × NZW)F1 FL recipient mice. Thus, even in the presence of testosterone, female HCs from lupus-prone (NZB × NZW)F1 mice cannot be held back and proceed to generate autoreactive B cells, followed by IgG-IC deposition, glomerulonephritis and renal failure.
Pre-B cell lines from fetal livers of lupus-prone (NZB × NZW)F1 mice have previously been shown to possess intrinsic autoimmune competencies when compared with pre-B cell lines established from non-lupus prone strains42, however, whether these cells lines were of a male or female origin was not reported and remains unknown. Since the immune system of FL chimera mice originates from transferred stem cells and progenitor cells, rather than more mature lymphocytes, our data suggest that the defect is genetically encoded. A major player in B cell development and differentiation is Bruton’s tyrosine kinase (Btk) encoded by the X chromosome43. Although not much is known about Btk levels and activity in lupus, a recent study reported amelioration of end-stage lupus-like disease in older female (NZB × NZW)F1 mice treated with a Btk inhibitor44. The study did not investigate if males were equally susceptible, and thus any sex-driven abnormality remains to be identified. Another candidate gene is Cd40l, also encoded by the X chromosome. CD40L is essential for T-cell dependent B cell activation and has been assigned an essential role in (NZB × NZW)F1 lupus-like disease development45,46, however whether CD40L-mediated B cell activation is differentially active in males and females remains unknown.
IFNα is recognized as a key cytokine in SLE and mouse lupus-like disease19,39–41,47,48. IFNα is predominantly induced in response to viral or intracellular bacterial infections, but although tempting, a cause-and-effect relationship between infections and SLE remains elusive. We observed elevated levels of serum IFNα in chimera mice that had received female HCs, suggesting that dysregulated IFNα production, either directly or via exorbitant endogenous stimuli, is also intrinsically driven by female HCs. Several genes known to be involved in IFNα production and/or responsiveness have been associated with SLE in genome wide association studies, including Irf5, Irf7, Irf8, Irak1, Tyk2, Stat4 and Fcgr2a among others (reviewed in49). Of these, Irak1 is particularly interesting. First, the Irak1 gene is encoded on the X chromosome making it an attractive candidate when studying sex-dependent disease patterns. Secondly, IRAK1 protein is essential for most TLR-mediated intracellular signaling resulting in IFNα production. And finally, IRAK1 has been shown to be required for SLE serum (i.e. IgG-IC)-induced pDC activation and IFNα production50.
Tlr7 is another X-linked gene that has been strongly associated with mouse lupus-like disease16,27, although not directly identified in GWAS studies. Recently, however, TLR7 expression levels were found to correlate with elevated levels of anti-RNA antibodies in SLE patients51. TLR7 is expressed by both pDCs and B cells resulting in IFNα production and B cell differentiation52,53, and TLR7-deficient lupus-prone mice fail to develop significant levels of ANAs27. Whether female pDCs and/or B cells from (NZB × NZW)F1 mice respond better to TLR7 cross-linking than male cells remains to be determined, however, it is tempting to speculate such a relationship since 1) autoantibody producing CD11c+ B cells depend on TLR7 signaling and are found predominantly in young autoimmune females15, and 2) TLR7 agonist stimulation of human PBMCs resulted in significantly more IFNα production from female, than male cells54,55.
Since estrogen receptor alpha (ERα) expression is required for disease development in female (NZB × NZW)F1 mice, it is interesting to note that IFNα induces transcription of the Esr1 gene and subsequent expression of the estrogen receptor alpha (ERα)56,57. Oppositely, estrogen treatment has been suggested to drive dendritic cell activation and enhance IFNα production upon at least TLR9 ligation58,59. Whether male and female HCs respond equally to estrogens has been evaluated both in vivo and in vitro in (NZB × NZW)F1 and non-autoimmune mice, showing no significant differences58,60,61. Based on these observations, we do not believe the disease promoting effect of female HCs is due to increased responsiveness of female cells to low levels of estrogens present in male recipients, although further experiments are needed to firmly rule out this possibility.
So what about testosterone and its well established protective effect? We have recently described the presence of a population of testosterone-induced immunosuppressive myeloid cells (Gr1highCD11b+) in male (NZB × NZW)F1 mice62. These cells have the capacity to directly suppress B cell differentiation in vitro, while depletion in vivo promotes autoantibody production in male mice. In our chimera system, male recipients experienced a later onset of disease, although autoantibody production seemed to be only marginally lower in male versus female recipients. Comparing intact and castrated male recipient mice after reconstitution with female FL-derived HCs confirmed that the percentage of Gr1highCD11b+ cells were in fact reduced in castrated male FL recipients (p < 0.05), however autoantibody levels were unchanged (unpublished results). Based on these observations, we propose that testosterone may act by promoting the development of immunosuppressive Gr1highCD11b+ cells capable of delaying, but not inhibiting, disease development. The presence of these cells is subsequently enough to control intrinsic disease promoting signals in male HCs, but not in female HCs, the latter promoting elevated IFNα production, B cell differentiation, ANA production and fatal renal disease. Taken together, we have found that (NZB × NZW)F1 female HCs have an intrinsic ability to drive autoimmune lupus-like disease, regardless of the hormonal environment of the host. This strongly implicates genetic rather than hormonal factors as the underlying mechanism driving the increased incidence of autoimmunity in females as compared to males.
MATERIALS AND METHODS
Mice and cells
Three week old male and female (NZB × NZW)F1 mice were obtained from The Jackson Laboratory and kept in a specific pathogen-free environment at National Jewish Health, Denver, CO. All mouse experiments were approved by the local IACUC committee. Bone Marrow chimera mice were generated by lethal irradiation (1000 rad) using a Cs137-irradiator of 4 wk old male or female prepubertal (NZB × NZW)F1 mice. Mice were given acidified water (pH 2.7) to drink throughout the experiment. Bone marrow cells were obtained from 4 wk old male or female (NZB × NZW)F1 mice and 5×106 cells were injected intravenously 2–4 hours after irradiation. Fetal liver single cells were obtained from E13.5-E14.5 embryos and frozen in 90% fetal bovine serum, 10% DMSO at −80°C. The sex of each embryo was determined visually as well as by real-time RT-PCR analyses of Uty and Xist expression levels (see below). Each fetal liver provided enough cells to reconstitute 3 recipients via tail vein injection.
Real-time reverse transcriptase-PCR
PBMCs were obtained from chimera mice 6 weeks post reconstitution. RNA was isolated using RNeasy Plus Micro Kit (Qiagen) and cDNA prepared using the qScript cDNA SuperMix (Quanta BioSciences). PCR was run on a real-time PCR machine (ABI7300, Applied Biosystems, CA) using the following primers: Xist-F: 5’-GGAGGAACGAAAGACCAAATTG-3’; Xist-R: 5’-GTCCCACCCTCTGT GAGTGAA-3’; Uty-F: 5’-TGCCATCACAAGTCA AAGCAA-3’; Uty-R: 5’-TGGTGCATCCA ACCTAACTGTT-3’. Unmanipulated male and female samples were used separately or in different ratios (1:1, 1:3, 1:9, 1:27) to generate a standard curve. The levels of transcripts in chimera mice were calculated as % of total.
Detection of sex hormones
Estradiol and testosterone were measured by ELISA using the manufacturer’s protocols (US Biological, MA). Testosterone levels were measured on 10 times diluted serum. Estradiol levels were measured after extraction. Briefly, 50 µl of serum was vortexed with 500 µl ethyl ether for 30 seconds. Phases were allowed to separate and the organic phase was transferred to a fresh glass tube. Solvent was allowed to evaporate before the residue was dissolved in 250 µl of extraction buffer.
Antibody ELISA
Serum was obtained from chimera mice every 4 weeks starting 8 weeks post irradiation and reconstitution. For detection of total IgG and IgM, serum was diluted 1:50.000–1:200.000 in serum diluent (sterile filtered 0.5% bovine g-globulin, 5% gelatin, 0.05mM Tween in 1× PBS). For detection of ANAs, serum was diluted 1:300. Levels of anti-chromatin, anti-histone and anti-dsDNA IgG autoantibodies were measured as previously described62. All reactions were developed using 10mg/ml 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)(ABTS) in McllWain’s buffer (0.09 M Na2HPO4,0.06 M citric acid, pH 4.6). Anti-dsDNA IgG levels were determined using the manufacturer’s protocol (Alpha Diagnostic International Inc., TX).
Cytokine ELISA
IFNα and BAFF were measured in 1:4 diluted serum obtained 12 and 16 weeks post irradiation and reconstitution, respectively, using the manufacturer’s protocols (IFNα ELISA: PBL Interferon Source, NJ; BAFF ELISA: R & D Systems, MN).
Flow cytometry
Flow cytometry was performed using a Cyan Flow cytometer ADP (Beckman Coulter) and all analyses were done using FloJo version 9.5.2. Antibodies with the following specificities were used for all analyses: CD11b, CD11c, CD19, CD21, CD23, CD38, CD40, B220 (CD45R), CD138, F4/80, Gr1 (Ly6C/6G), IgM, IgD, (all from eBiosciences). Peanut agglutinin (PNA) was obtained from Vector inc.
Immunofluorescence staining
IgG deposition and complement factor 3 (C3) fixation was measured by immunofluorescence staining. Briefly, half kidneys were quick-frozen in OCT™ and 5µm sections were prepared. Sections were stained using TexasRed-conjugated anti-mouse IgG (Invitrogen) and FITC-conjugated anti-mouse C3 specific antibodies (ICL, inc.). Images were collected using an HC Plan Apo 20×/0.7NA objective lens on a Leica DMR upright microscope (Leica Microsytems) equipped with a Retiga EXi Cooled CCD Camera (QImaging).
Statistical analyses
All statistical analyses were done using GraphPad Prism v. 5.04. Analyses of cumulative incidence were done using a Log-rank test (Mantel Cox test). Comparisons of average time of onset, cellular proportions, and serum cytokine levels between two groups were done using a two-tailed non-parametric Mann Whitney test. P values < 0.05 were considered statistical.
ACKNOWLEDGEMENTS
The authors would like to thank Shirley Sobus and Joshua Loomis for their help with flow cytometric experiments. This study was supported in part by the Denver Autoimmune Center of Excellence and USPHS grants AI18785 and AI22295 (P.M.) and R21AI083804 (T.N.J.).
Footnotes
CONFLICT OF INTEREST
The authors declare no financial conflicts of interest.
References
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Mellors RC. Autoimmune and immunoproliferative diseases of NZB/Bl mice and hybrids. Int Rev Exp Pathol. 1966;5:217–252. [PubMed] [Google Scholar]
- 3.Gubbels Bupp MR, Jorgensen TN, Kotzin BL. Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus. Genes Immun. 2008;9:47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- 4.Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- 5.Morton JI, Siegel BV, Moore RD. Transplantation of autoimmune potential. II. Glomerulonephritis in lethally irradiated DBA/2 recipients of NZB bone marrow cells. Transplantation. 1975;19:464–469. [PubMed] [Google Scholar]
- 6.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti- nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roubinian J, Talal N, Siiteri PK, Sadakian JA. Sex hormone modulation of autoimmunity in NZB/NZW mice. Arthritis Rheum. 1979;22:1162–1169. doi: 10.1002/art.1780221102. [DOI] [PubMed] [Google Scholar]
- 8.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59:1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52:393–400. doi: 10.1046/j.1365-3083.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 10.Talal N, Ahmed SA, Dauphinee M. Hormonal approaches to immunotherapy of autoimmune disease. Ann N Y Acad Sci. 1986;475:320–328. doi: 10.1111/j.1749-6632.1986.tb20880.x. [DOI] [PubMed] [Google Scholar]
- 11.Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 14.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol Res. 2013;55:210–216. doi: 10.1007/s12026-012-8365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago-Raber ML, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 19.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 20.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 21.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 22.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 24.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen TN, Alfaro J, Enriquez HL, Jiang C, Loo WM, Atencio S, et al. Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;184:775–786. doi: 10.4049/jimmunol.0901322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, et al. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9- deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Kincade PW, Igarashi H, Medina KL, Kouro T, Yokota T, Rossi MI, et al. Lymphoid lineage cells in adult murine bone marrow diverge from those of other blood cells at an early, hormone-sensitive stage. Semin Immunol. 2002;14:385–394. doi: 10.1016/s1044532302000738. [DOI] [PubMed] [Google Scholar]
- 29.Peeva E, Venkatesh J, Diamond B. Tamoxifen Blocks Estrogen-Induced B Cell Maturation but Not Survival. J Immunol. 2005;175:1415–1423. doi: 10.4049/jimmunol.175.3.1415. [DOI] [PubMed] [Google Scholar]
- 30.Gordon RD, Simpson E, Samelson LE. In vitro cell-mediated immune responses to the male specific(H–Y) antigen in mice. J Exp Med. 1975;142:1108–1120. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen PL, Ziff M. Abnormal polyclonal B cell activation in NZB/NZW F1 mice. J Immunol. 1977;119:1534–1537. [PubMed] [Google Scholar]
- 32.Klinman DM. Polyclonal B cell activation in lupus-prone mice precedes and predicts the development of autoimmune disease. J Clin Invest. 1990;86:1249–1254. doi: 10.1172/JCI114831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stohl W, Xu D, Kim KS, Koss MN, Jorgensen T, Deocharan B, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 35.Giltiay NV, Lu Y, Allman D, Jorgensen TN, Li X. The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation. J Immunol. 2010;185:99–109. doi: 10.4049/jimmunol.0903312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang NH, Cheung YH, Loh C, Pau E, Roy V, Cai YC, et al. B cell activating factor (BAFF) and T cells cooperate to breach B cell tolerance in lupus-prone New Zealand Black (NZB) mice. PLoS One. 2010;5:e11691. doi: 10.1371/journal.pone.0011691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62:1457–1468. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;208:1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, et al. Type-I Interferon Receptor Deficiency Reduces Lupus-like Disease in NZB Mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 × NZW)F(1) mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 41.Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183:6021–6029. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reininger L, Radaszkiewicz T, Kosco M, Melchers F, Rolink AG. Development of autoimmune disease in SCID mice populated with long-term "in vitro" proliferating (NZB × NZW)F1 pre-B cells. J Exp Med. 1992;176:1343–1353. doi: 10.1084/jem.176.5.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 44.Mina-Osorio P, Lastant J, Keirstead N, Whittard T, Ayala J, Stefanova S, et al. Suppression of Glomerulonephritis in Lupus-Prone NZB × NZW Mice by RN486, a Selective Inhibitor of Bruton's Tyrosine Kinase. Arthritis Rheum. 2013;65:2380–2391. doi: 10.1002/art.38047. [DOI] [PubMed] [Google Scholar]
- 45.Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibody treatment prevents the development of lupus- like nephritis in a subset of New Zealand black × New Zealand white mice. Response correlates with the absence of an anti-antibody response. J Immunol. 1996;157:3159–3164. [PubMed] [Google Scholar]
- 46.Wang X, Huang W, Schiffer LE, Mihara M, Akkerman A, Hiromatsu K, et al. Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. Arthritis Rheum. 2003;48:495–506. doi: 10.1002/art.10929. [DOI] [PubMed] [Google Scholar]
- 47.Ronnblom L, Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001;22:427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- 48.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronson PG, Chaivorapol C, Ortmann W, Behrens TW, Graham RR. The genetics of type I interferon in systemic lupus erythematosus. Curr Opin Immunol. 2012;24:530–537. doi: 10.1016/j.coi.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Chiang EY, Yu X, Grogan JL. Immune complex-mediated cell activation from systemic lupus erythematosus and rheumatoid arthritis patients elaborate different requirements for IRAK1/4 kinase activity across human cell types. J Immunol. 2011;186:1279–1288. doi: 10.4049/jimmunol.1002821. [DOI] [PubMed] [Google Scholar]
- 51.Chauhan SK, Singh VV, Rai R, Rai M, Rai G. Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J Clin Immunol. 2013;33:954–964. doi: 10.1007/s10875-013-9887-0. [DOI] [PubMed] [Google Scholar]
- 52.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 53.Clingan JM, Matloubian M. B Cell-Intrinsic TLR7 Signaling Is Required for Optimal B Cell Responses during Chronic Viral Infection. J Immunol. 2013;191:810–818. doi: 10.4049/jimmunol.1300244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 55.Wang JP, Zhang L, Madera RF, Woda M, Libraty DH. Plasmacytoid dendritic cell interferon-alpha production to R-848 stimulation is decreased in male infants. BMC Immunol. 2012;13:35-. doi: 10.1186/1471-2172-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PLoS One. 2010;5:e10868-. doi: 10.1371/journal.pone.0010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bynote KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor-alpha deficiency attenuates autoimmune disease in (NZB × NZW)F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 58.Seillet C, Rouquie N, Foulon E, Douin-Echinard V, Krust A, Chambon P, et al. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol. 2013;190:5459–5470. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Xu Y, Ma L, Sun L, Fu G, Hou Y. 17beta-estradiol enhances the response of plasmacytoid dendritic cell to CpG. PLoS One. 2009;4:e8412-. doi: 10.1371/journal.pone.0008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, et al. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38:501–508. doi: 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aronica SM, Dozier A, Fanti P, Nazareth M. Altered bone marrow cell sensitivity in the lupus-prone NZB/W mouse: regulation of CFU-GM colony formation by estrogen, tamoxifen and thrombopoietin. Lupus. 2000;9:271–277. doi: 10.1191/096120300680198962. [DOI] [PubMed] [Google Scholar]
- 62.Trigunaite A, Khan A, Der E, Song A, Varikuti S, Jorgensen TN. Gr1 CD11b cells suppress B cell differentiation and lupus-like disease in lupus-prone male mice. Arthritis Rheum. 2013;65:2392–2402. doi: 10.1002/art.38048. [DOI] [PubMed] [Google Scholar]