Abstract
Transport proteins have sometimes gained secondary regulatory functions that influence gene expression and metabolism. These functions allow communication with the external world via mechanistically distinctive signal transduction pathways. In this brief review we focus on three transport systems in Escherichia coli that control and coordinate carbon, exogenous hexose-phosphate and phosphorous metabolism. The transport proteins that play central roles in these processes are (1) the phosphoenolpyruvate (PEP)-dependent phosphotransferase system, PTS, (2) the glucose-6-phosphate receptor, UhpC, and (3) the phosphate-specific transporter, PstSABC, respectively. While the PTS participates in multiple complex regulatory processes, three of which are discussed here, UhpC and the Pst transporters exemplify differing strategies.
Keywords: Transport, metabolism, gene expression, signal transduction, phosphotransferase system, PTS, UhpC, PstSABC, Escherichia coli
“No man ever steps in the same river twice”–Heraclitus
Introduction
Transport proteins evolved early as their functions are essential to all life, but regulation was late-evolving and consequently often phylum-specific. Therefore, although transport mechanisms are universal, regulation is not. For example, while the constituents of the prokaryotic sugar-transporting phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS) are essentially the same in all bacterial and archaeal phyla that possess the system, its involvement in regulation differs drastically depending on the organismal type, often even within a single phylum. Similarly, glucose is taken up by the same transport mechanism in most eukaryotes, from yeast to humans, but its regulation is completely different depending on the organismal type. In this brief review, we examine the roles played by transport systems in the regulation of carbon, glucose-6-phosphate (G6P) and phosphorous (Pho) metabolism in a single organism, Escherichia coli. We anticipate that, while the principles revealed by the studies described here will prove to be universal, the specific mechanisms will be organism-specific.
The E. coli phosphotransferase system (PTS)
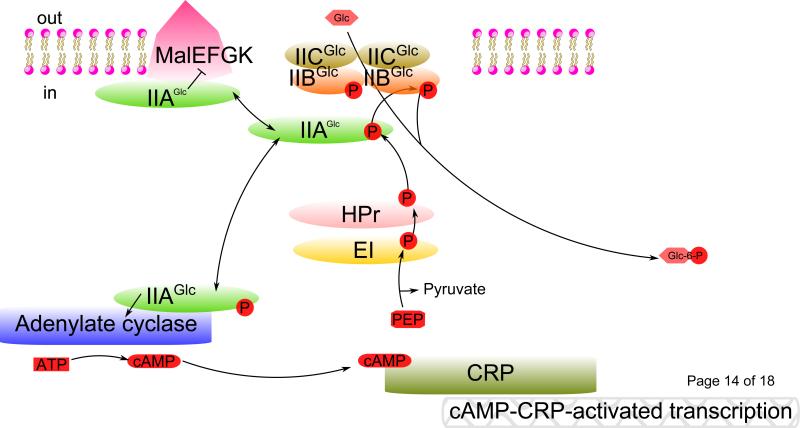
The PTS, discovered just 50 years ago [1,2], consists of several proteins and/or protein domains: (1) a PEP-dependent protein phosphorylating kinase, termed Enzyme I, (2) a heat-stable phosphoryl carrier protein, HPr and (3) sugar-specific Enzyme II complexes which consist of IIA, IIB, IIC and sometimes IID proteins and/or protein domains [3-7]. Enzyme I and HPr are the general energy-coupling proteins, common to all Enzyme II complexes of the PTS, catalyzing phosphoryl transfer from PEP to HPr. Phosphorylated HPr then transfers the phosphoryl group to IIA, which phosphorylates IIB. The IIC permease then transfers the phosphoryl group from IIB~P to a sugar in a process that couples sugar phosphorylation to the concomitant IIC-mediated transport of the extracellular sugar into the cytoplasm. The five step phosphoryl transfer process can be schematically described as: PEP → EI~P → HPr~P → IIA~P → IIB~P —(IIC)→ Sugar-P. In all PTS phosphoryl transfer proteins, the phosphoryl moiety is linked to a histidyl residue, except for some of the IIB proteins in which a cysteyl residue is phosphorylated. In each case, a high energy phosphoryl protein forms reversibly with energies of hydrolysis close to that of PEP [8], and hence, only the last step, sugar phosphorylation, is physiologically irreversible. If the relevant sugar of a PTS transporter is present in the extracellular medium, the sugar drains the phosphoryl groups off of all of the PTS phosphoryl carrier proteins and induces synthesis of the EII complex, a prerequisite for rapid sugar uptake (Figure 1).
Fig. 1. PTS-mediated catabolite repression and inducer exclusion.
Principal signal transduction pathways for carbon regulation in E. coli. A sugar such as glucose (Glc) is transported into the cell cytoplasm and concomitantly phosphorylated by the PTS, resulting in dephosphorylation of IIAGlc. Non-phosphorylated IIAGlc inhibits non-PTS sugar catabolic enzymes and transporters causing inducer exclusion. Phosphorylated IIAGlc, on the other hand, promotes cAMP formation by binding to adenylate cyclase and allosterically activating it. cAMP-driven transcription results when cAMP binds to CRP, a transcriptional activator. The rate-limiting step in the PTS phosphoryl transfer chain appears to be Enzyme I-catalyzed phosphorylation of HPr. ⊥, inhibition; →, activation.
Normally, a distinction is made between so called PTS-sugars (mostly hexoses and hexitols, e.g., glucose, mannose, fructose and mannitol) and non-PTS sugars (e.g., maltose, lactose, glycerol and melibiose) [8]. For the hexoses, the main enzymes II are: IIGlc, IIMan and IIFru. The phosphorylated forms of these substrates are converted to fructose-1,6-bisphosphate in preparation for glycolytic metabolism. There is considerable organismal variation concerning the sugars transported via the PTS. For example, in Rhodospirillum rubrum, only fructose is utilized by the PTS while in Spirochaeta aurantia, only mannitol is utilized via this system [8,9].
Phosphorylated sugars, released from their Enzyme II complexes into the cytoplasm and metabolized via glycolysis, generate both PEP and ATP. Hence, the glycolytic pathway, including the PTS, constitutes a cycle starting with PEP and generating PEP. However, in some bacteria, PTS EII-mediated transport may not always be coupled to sugar phosphorylation. Exceptions to the usual mechanism of group translocation in which transport is tightly coupled to substrate modification can be found in references [10-13].
There can be many different Enzyme II complexes in any given bacterial cell, and each one exhibits specificity for just one or a few closely related sugars. In fact, the IIC constituents have evolved independently at least four different times, giving rise to proteins of differing topologies (0, 6, 8 and 11 α-helical transmembrane segments (TMSs), respectively) [12]. The Enzyme II transporter genes are in general localized to their respective operons which also contain the genes required for conversion of the sugar substrate into a common glycolytic intermediate. Two exceptions to this generalization are the operons encoding the glucose and mannose Enzyme II complexes. These operons encode only the PTS enzyme II constituents.
PTS-mediated catabolite repression and inducer exclusion
The name carbon catabolite repression derives from the notion that the catabolites of sugar metabolism are responsible for repression. In fact, the metabolism of any sugar can cause repression, and in general, the more rapidly an exogenous sugar is transported, the stronger the catabolite repression. However, in E. coli, the phosphorylation state of IIAGlc provides the dominant mechanism. This mechanism is operative in enteric Gram-negative bacteria closely related to E. coli, but different versions are present in Gram-positive firmicutes and actinobacteria, in spirochaetes and even in other proteobacteria. For example, in the firmicute, Bacillus subtilis [14], the PTS plays a role in catabolite repression, but the mechanism involves an ATP-dependent HPr kinase, not the phosphorylation state of IIAGlc. In all bacteria, including E. coli, there are probably multiple mechanisms of catabolite repression [15-17].
In its phosphorylated forms, IIAGlc binds to and activates adenylate cyclase, which converts ATP to cyclic adenosine 3’,5’-monophosphate (cAMP), a cytoplasmic second messenger indicating the extracellular availability of a PTS carbon and energy source [18]. It is noteworthy that although most Enzyme II complexes contain the full complement of IIA, IIB, IIC and sometimes IID proteins or protein domains, IIAGlc can be promiscuous within the glucose family, replacing the sugar-specific IIA for some systems. Thus, for example, the E. coli sucrose Enzyme II does not have its own IIA and uses IIAGlc. This facilitates the direct regulation of the IIAGlc phosphorylation state by several related sugars. Furthermore, if phosphoryl groups are drained off of HPr, all of the systems that use HPr are affected. To the extent that signals are conveyed through the same second messenger (cAMP), signals from different complexes cannot be distinguished [19,20].
A decrease of intracellular cAMP leads to the dissociation of the cAMP-cAMP receptor protein (CRP) complex from its operator sites in the control regions of operons subject to CRP control. This thereby deactivates cAMP-CRP-directed transcription of catabolite repressible genes responsible for the catabolism of exogenous carbon sources [21,22]. Non-PTS carbohydrate enzymes and transporters, including glycerol kinase (GlpK), the maltose permease (MalEFGK) and the lactose permease (LacY) of E. coli are regulated by direct binding of IIAGlc [23], inhibiting their activities if a PTS sugar is abundant, a phenomenon called “inducer exclusion” (Fig. 1).
Regulation of glycogen breakdown by HPr of the PTS
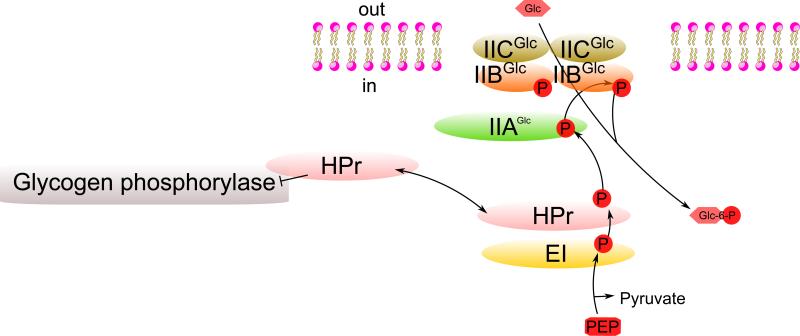
In addition to its role in the PTS phosphorylation cycle, HPr allosterically regulates the enzyme, glycogen phosphorylase, which catalyzes the rate-limiting step of glycogenolysis [24], the release of glucose 1-phosphate from cellular glycogen, a storage form of energy consisting of glucose polymers linked α-1,4. When phosphate is drained off of HPr because a PTS sugar is available in the medium, HPr binds to glycogen phosphorylase, inhibiting its activity so that utilization of glycogen is blocked [24] (Fig. 2). Thus, the PTS proteins, HPr, IIAGlc, and IIBCGlc, all have direct but distinct signaling activities, secondary functions that undoubtedly evolved late, after the evolution of the PTS as a concerted sugar transport/kinase system.
Fig. 2. Regulation of glycogen breakdown by HPr of the PTS.
HPr, the phosphate donor to PTS IIA proteins, becomes dephosphorylated when exogenous PTS sugars such as glucose are available. Dephospho-HPr then binds to glycogen phosphorylase, preventing glucose-1-phosphate release from glycogen. ⊥, inhibition.
Direct binding of a transcriptional repressor, Mlc, to the PTS Glucose Enzyme II
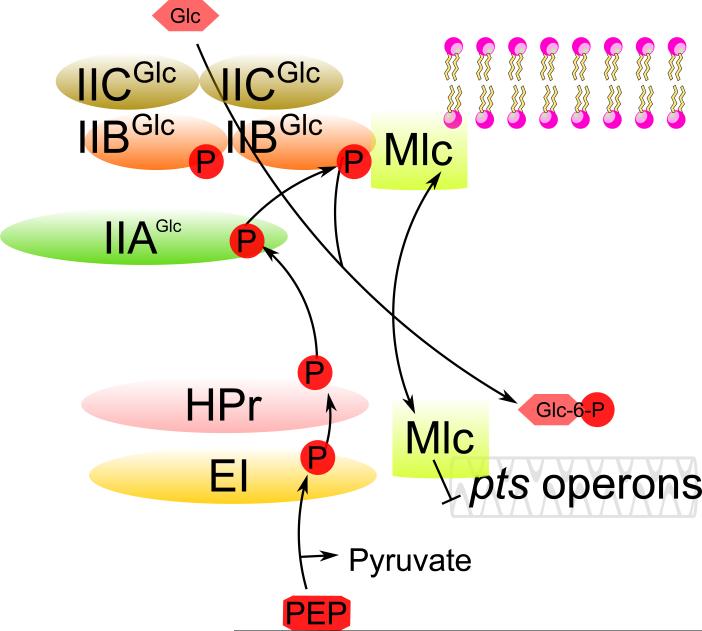
In addition to the signal transduction pathways described above for the regulation of non-PTS sugar metabolism, another mechanism, influencing primarily the expression of pts genes, involves the transcription factor, Mlc, which binds to and is regulated by IIBCGlc. Mlc primarily regulates the pts operon, encoding Enzyme I and HPr, as well as genes encoding the glucose (PtsG) and mannose (Man) EII complexes [25]. If glucose is transported by IIBCGlc, this transporter sequesters Mlc. Then, Mlc cannot diffuse to the DNA to repress transcription. This results in glucose activation (or more accurately, derepression) of the ptsG, man and pts operons, all of which are concerned with glucose metabolism. The PTS thus exhibits autogenous induction [26,27], where synthesis of Enzyme I, HPr, IIGlc and IIMan is enhanced by transport of glucose through IICGlc. The sequestration of Mlc by IIBCGlc when exogenous glucose is available thus mediates the autogenous induction of the entire glucose PTS [28] (Fig. 3).
Fig. 3. Direct binding of a transcriptional repressor, Mlc, to the PTS Glucose Enzyme II.
IIBCGlc in the unphosphorylated state (ie., when glucose is present) sequesters Mlc, preventing repression of pts genes. However, when glucose is absent, IIBCGlc is phosphorylated, causing release of Mlc so it represses expression of these genes. ⊥, repression.
Glucose-6-phosphate regulation
The Uptake hexose phosphate Transport (UhpT) system of E. coli is specifically induced by glucose-6-phosphate (G6P) although the transporter itself has broad specificity for a wide range of sugar phosphates [29-31] (Fig. 4). Although the transporter consists of a single protein, UhpT, the regulatory system controlling its expression is composed of three proteins, UhpA, B and C [32]. UhpT (TC# 2.A.1.4.1) and UhpC (TC# 2.A.1.4.4) are homologous sugar phosphate:inorganic phosphate (Pi) exchangers functioning as transporter and receptor/transporter, respectively. They are close homologues within the Organophosphate:Pi Antiporter (OPA) family of the Major Facilitator Superfamily of secondary carriers (TC# 2.A.1.4). The differences between UhpT and UhpC are that while the low specificity UhpT is an efficient transporter expressed at high levels following induction by exogenous G6P, the G6P-specific UhpC works as a receptor expressed at extremely low levels [33]. UhpC has high affinity for G6P despite its low rate of transport relative to UhpT, conveying a signal to UhpB and UhpA that upregulates the expression of the uhpT gene when external G6P is available. UhpC was first characterized in 1980 as a receptor (initially called UhpR) that could induce UhpT expression [34], and high degree of sequence similarity between UhpT and UhpC was demonstrated in 1992 [35].
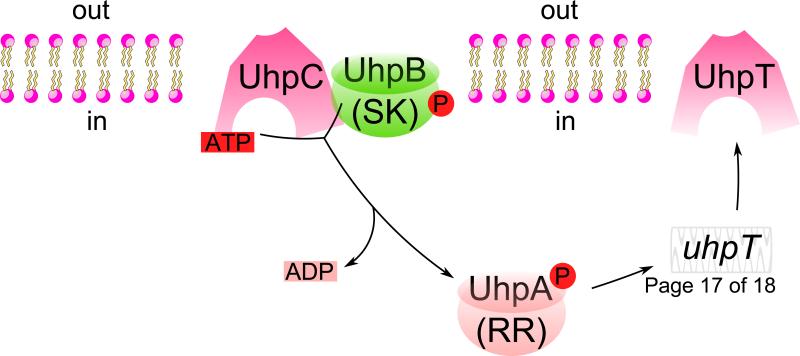
Fig. 4. The uptake hexose phosphate (UHP) transport system.
The Uhp system consists of four proteins: UhpA, B, C, and T. UhpC and UhpT are homologous members of the major facilitator superfamily, serving the function of receptor/transporter and transporter, respectively. UhpB and A are the sensor kinase and response regulator of the regulatory two component system, serving to convey the signal of glucose-6-phosphate (G6P) availability to upregulate the expression of the uhpT gene. The 2 TMS membrane domain of the UhpB protein senses when the substrate, G6P, for which UhpC is highly specific, is bound to its external surface. Then with G6P bound to UhpC, and with UhpC in direct contact with UhpB, UhpB utilizes ATP to autophosphorylate its central domain. Once UhpB is phosphorylated, UhpB can donate its phosphoryl group to UhpA, which binds to multiple sites between -80 and -32 upstream of the start site of the uhpT gene, functioning as an enhancer in the promoter region. →, transcriptional activation.
UhpB is a sensor kinase (SK) while UhpA is a DNA-binding response regulator (RR) of a two-component (SK/RR) regulatory system [36]. UhpB, which interacts directly with UhpC in its N-terminal 2 TMS membrane domain has a kinase activity in its C-terminal domain [37], that phosphorylates a histidyl residue in its central domain, H313, using ATP as the phosphoryl donor. UhpB is activated only when G6P is bound to the receptor. Phosphorylated UhpB then transfers the phosphoryl group to an aspartyl residue in UhpA, which promotes gene expression of uhpT using σ70 RNA polymerase [38], by binding to multiple sites in positions between -80 and -32 upstream of the uhpT gene in the transcriptional regulatory promoter region. UhpA~P functions as an enhancer, binding upstream of the promoter. The mechanism of genetic regulation of the synthesis of UhpT [39] is analogous to the mechanism by which hormones, such as insulin, regulates gene expression in mammals, and is an example of a transporter that directly controls gene expression. In humans, deficiencies in a close homologue, TC# 2.A.1.4.5, is responsible for glycogen storage disease (Gierke's disease) [40].
It has been shown that higher levels of G6P are needed to induce UhpT transcription under phosphate limitation. Products of the pho regulon can hydrolyze G6P in the periplasm (inducer degradation), to glucose and free phosphate [41]. In an example of Uhp cross talk, adenylate cyclase activity is inhibited by hexose phosphate transport although the mechanism has not been defined [42]. The more UhpT that is expressed in the presence of exogenous G6P, the lower the cytoplasmic cAMP concentration. If glucose is transported, IIAGlc in the free form is generated, and hence less cAMP is produced. Since the uhpT, ptsHI, ptsG and man genes are all under cAMP-CRP control, this mechanism allows glucose to inhibit sugar phosphate uptake and sugar phosphate to inhibit sugar uptake [43,44]. Thus, rates of cAMP-CRP-activated transcription are reduced by transport of either glucose or G6P.
Phosphorous (pho) regulation
In the presence of excess external inorganic phosphate (Pi), this anion can pass the outer membrane of E. coli through the PhoE porin [45] and then enter the cytoplasm via the low affinity PiT transporter. Under these conditions, the high affinity phosphate-specific transporter, Pst is repressed. In the case of phosphate limitation (sub-μM external levels), a periplasmic phosphate sensory binding protein, PstS, a constituent of the high affinity ABC phosphate transporter, PstSABC, binds inorganic phosphate and facilitates transport across the cytoplasmic membrane. PstA and PstC are the integral membrane proteins of the system while PstB hydrolyzes ATP to energize uptake. This transport system transmits a signal, probably via the accessory protein, PhoU, to the sensor histidine kinase, PhoR, which positively controls transcription of the many phosphate-yielding (pho) operons via the phosphorylated response regulator, PhoB (Fig. 5). PhoU may also regulate both Pi uptake via the Pst system and Pst-mediated signal transduction to PhoR [46]. Interestingly, full activation of the pho regulon requires the non-phosphorylated form of a cytoplasmic PTS regulatory protein, EIIANtr [47], suggesting that the transcriptional responses to phosphate limitation are dependent on nitrogen and carbon sufficiency, mediated by PTSNtr [48].
Fig 5. The phosphate-specific transporter, PstSABC, a transporter functioning in signal transduction.
The phosphate (pho) regulon uses PstS, a periplasmic inorganic phosphate (Pi) binding receptor that serves as a constituent of the ABC transport complex, PstSABC. It also serves as a sensor of external Pi concentration. PstS senses the phosphate concentration in the periplasmic space, transmits a signal via PstABC and PhoU to the sensor kinase, PhoR, independently of the transport activity of PstSABC, thereby influencing expression of pho regulon genes. Therefore, the transporter is a sensor that senses extracellular phosphate to control gene expression. ⊥, inhibition; →, activation.
PhoR self-phosphorylates on a histidyl residue before transferring the phosphoryl group to an aspartyl residue in PhoB [49]. PhoB~P then binds to so-called PHO boxes upstream of pho operons to activate transcription of the many pho genes, including those in the pst operon [50]. Most of the enzymes encoded by pho genes degrade organic phosphates, converting them to Pi, which is the preferred source of phosphorous.
Under conditions of excess exogenous phosphate, with Pi bound to PstS, the products of the pst operon, together with PhoU, transmit an inhibitory signal to the PhoR kinase and thereby inhibits PhoB phosphorylation and hence pho regulon expression. Deleting any one gene in the pst operon, including phoU, results in constitutive expression of pho operons [51]. This shows that the signal transmitted by the Pst-PhoU system to PhoR is a negative one, dependent on all constituents of the Pst transporter, causing inhibition of the otherwise constitutively high level of PhoR activity [46]. Surprisingly, the effect of signal transduction via the Pst transporter on gene regulation is not a consequence of Pi transport. Although the same proteins are involved in both transport and signal transduction, these two functions are independent of each other as shown by experiments demonstrating that specific point mutations in the pst genes can eliminate either one or the other of these functions without affecting the other [46]. Thus, while the two functions use the same proteins, they do not use the same mechanism [52].
Conclusions and perspectives: Integration of, and parallels between carbon, Glc6P, and phosphorous regulation in E. coli
In the sugar-transporting PTS, effects on transcription are carried out (1), by the binding of Mlc to IIBCGlc, (2) by controlling cytoplasmic inducer levels by direct interaction of IIAGlc with target non-PTS catabolic enzymes and transporters, and (3) by regulating cAMP levels by direct interaction of IIAGlc~P with adenylate cyclase. Additionally, (4) the PTS limits the utilization of other carbon sources such as exogenous non-PTS sugars and endogenous glycogen when glucose or another PTS sugar is available and transported. These mechanisms allow E. coli to create a hierarchy of preferred carbon sources, enabling rapid growth while preventing waste.
Regulation of G6P utilization is controlled by the Uhp signal transduction system. UhpC, which is a major facilitator superfamily transporter, highly specific for G6P, works as a receptor, conveying a signal through a two-component system, the UhpB sensor kinase and the UhpA response regulator, to increase rates of synthesis of UhpT, the low specificity sugar-P transporter. This mechanism allows the bacteria to respond to exogenous G6P, preventing induction by intracellular G6P and leakage of sugar phosphates from the cell.
Fewer details are established for phosphate (pho) gene regulation via the ABC transporter complex, PstSABC. In the presence of sufficient external phosphate. The PstS receptor binds periplasmic inorganic phosphate, which binds to the PstABC complex, transmitting via PhoU an inhibitory signal to PhoR, the sensor kinase. Inhibition of the phosphorylation state of PhoR causes PhoB to be dephosphorylated, preventing expression of the entire pho regulon by a negative feedback mechanism that once again prevents energy wastage.
There are reciprocal effects of PTS-mediated transport on uhp transcription, and vice versa. UhpT is under catabolite repression [53], as are all PTS enzymes. When UhpT is transporting sugar phosphates, catabolite repression results from inhibition of adenylate cyclase activity [42], just as glucose transport by the PTS induces catabolite repression. Thus, the systems repress themselves and each other, maintaining a controlled rate of carbon uptake that allows optimal growth with minimal wastage.
The transcriptional responses to phosphate limitation are dependent on carbon and nitrogen sufficiency, mediated by PTS proteins [48], again revealing interconnectivity of the regulatory systems. These interactions prevent wasteful metabolism of nutrients under conditions that are not beneficial to the cell. Moreover, pho regulon periplasmic phosphatases break down G6P, inhibiting uhpT induction when inorganic phosphates is present in excess [41].
These examples emphasize the interconnectivity of the systems considered in this review article. Future work is likely to reveal additional aspects of the integrative control systems that allow coordination of these different metabolic processes. Additional details concerning the involvement of key transporters in metabolic and transcriptional regulation are also likely to come to light.
Highlights.
Transporters can transduce signals, regulating metabolism and gene expression.
In E. coli, Different PTS transport proteins, HPr, IIAGlc, and IIBCGlc, have distinct signaling activities.
The UhpC transporter serves as a receptor, promoting expression of the sugar-phosphate uptake system, UhpT.
The PstSABC phosphate transporter transduces signals independently of transport to regulate the phosphorous (pho) regulon.
These E. coli systems illustrate mechanistic principles involved in the use of transport proteins to coordinate cellular activities.
Acknowledgements
The work reported was supported by NIH Grants GM077402-05A1 and GM 094610-01. We thank Professors Alex Ninfa and Mike Merrick for critically reading the manuscript prior to submission.
Abbreviations
- PTS
Phosphotransferase system
- EI
Enzyme I of the PTS
- HPr
heat-stable phosphoryl carrier protein
- IIA
Enzyme IIA phosphocarrier protein of a PTS EII complex
- IIB
Enzyme IIB phosphocarrier protein of a PTS EII complex
- IIC
Enzyme IIC permease of a PTS EII complex
- cAMP
Cyclic adenosine 3’,5’-monophosphate
- CRP
cAMP receptor protein
- G6P
Glucose-6-phosphate
- Uhp
Uptake hexose phosphates
- PstSABC
ABC-type high affinity inorganic phosphate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, are highlighted as:
• of special interest
•• of outstanding interest
- 1••.Kundig W, Ghosh S, Roseman S. Phosphate Bound to Histidine in a Protein as an Intermediate in a Novel Phospho-Transferase System. Proceedings of the National Academy of Sciences of the United States of America. 1964;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [Represents the discovery of the PTS in 1964.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Saier MH, Jr., Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Molecular microbiology. 1994;13:755–764. doi: 10.1111/j.1365-2958.1994.tb00468.x. [Summarizes the developments until 1994.] [DOI] [PubMed] [Google Scholar]
- 3.Herzberg O, Reddy P, Sutrina S, Saier MH, Jr., Reizer J, Kapadia G. Structure of the histidine-containing phosphocarrier protein HPr from Bacillus subtilis at 2.0-A resolution. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2499–2503. doi: 10.1073/pnas.89.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CA, Saier MH., Jr. Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. The Journal of biological chemistry. 1983;258:10761–10767. [PubMed] [Google Scholar]
- 5.Fairbrother WJ, Gippert GP, Reizer J, Saier MH, Jr., Wright PE. Low resolution solution structure of the Bacillus subtilis glucose permease IIA domain derived from heteronuclear three-dimensional NMR spectroscopy. FEBS letters. 1992;296:148–152. doi: 10.1016/0014-5793(92)80367-p. [DOI] [PubMed] [Google Scholar]
- 6.Ab E, Schuurman-Wolters GK, Nijlant D, Dijkstra K, Saier MH, Robillard GT, Scheek RM. NMR structure of cysteinyl-phosphorylated enzyme IIB of the N,N'-diacetylchitobiose-specific phosphoenolpyruvate-dependent phosphotransferase system of Escherichia coli. Journal of molecular biology. 2001;308:993–1009. doi: 10.1006/jmbi.2001.4623. [DOI] [PubMed] [Google Scholar]
- 7•.Barabote RD, Saier MH., Jr. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiology and molecular biology reviews : MMBR. 2005;69:608–634. doi: 10.1128/MMBR.69.4.608-634.2005. [Provides a comprehensive survey of PTS proteins encoded within dozens of bacterial genomes, revealing the diversity of such proteins and impying the existence of many yet-to-be discovered regulatory mechanisms.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saier MH., Jr. Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977;41:856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saier MH, Jr., Newman MJ, Rephaeli AW. Properties of a phosphoenolpyruvate: mannitol phosphotransferase system in Spirochaeta aurantia. J Biol Chem. 1977;252:8890–8898. [PubMed] [Google Scholar]
- 10.Rodriguez-Diaz J, Rubio-del-Campo A, Yebra MJ. Lactobacillus casei ferments the N-Acetylglucosamine moiety of fucosyl-alpha-1,3-N-acetylglucosamine and excretes L-fucose. Appl Environ Microbiol. 2012;78:4613–4619. doi: 10.1128/AEM.00474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hvorup R, Chang AB, Saier MH., Jr. Bioinformatic analyses of the bacterial L-ascorbate phosphotransferase system permease family. Journal of molecular microbiology and biotechnology. 2003;6:191–205. doi: 10.1159/000077250. [DOI] [PubMed] [Google Scholar]
- 12•.Saier MH, Hvorup RN, Barabote RD. Evolution of the bacterial phosphotransferase system: from carriers and enzymes to group translocators. Biochem Soc Trans. 2005;33:220–224. doi: 10.1042/BST0330220. [Provides evidence that PTS Enzyme II complexes evolved independently several times.] [DOI] [PubMed] [Google Scholar]
- 13.Saier MH, Jr., Simoni RD, Roseman S. The physiological behavior of enzyme I and heat-stable protein mutants of a bacterial phosphotransferase system. J Biol Chem. 1970;245:5870–5873. [PubMed] [Google Scholar]
- 14•.Saier MH, Jr., Chauvaux S, Deutscher J, Reizer J, Ye JJ. Protein phosphorylation and regulation of carbon metabolism in gram-negative versus gram-positive bacteria. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [Compares two PTS-mediated carbon regulatory mechanisms in two phylogenetically divergent bacteria.] [DOI] [PubMed] [Google Scholar]
- 15•.Lengeler JW, Jahreis K. Bacterial PEP-dependent carbohydrate: phosphotransferase systems couple sensing and global control mechanisms. Contributions to microbiology. 2009;16:65–87. doi: 10.1159/000219373. [Summarizes PTS signal transduction in E. coli.] [DOI] [PubMed] [Google Scholar]
- 16.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Current opinion in microbiology. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 17•.Saier MH., Jr. Cyclic AMP-independent catabolite repression in bacteria. FEMS microbiology letters. 1996;138:97–103. doi: 10.1111/j.1574-6968.1996.tb08141.x. [Discusses catabolite repression other than those dependent on cAMP.] [DOI] [PubMed] [Google Scholar]
- 18•.Aboulwafa M, Saier MH., Jr. Biophysical studies of the membrane-embedded and cytoplasmic forms of the glucose-specific Enzyme II of the E. coli phosphotransferase system (PTS). PLoS One. 2011;6:e24088. doi: 10.1371/journal.pone.0024088. [Reveals that PTS Enzyme II complexes can exist in multiple principal states inside the E. coli cell.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bywater RP. Membrane-spanning peptides and the origin of life. J Theor Biol. 2009;261:407–413. doi: 10.1016/j.jtbi.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Bywater RP, Sorensen A, Rogen P, Hjorth PG. Construction of the simplest model to explain complex receptor activation kinetics. J Theor Biol. 2002;218:139–147. doi: 10.1006/jtbi.2002.3036. [DOI] [PubMed] [Google Scholar]
- 21.Escalante A, Salinas Cervantes A, Gosset G, Bolivar F. Current knowledge of the Escherichia coli phosphoenolpyruvate-carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation. Appl Microbiol Biotechnol. 2012;94:1483–1494. doi: 10.1007/s00253-012-4101-5. [DOI] [PubMed] [Google Scholar]
- 22.Peterkofsky A, Wang G, Garrett DS, Lee BR, Seok YJ, Clore GM. Three-dimensional structures of protein-protein complexes in the E. coli PTS. Journal of molecular microbiology and biotechnology. 2001;3:347–354. [PubMed] [Google Scholar]
- 23.Chen S, Oldham ML, Davidson AL, Chen J. Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography. Nature. 2013 doi: 10.1038/nature12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seok YJ, Koo BM, Sondej M, Peterkofsky A. Regulation of E. coli glycogen phosphorylase activity by HPr. J Mol Microbiol Biotechnol. 2001;3:385–393. [PubMed] [Google Scholar]
- 25.Joyet P, Bouraoui H, Ake FM, Derkaoui M, Zebre AC, Cao TN, Ventroux M, Nessler S, Noirot-Gros MF, Deutscher J, et al. Transcription regulators controlled by interaction with enzyme IIB components of the phosphoenolpyruvate: sugar phosphotransferase system. Biochimica et biophysica acta. 2013;1834:1415–1424. doi: 10.1016/j.bbapap.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26••.Plumbridge J. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Current opinion in microbiology. 2002;5:187–193. doi: 10.1016/s1369-5274(02)00296-5. [Summarizes details of the transcriptional regulation of pts genes by Mlc.] [DOI] [PubMed] [Google Scholar]
- 27.Plumbridge J. Regulation of PTS gene expression by the homologous transcriptional regulators, Mlc and NagC, in Escherichia coli (or how two similar repressors can behave differently). Journal of molecular microbiology and biotechnology. 2001;3:371–380. [PubMed] [Google Scholar]
- 28.Schlegel A, Bohm A, Lee SJ, Peist R, Decker K, Boos W. Network regulation of the Escherichia coli maltose system. J Mol Microbiol Biotechnol. 2002;4:301–307. [PubMed] [Google Scholar]
- 29.Shattuck-Eidens DM, Kadner RJ. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. Journal of bacteriology. 1981;148:203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shattuck-Eidens DM, Kadner RJ. Molecular cloning of the uhp region and evidence for a positive activator for expression of the hexose phosphate transport system of Escherichia coli. Journal of bacteriology. 1983;155:1062–1070. doi: 10.1128/jb.155.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weston LA, Kadner RJ. Role of uhp genes in expression of the Escherichia coli sugar-phosphate transport system. Journal of bacteriology. 1988;170:3375–3383. doi: 10.1128/jb.170.8.3375-3383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weston LA, Kadner RJ. Identification of uhp polypeptides and evidence for their role in exogenous induction of the sugar phosphate transport system of Escherichia coli K-12. Journal of bacteriology. 1987;169:3546–3555. doi: 10.1128/jb.169.8.3546-3555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwoppe C, Winkler HH, Neuhaus HE. Properties of the glucose-6-phosphate transporter from Chlamydia pneumoniae (HPTcp) and the glucose-6-phosphate sensor from Escherichia coli (UhpC). Journal of bacteriology. 2002;184:2108–2115. doi: 10.1128/JB.184.8.2108-2115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenbaum PE, Farmer KS. uhp-directed, glucose 6-phosphate membrane receptor in Escherichia coli. Journal of bacteriology. 1980;142:347–349. doi: 10.1128/jb.142.1.347-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Island MD, Wei BY, Kadner RJ. Structure and function of the uhp genes for the sugar phosphate transport system in Escherichia coli and Salmonella typhimurium. Journal of bacteriology. 1992;174:2754–2762. doi: 10.1128/jb.174.9.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JS, 3rd, Kadner RJ. The phosphoryl transfer domain of UhpB interacts with the response regulator UhpA. Journal of bacteriology. 2001;183:3149–3159. doi: 10.1128/JB.183.10.3149-3159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Island MD, Kadner RJ. Interplay between the membrane-associated UhpB and UhpC regulatory proteins. Journal of bacteriology. 1993;175:5028–5034. doi: 10.1128/jb.175.16.5028-5034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olekhnovich IN, Kadner RJ. RNA polymerase alpha and sigma(70) subunits participate in transcription of the Escherichia coli uhpT promoter. Journal of bacteriology. 1999;181:7266–7273. doi: 10.1128/jb.181.23.7266-7273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadner RJ, Webber CA, Island MD. The family of organo-phosphate transport proteins includes a transmembrane regulatory protein. Journal of bioenergetics and biomembranes. 1993;25:637–645. doi: 10.1007/BF00770251. [DOI] [PubMed] [Google Scholar]
- 40.Pan CJ, Chen SY, Jun HS, Lin SR, Mansfield BC, Chou JY. SLC37A1 and SLC37A2 are phosphate-linked, glucose-6-phosphate antiporters. PloS one. 2011;6:e23157. doi: 10.1371/journal.pone.0023157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffer SM, van Uden N, Tommassen J. Expression of the pho regulon interferes with induction of the uhpT gene in Escherichia coli K-12. Archives of microbiology. 2001;176:370–376. doi: 10.1007/s002030100339. [DOI] [PubMed] [Google Scholar]
- 42.Dumay V, Danchin A, Crasnier M. Regulation of Escherichia coli adenylate cyclase activity during hexose phosphate transport. Microbiology. 1996;142(Pt 3):575–583. doi: 10.1099/13500872-142-3-575. [DOI] [PubMed] [Google Scholar]
- 43.Olekhnovich IN, Kadner RJ. Mutational scanning and affinity cleavage analysis of UhpA-binding sites in the Escherichia coli uhpT promoter. Journal of bacteriology. 2002;184:2682–2691. doi: 10.1128/JB.184.10.2682-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olekhnovich IN, Dahl JL, Kadner RJ. Separate contributions of UhpA and CAP to activation of transcription of the uhpT promoter of Escherichia coli. Journal of molecular biology. 1999;292:973–986. doi: 10.1006/jmbi.1999.3127. [DOI] [PubMed] [Google Scholar]
- 45.Jansen C, Heutink M, Tommassen J, de Cock H. The assembly pathway of outer membrane protein PhoE of Escherichia coli. European journal of biochemistry / FEBS. 2000;267:3792–3800. doi: 10.1046/j.1432-1327.2000.01417.x. [DOI] [PubMed] [Google Scholar]
- 46.Muda M, Rao NN, Torriani A. Role of PhoU in phosphate transport and alkaline phosphatase regulation. Journal of bacteriology. 1992;174:8057–8064. doi: 10.1128/jb.174.24.8057-8064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luttmann D, Gopel Y, Gorke B. The phosphotransferase protein EIIA(Ntr) modulates the phosphate starvation response through interaction with histidine kinase PhoR in Escherichia coli. Molecular microbiology. 2012;86:96–110. doi: 10.1111/j.1365-2958.2012.08176.x. [DOI] [PubMed] [Google Scholar]
- 48.Pfluger-Grau K, Gorke B. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends in microbiology. 2010;18:205–214. doi: 10.1016/j.tim.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 49•.Hsieh YJ, Wanner BL. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol. 2010;13:198–203. doi: 10.1016/j.mib.2010.01.014. [Summarizes the transcriptional regulatory system for the pho regulon.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguena M, Spira B. Transcriptional processing of the pst operon of Escherichia coli. Curr Microbiol. 2009;58:264–267. doi: 10.1007/s00284-008-9319-1. [DOI] [PubMed] [Google Scholar]
- 51.Torriani A. From cell membrane to nucleotides: the phosphate regulon in Escherichia coli. Bioessays. 1990;12:371–376. doi: 10.1002/bies.950120804. [DOI] [PubMed] [Google Scholar]
- 52.Rao NN, Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990;4:1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 53.Kadner RJ, Murphy GP, Stephens CM. Two mechanisms for growth inhibition by elevated transport of sugar phosphates in Escherichia coli. Journal of general microbiology. 1992;138:2007–2014. doi: 10.1099/00221287-138-10-2007. [DOI] [PubMed] [Google Scholar]