Abstract
Chronic nicotine administration increases the density of brain α4β2* nicotinic acetylcholine receptors (nAChRs), which may contribute to withdrawal symptoms associated with smoking cessation. Varenicline, a smoking cessation drug, also increases these receptors in rodent brain. The maintenance of this increase by varenicline as well as nicotine replacement may contribute to the high rate of relapse during the first year after smoking cessation. Recently we found that sazetidine-A, a potent partial agonist that desensitizes α4β2* nAChRs, does not increase the density of these receptors in brain at doses that decrease nicotine self-administration, increase attention in rats, and produce anxiolytic effects in mice. Here we investigated whether chronic sazetidine-A and varenicline maintain the density of nAChRs after their up-regulation by nicotine. In addition, we examined the effects of these drugs on a measure of anxiety in mice and weight gain in rats. After increasing nAChRs in the rodent brain with chronic nicotine, replacing nicotine with chronic varenicline maintained the increased nAChR binding, as well as the subunit proteins measured by western blots. In contrast, replacing nicotine treatments with chronic sazetidine-A resulted in the return of the density of nAChRs to the levels seen in saline controls. Nicotine, sazetidine-A and varenicline each demonstrated anxiolytic effects in mice, but only sazetidine-A and nicotine attenuated the gain of weight over a 6-week period in rats. These findings suggest that apart from its modest anxiolytic and weight control effects, sazetidine-A, or drugs like it, may be useful in achieving long-term abstinence from smoking.
Keywords: nicotinic receptor, nicotine dependence, receptor up-regulation, sazetidine-A, varenicline, nicotine
Introduction
Despite the widely known health risks of smoking, ~18% to 50% of adults in industrialized countries continue to smoke (CDC, 2012). This continued smoking in the face of the health risks is related primarily to the strong addictive properties of inhaled nicotine.
The nicotinic acetylcholine receptor (nAChR) most closely associated with nicotine addiction is the α4β2* subtype. The basis for this association is: 1) The α4β2* nAChR predominates in most areas of mammalian brain (Flores et al., 1992; Marks et al., 1992; Perry et al., 2002; Mao et al. 2008; Gotti et al., 2009); 2) It is located within brain reward circuits that are targets of drugs of abuse (Marks et al., 1992; Zoli et al., 2002) and, more specifically, it is found on axons and cell bodies of dopamine neurons (Schwartz et al., 1984; Clarke et al., 1985; Zoli et al., 2002), where it mediates nicotine-stimulated dopamine release (Rapier et al., 1988; Rowell and Wonnacott, 1990; Grady et al., 1992; 1994); 3) Genetic evidence from knockout and knockin mice implicates α4 and β2 subunits either on dopamine neurons (Picciotto et al. 1998; Maskos et al., 2005) or on GABA neurons (Tapper et al., 2004; Nashmi et al. 2007) in nicotine self-administration, reinforcement, and tolerance; and 4) Chronic administration of nicotine increases the density of α4β2* nAChRs in rat and mouse brain (Schwartz and Kellar 1983; 1985; Marks et al., 1983, 1985; Flores et al., 1992; Mao et al., 2008; Moretti et al., 2010; Marks et al., 2011), and a similar increase is found in autopsied brains from humans who smoked (Benwell et al., 1988; Breese et al., 1997; Perry et al., 1999), as well as in brain imaging studies of current smokers (Staley et al., 2006; Wüllner et al., 2008; Cosgrove et al., 2009).
In vivo administration of other nAChR ligands, including cytisine (Schwartz and Kellar, 1985), anatoxin (Rowell and Wonnacott, 1990) and varenicline (Turner et al., 2011), also increase the density of α4β2* nAChRs. In contrast, sazetidine-A (saz- A), a ligand with high affinity for α4β2* nAChRs, does not increase these receptors in rat or mouse brain, even when administered chronically at behaviorally active doses (Hussmann et al., 2012). Saz-A is a partial to full agonist at α4β2* nAChRs, depending on the receptor subunit stoichiometry, and stimulates dopamine release in rat brain slices (Zwart et al., 2008). Importantly, saz-A potently and selectively desensitizes α4β2* nAChRs (Xiao et al., 2006).
Because up-regulated α4β2* nAChRs may directly contribute to the cellular mechanisms underlying nicotine addiction, we determined whether saz-A would sustain the increase in receptors after they were up-regulated by chronic administration of nicotine. This paradigm would be analogous to a nicotine-addicted individual with up-regulated nAChRs who stops smoking with the aid of a drug (e.g., varenicline), and eventually stops taking that drug but tries to remain abstinent from nicotine. Therefore we measured nAChRs in the cerebral cortex from rats and mice that were treated chronically with nicotine and then either maintained on nicotine or switched to saline, the smoking cessation drug varenicline or saz-A.
Withdrawal from smoking is usually accompanied by some degree of anxiety (Hogle et al., 2010; Weinberger et al., 2010; Piper et al. 2011) and often leads to weight gain (Aubin et al., 2012); therefore, we also examined these two manifestations of nicotine treatment and withdrawal in the absence or presence of varenicline or saz-A.
Methods
Materials
[3H]Epibatidine ([3H]EB, ~55 Ci/mmol) was purchased from Perkin Elmer Life Science (Boston, MA). Sazetidine-A dihydrochloride (Xiao et al., 2006) was synthesized by RTI, International (Research Triangle, NC) and supplied by NIDA. Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO). Varenicline tartrate was a kind gift from Ms. Carolyn Kelman (Pfizer, Groton, CT). Osmotic minipumps were purchased from Alzet (Cupertino, CA). The monoclonal antibody mAb 290, which binds β2 subunits incorporated in assembled nAChRs (Sallette et al., 2005), was purchased from Sigma Aldrich, and the polyclonal antibodies sc-1772 and sc-11372, which bind the α4 and β2 subunits of nAChRs, respectively, were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Odyssey blocking buffer and the fluorescently tagged antibodies donkey anti-goat 800CW and donkey anti-rabbit 680LT were purchased from LI-COR (Lincoln, NE). Protein G Ultra Link Resin, dimethyl pimelimidate (DMP), Triton X-100 detergent and Coomassie Plus Protein Assay were purchased from Thermo Fisher Scientific (Pittsburgh, PA). All other reagents were purchased from Sigma Aldrich unless noted otherwise.
Animals
Male adult Sprague Dawley rats (~8 weeks old, 240 g) were purchased from Harlan Laboratories (Frederick, MD). Male 129SvJ;C57BL/6J F1 hybrid mice (~ 6weeks old, 25–30 g) were bred and housed at the University of Pennsylvania (Philadelphia, PA). Rats and mice were housed in groups in AAALAC-approved facilities at Georgetown University or the University of Pennsylvania. All rodents were maintained on a 12 hr light/12 hr dark cycle with free access to food and water. Treatment, care and housing were carried out in accordance with the National Institutes of Health guidelines on animal care. All experimental procedures were approved by each university’s animal care and use committee, and animal use was reported in compliance with ARRIVE guidelines.
Drug administration
Drugs were dissolved in sterile saline and administered subcutaneously via implanted osmotic minipumps. The drug doses reported here are expressed as the free base. For rats, the doses were: nicotine, 6 mg/kg/day; saz-A, 4.7 mg/kg/day; and varenicline, 1.2 mg/kg/day. For mice, the doses were nicotine, 18 mg/kg/day; saz-A 1.8 mg/kg/day; and varenicline 1.8 mg/kg/day. These doses of saz-A and varenicline are within the range that produce anxiolytic and/or antidepressant activity in preclinical tests in rats and mice (Kozikowski et al., 2009 Turner et al., 2010; 2011; Caldarone et al., 2011); reduce nicotine and alcohol self-administration in rats (Levin et al., 2010; Rezvani et al., 2010; George et al., 2011; Wouda et al., 2011; Johnson et al., 2012;); and increase performance in tests of attention in rats (Rezvani et al., 2011; 2012).
Animals received two phases of drug treatment, which are illustrated in figure 1A. To begin phase 1 of treatment, the rats and mice were anesthetized with isoflurane and a small incision was made between the shoulders. An osmotic minipump filled with saline or nicotine was then implanted subcutaneously and the incision was closed with wound clips. Buprenorphine (0.01 mg/kg) or ketoprofen (0.5 mg/kg) was injected subcutaneously one time for analgesia. The animals were allowed to recover and then moved to their home cages. After two weeks with these pumps delivering saline or nicotine, phase 2 of treatment began. The animals were anesthetized, the pumps removed and a new pump inserted in an incision made ~ 2 cm away from the first. These pumps contained either saline, nicotine, varenicline or saz-A. Again, the wounds were closed and the animals were given a single injection of analgesic, as described above.
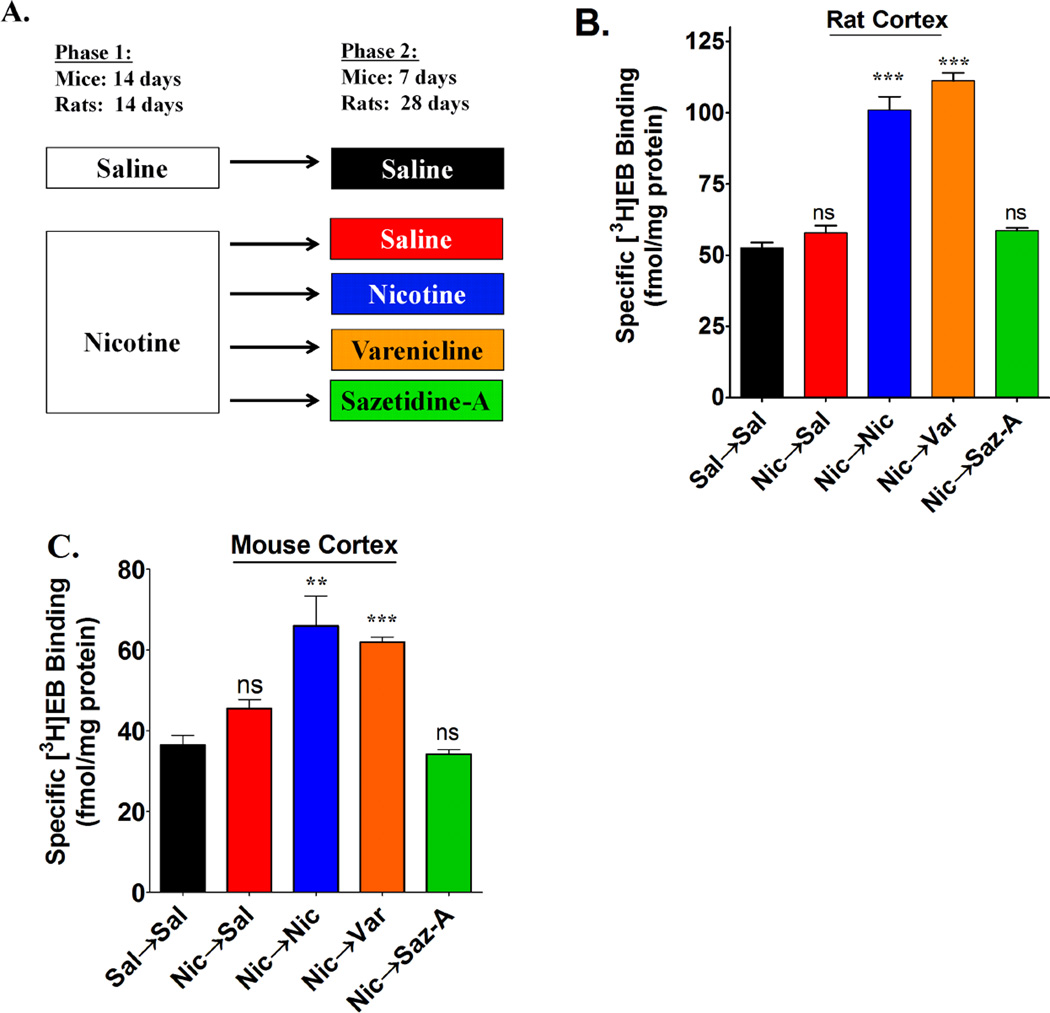
Figure 1. Measuring nAChR binding sites after 2-phase chronic treatments.
(A) Male mice and rats underwent a 2-phase drug treatment paradigm as illustrated in the figure. Saline (Sal) and drugs were delivered by osmotic minipumps at the following doses (free base): Rats; 6 mg/kg/day nicotine (Nic), 4.7 mg/kg/day saz-A, and 1.2 mg/kg/day varenicline (Var). Mice; 18 mg/kg/day nicotine; 1.8 mg/kg/day saz-A; and 1.8 mg/kg/day varenicline. For rats, osmotic minipumps were removed following the completion of phase 2 treatments to allow drug elimination and were sacrificed 2 days later. Mice were sacrificed while pumps were still implanted following completion of phase-2 treatments. The density of nAChR binding sites were measured in cortical membrane homogenates from both (B) rats and (C) mice using 2 nM [3H]EB. These densities were graphed as the mean ± SEM from 4 – 8 animals in each group. Statistical analysis was compared to Sal→Sal treatments by a one-way ANOVA with a post-hoc Bonferroni test (ns = not significant; **p<0.01; ***p<0.001).
After 7 days of phase 2 treatment, a behavioral assessment of the mice was carried out using the novelty-induced hypophagia test, as described below. The mice were then anesthetized and decapitated while the pumps were still implanted. The rats were maintained in the phase 2 treatment for 28 days before the pumps were removed. They were then anesthetized and decapitated 2 days later. All brains were quickly removed, dissected, frozen on dry ice and stored at −80° until use. The rat weights were monitored and recorded periodically during both phase 1 and phase 2 treatments.
Preparation of membrane homogenates for binding assays
Dissected cerebral cortex was weighed and homogenized in 30 mL of 50 mM Tris HCl buffer (pH 7.4) with a polytron homogenizer. The homogenates were then centrifuged at 35,000 g for 10 min at 4° C. The resulting supernatants were discarded and the pellets were re-suspended and homogenized again in 30 mL of 50 mM Tris buffer. The homogenates were incubated at 37° C for 1 hr while gently shaking to allow drugs to dissociate from the nAChR binding sites before undergoing another high speed centrifugation. This procedure was repeated until the pellets underwent a total of 4 high-speed centrifugations and 3 incubations for 1 hr each at 37° C. The final pellet was re-suspended in 20 volumes of 50 mM Tris buffer.
Radioligand binding
To determine total binding, membrane homogenates equivalent to 10 mg of frozen cortex (~ 400 µg protein) were added to test tubes containing 2 nM [3H]EB in a final volume of 0.5 mL of 50 mM Tris HCl buffer (pH 7.4). Nonspecific binding was determined in parallel in similar tubes containing 300 µM nicotine. The tubes were incubated for at least 4 hr at room temperature while gently shaking in the dark. After incubation, bound [3H]EB was collected by vacuum filtration over GF/C filters presoaked in 0.5% polyethyleneimine. Radioactivity in the filters was measured by scintillation counting. Specific [3H]EB binding was calculated by subtracting the nonspecific binding from the total binding. All specific binding was then normalized to the total protein in the binding assay as measured by a BCA protein assay.
Preparation of membrane homogenates for immunoassays
One hemisphere of rat cortex was suspended in ice-cold 10 mM Tris buffer containing 5 mM EDTA, and 5 mM EGTA pH 7.4 (TEE buffer) and homogenized with a Polytron homogenizer. The homogenates were centrifuged at 35,000 g and the pellets were resuspended in fresh ice-cold buffer and sonicated with a Tekmar Sonicator. This procedure was repeated and the final pellet was frozen at −80° C until used for western blotting.
Immunoprecipitation and Western blotting
Homogenates were thawed at 37° C and then immediately placed on ice. 150 µL aliquots of membrane homogenates were solubilized in 2% Triton X-100 detergent, 1.8 mM phosphatidylcholine, and 0.05 % SDS for 5–6 hours with rotation at 4° C. Solubilized proteins were separated from membrane homogenates by pelleting the non-solubilized membrane fraction at 35,000 g for 30 minutes. The solubilized supernatant fractions were separated and protein assays were performed using the Coomassie Protein Plus Assay. To concentrate the samples and to avoid interference from free α and β subunits in the quantitative analyses of the western blots, the solubilized proteins were first immunoprecipitated with mAb 290, which is a conformationally specific antibody that selectively binds to β2 subunits incorporated in nAChRs (Sallette et al., 2005). The solubilized samples (500 µg protein) were immunoprecipitated with 2.7 – 3.5 µg of mAb 290 covalently coupled to Protein G UltraLink Resin (performed previously using dimethyl pimelimidate, according to manufacturer’s instructions). Immunoprecipitation (IP) samples were rotated overnight (~18 hours) at 4° C and then centrifuged at low speed to pellet the Protein G-mAb 290-nAChR complexes. The supernatants were removed and the pellets were washed 3× with 1 % thesit detergent in TEE. The final pellets were re-suspended in 40 µL of western blot denaturing buffer and stored at −80° C until use.
For western blotting, IP pellet samples were denatured in Tris buffer containing 50 mM dithiothreitol, 2% SDS and 10%glycerol, and 10 µL of each sample was separated by SDS-PAGE on 9% acrylamide gels. Samples were run in triplicate across three different gels. Proteins were transferred to Immobilon-FL PVDF membranes and blocked for 1 hour with Odyssey Blocking Buffer diluted 50 % with PBS. Membranes were incubated overnight at 4° C with 1 µg/mL of each of the primary polyclonal antibodies, directed against the α4 subunit (sc-1772) and the β2 subunit (sc-11372), washed 5× with phosphate-buffered saline with Tween-20, incubated for 1 hour with secondary antibodies (1:25,000 each donkey anti goat 800CW and donkey anti rabbit 680LT), washed again 5× with the buffer, and imaged using the Odyssey Infrared Imaging System with dual color detection. Integrated Intensity values for α4 and β2 bands were obtained using the Odyssey Application Software (Version 3.0). Median integrated intensity values for each sample from rat brain were normalized against a standard curve prepared from HEK293 cells stably expressing rat α4β2 nAChRs. The standard curve was fit to a 4 parameter logistic equation as shown below:
In this equation, A = maximum, B = slope, C = inflection and D = minimum. Values for µg of α4 or β2 for each rat were calculated using the standard curve and these values were analyzed for each group using GraphPad Prism 5 with One-Way ANOVA and Bonferroni’s post-hoc test.
Novelty-Induced Hypophagia (NIH) Test
The NIH test was used as a measurement of anxiety and was carried out as described previously (Turner et al., 2010; Dulawa et al., 2004). Male mice (n= 8 to 16/group) were housed in pairs starting from 1 week prior to training until the end of the experiment. For training, mouse pairs were separated in their home cage with a plastic divider and left to acclimate for 1 hr. Then a highly palatable food (peanut butter chips; Nestle, Glendale, CA) was placed in the cage for 15 min and the latency to feed was recorded. Training was performed daily until the variability of latency to feed was less than 20% (~12 days). Mice then underwent phase 1 treatment for 2 weeks and phase 2 treatments for 1 week. Training was continued daily during both treatment phases. On the 6th day of phase 2 treatments, the latency for mice to feed in their home environment was measured. One day later, their latency to feed in a novel environment was measured. For this measurement, individual mice were placed in new cages without bedding. The cages had been wiped clean with Pine Sol to emit a novel odor and placed in a white box with bright light illumination (2150 lux). The time to feed in this novel environment was then measured.
Results
Brain nAChRs return to control levels during treatment with sazetidine-A
The treatment paradigm for these studies is shown in figure 1A. During the phase 1 treatment, saline or nicotine was administered subcutaneously to rats and mice for 2 weeks via osmotic pumps. At the end of the phase 1 treatment period, the pumps were removed and replaced with a pump that contained either saline (groups designated Sal→Sal and Nic→Sal), nicotine (Nic→Nic), varenicline (Nic→Var), or saz-A (Nic→Saz-A). This phase 2 treatment lasted for 4 weeks in rats and 1 week in mice.
Treatment with nicotine consistently increases the density of nAChRs in rat and mouse cerebral cortex (Schwartz and Kellar, 1983; Marks et al., 1983). For example, a similar 2-week treatment of rats and mice with nicotine via osmotic pumps increased nAChRs measured with [3HEB] by nearly 100% (Hussmann et al., 2012). A similar increase in [3HEB] binding sites was found here (Fig. 1B and 1C) in animals that received nicotine in both treatment phases, a duration of 6 weeks in rats and 3 weeks in mice. This indicates that in rats and mice a new steady-state level of nAChRs in the cerebral cortex is set within the first 2 weeks of continuous administration of nicotine via osmotic pumps. The nicotine-induced increase in nAChRs is reversible; thus, when the initial two-week treatment with nicotine was replaced by saline treatment for 4 weeks in rats and 1 week in mice, nAChRs labeled by [3H]EB in cerebral cortex returned to a density level similar to that in control animals treated with saline throughout both periods (Fig. 1B and 1C).
In rats and mice treated with varenicline in phase 2, the density of nAChRs measured in the cerebral cortex with [3H]EB was nearly identical to the density in the cortex from animals treated with nicotine in both phases of treatment (Fig. 1B and 1C). In contrast, in rats and mice that received nicotine in the phase 1 treatment period followed by saz-A in the phase 2 treatment period, the density of nAChRs was nearly identical to the density in the saline treated groups. These results indicate that treatment with saz-A following chronic nicotine treatment does not sustain the nicotine-induced increase in brain nAChRs.
nAChR subunit protein after treatments
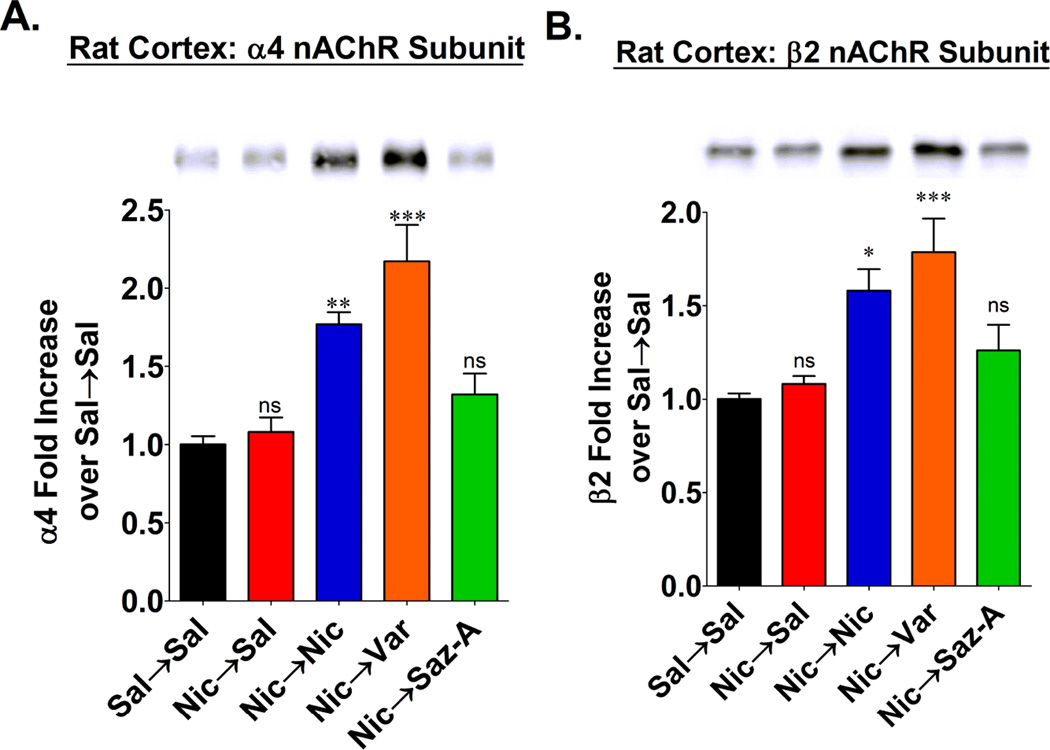
To examine the effects of these chronic treatments with these nicotinic drugs on rat α4β2* nAChRs independent of the [3H]EB binding sites, we evaluated the receptor’s constituent subunit proteins by quantitative analysis of western blots. To avoid inclusion of unassembled α4 and/or β2 subunits in these measurements, we first immunoprecipitated the α4β2* nAChRs with mAb 290, a conformationally specific antibody that binds to β2 subunits assembled in nAChRs (Sallette et al., 2005). As shown in the western blots in figure 2A and 2B, both the α4 and β2 nAChR subunit proteins were increased in the cerebral cortex from rats that received either nicotine or varenicline during the 4 week phase 2 treatment period; while in contrast, the levels of subunit proteins from rats that received either saline or saz-A during the phase 2 treatment were not significantly different from the subunit levels in control rats treated with saline in both phases. These results thus demonstrate that the up-regulation of α4β2* nAChR binding sites following treatment with nicotine or varenicline is associated with increases in the constituent subunit proteins assembled as nAChRs. This is consistent with a recent study that used binding of [125I]monoclonal antibodies to α4 and β2 subunits to demonstrate an increase in nAChR subunit protein in brain after chronic nicotine treatment of mice (Marks et al., 2011). Moreover, the western blots from rats treated with nicotine followed by saline demonstrate that the nicotine-induced increase in subunit proteins is reversible. In addition to confirming the absence of an increase in nAChR binding sites after chronic treatment with saz-A, these western blots demonstrate that residual drug bound to the receptor does not explain the absence of up-regulation of nAChRs after treatment with saz-A.
Figure 2. Measuring α4 and β2 subunit protein levels after chronic treatments.
Dissected cerebral cortex from the same rats as in figure 1 were homogenized and solubilized. Assembled α4β2* nAChRs were then isolated by immunoprecipitation with mAb 290. Precipitated receptors were denatured and then used for western blotting. The α4 and β2 subunits were probed with polyclonal antibodies SC-1772 and sc-11372, respectively. All samples were run in triplicate. A representative blot of α4 and β2 subunits is shown in (A) and (B) respectively. Band densities were quantified and compared to standard curves of α4 and β2 subunits expressed in HEK293 cells. The quantified results for α4 and β2 subunits are shown in (A) and (B) respectively, and the data are expressed as the mean ± SEM from 8 individual animals in each group. Statistical analysis was compared to Sal→Sal treatments by a one-way ANOVA and a post-hoc Bonferroni test (ns = not significant;*p<0.05; **p<0.01; ***p<0.001).
Nicotinic drugs decrease anxiety in mice
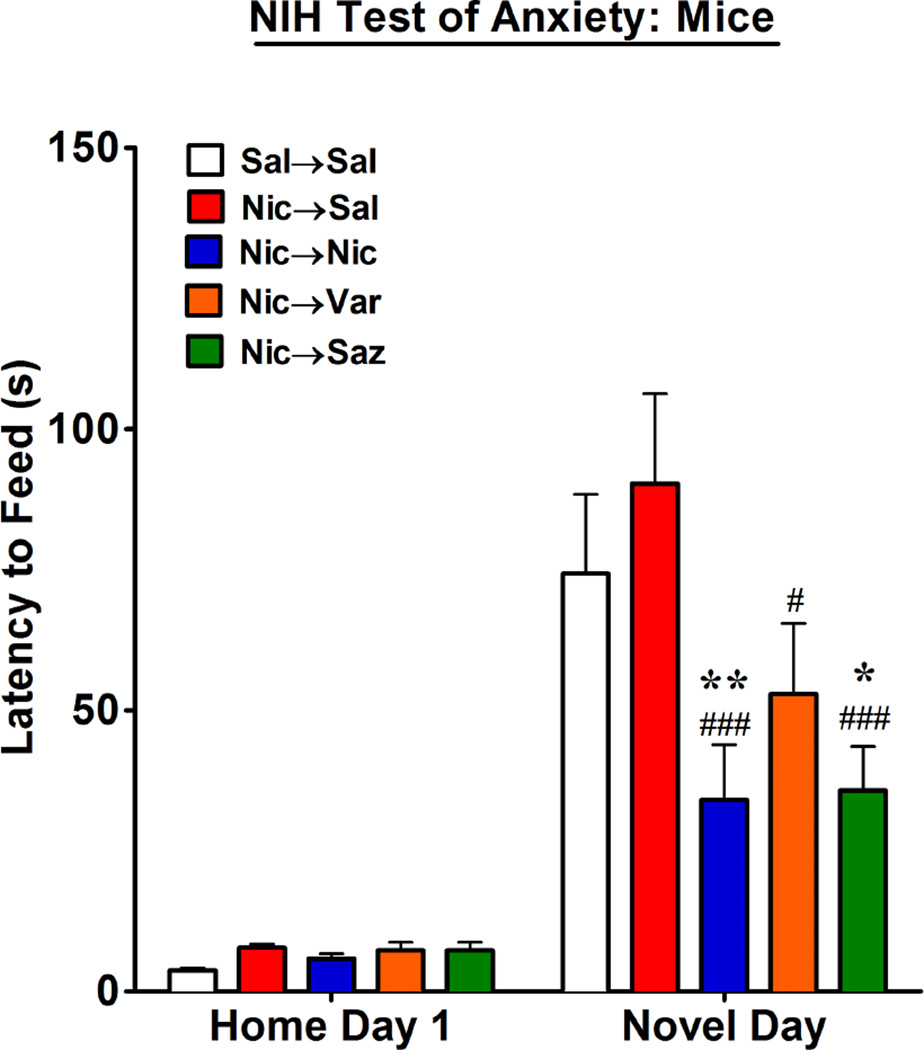
Increased anxiety during withdrawal from nicotine may be an important factor contributing to smoking relapse (Hogle et al., 2010; Piper et al, 2011). Therefore, it would very likely be advantageous for an effective smoking cessation therapy to demonstrate potential to blunt the anxiety producing effects of nicotine withdrawal. Nicotine, varenicline and saz-A all produce anxiolytic effects in rodents (File et al., 1998; Turner et al., 2010; 2011; McGranahan et al., 2011; Anderson and Brunzell, 2012; Varani et al., 2012). To examine this further, we determined if nicotine, varenicline and saz-A maintain anxiolytic activity in mice during withdrawal from chronic administration of nicotine. The mice from which the nAChR binding data in figure 1 were subsequently obtained were trained for the NIH test for anxiety (see methods), and on the 6th day of phase 2 treatments, the latency for the mice to feed in a familiar environment (their home cage) was measured. As shown in figure 3, none of the drugs used in phase 2 significantly affected the latency of the mice to feed in the familiar environment. The next day (the 7th day of phase 2 treatments), the latency of these mice to feed in a novel environment was measured. As shown in figure 3, the mice treated with saline in both phase 1 and 2 (Sal→Sal) showed the expected marked increase in their latency to feed in a novel environment compared to their latency in their familiar environment, which is indicative of increased anxiety. Mice treated with nicotine in phase 1 and 2 (Nic→Nic) showed the expected significant decrease in latency to feed consistent with an anxiolytic effect, as has been shown previously (Turner et al., 2010; 2011; Anderson and Brunzell, 2012). Mice treated with nicotine in phase 1 and then switched to saline (Nic→Sal) showed a latency similar to the animals treated with saline throughout both phases, which indicates that the anxiolytic effect of nicotine is reversible. The latency to feed in the Nic→Var group was significantly reduced compared to the Nic→Sal group (P<0.05) and showed a trend toward reduced latency to feed compared to the Sal→Sal. Finally, the latency to feed in the Nic→Saz group was significantly reduced compared to the latencies in the Nic→Sal group (P< 0.001), as well as compared to the Sal→Sal group (P<0.05).
Figure 3. NIH Test of Anxiety.
Male mice were trained for the NIH test of anxiety (see methods) and then underwent the 2-phase drug treatment paradigm as illustrated in figure 1A. On the 6th day of phase 2 treatments, the latency for mice to feed was measured in their home cage. The next day, the latency for mice to feed was measured in a novel environment. The data are expressed as the mean ± SEM from 8 – 16 individual animals per group and statistical analysis was performed by a two-way ANOVA with a post-hoc Bonferroni test (Compared to Sal→Sal: *p<0.05; **p<0.01. Compared to Nic→Sal; #p<0.05; ###p<0.001).
Rats on nicotine replacement drugs gain less weight
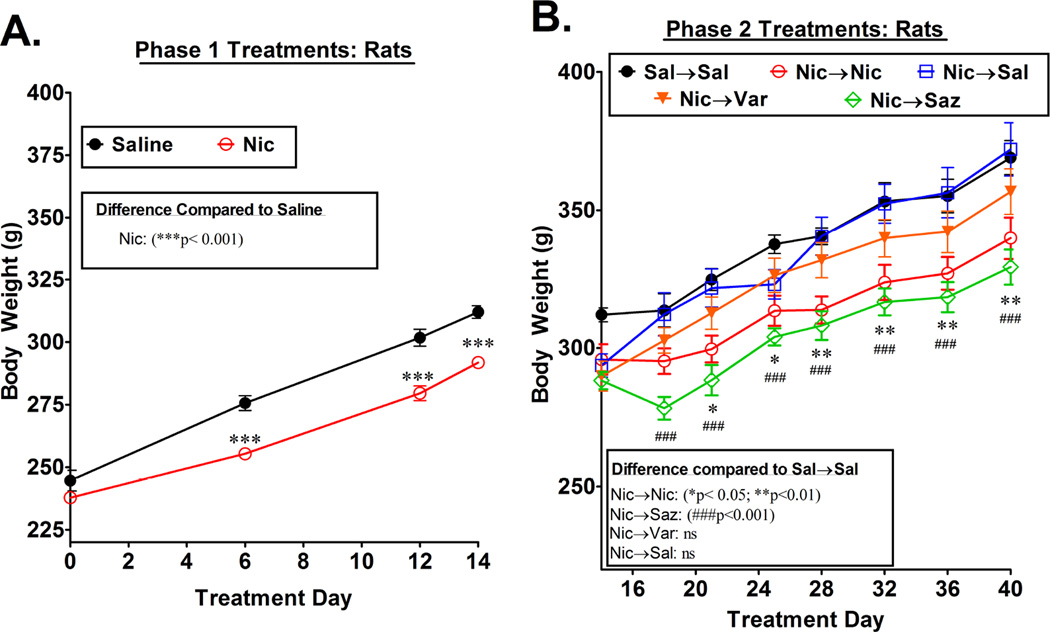
Chronic administration of nicotine and other nicotinic drugs is known to suppress appetite in rodents and result in a slower rate of weight gain (Grunberg et al., 1987; Mineur et al., 2011). Such an effect of nicotine is consistent with the gain of weight that often accompanies cessation of smoking (Aubin et al., 2012; Zoli and Picciotto, 2012). We therefore compared the body weights of the rats on saline and nicotine during phase 1 of the treatment paradigm and then in each group during the treatments in phase 2. The same rats from which the nAChR binding and western blot data in figures 1 and 2 were subsequently obtained were weighed periodically throughout the chronic treatments, and the results are shown in figure 4. During phase 1, both the saline and the nicotine-treated rats gained weight, but the nicotine-treated rats gained significantly less. This was particularly evident during the first 6 days of treatment, and by the end of the 14 day phase 1 treatment, the nicotine-treated rats weighed about 6% less than the saline-treated rats (Fig 4A). When the rats on nicotine during the first 2 weeks were switched to saline in phase 2 (Nic→Sal), they rapidly gained weight and reached a weight similar to that of the (Sal→Sal) controls within a week, following which their weights remained similar to that of controls throughout the remainder of the 4 week treatment period (fig. 4B). The rats that were maintained on nicotine during phase 2 treatment (Nic→Nic) continued to gain weight at a slower rate than the saline controls, and at the end of the 4-week phase 2 treatments weighed approximately 8% less than the saline controls (p<0.01). Similarly, the rats treated with saz-A during the phase 2 treatment (Nic→Saz) gained weight at a slower rate than controls, and they weighed approximately 11% less than the controls at the end of the 4-week treatment period (p<0.001). Although the weights of the rats treated with saz-A were slightly lower than the weights of the rats maintained on nicotine, this difference appears to be due to an initial decrease in body weight in the rats switched to saz-A, after which the weights of the two groups increased in parallel. Finally, the weights of the rats that were treated with varenicline during phase 2 (Nic→Var) were slightly lower than the two groups of saline-treated rats, but the differences did not reach statistical significance.
Figure 4. Body weight during chronic treatment with nicotinic drugs.
Male rats (n=6–8 per group) that underwent the 2-phase treatment paradigm illustrated in figure 1A were weighed periodically during both phases of treatment and the body weight for each group is expressed as the mean ± SEM. Statistical differences between groups were evaluated by two-way ANOVA, followed by a Bonferroni test. The results of these statistical analyses are shown within the figure.
Discussion
Based on nicotine’s up-regulation of α4β2* nAChRs and its high potency to desensitize nAChRs both in vivo (Sharp and Beyer, 1986; Hulihan-Giblin et al., 1990) and in vitro (Grady et al., 1994; Marks et al., 1994; Lester and Dani, 1995; Pidoplichko, et al., 1997), a hypothesis has emerged that can at least partially explain the addictive drive created by chronic administration of nicotine (Dani and Heinemann, 1996; Ortells and Barrantes, 2010; Hussmann et al., 2012). According to one version of this hypothesis (Hussmann et al., 2012), activation of the increased number of α4β2* nAChRs in a smoker’s brain by endogenous acetylcholine causes dysphoria/anxiety, which an addicted individual learns from experience can be relieved by smoking the next cigarette, which, in turn, then desensitizes the nAChRs and consequently titrates down their activity. This decreased activity lasts until the nicotine concentration falls below a critical threshold level, which then triggers the signals for the next cigarette; thus the cycle that we recognize as nicotine addiction is sustained.
In contrast to nicotine and varenicline, saz-A administered chronically to rats and mice does not increase nAChRs (Hussmann et al., 2012), even at doses that decrease nicotine and alcohol self-administration, increase attention on cognitive tasks, and produce anxiolytic effects (Levin et al., 2010; Rezvani et al., 2010; 2013; Turner et al., 2010; Caldarone et al., 2011). The explanation for this separation between behavioral effects and nAChR up-regulation may be related to the fact that the steady state brain concentration of saz-A (32 nM) during chronic administration is high enough to desensitize α4β2* nAChRs, but much lower than the concentrations reached by nicotine or varenicline, which concentrate in the brain and may enter compartments within the cell not accessed by saz-A (Hussmann et al., 2012). Thus, we hypothesize that desensitization of α4β2* nAChRs accounts for the behavioral effects of saz-A, but its concentration remains below the threshold necessary to induce up-regulation of the receptors (Hussmann et al., 2012), which is thought to occur in the endoplasmic reticulum (Sallette et al., 2004; 2005; Kuryatov et al., 2005; Srinivasan et al., 2011).
In the current study, we sought to extend this observation by testing whether saz-A would maintain the increased number of nAChRs once they had been up-regulated by chronic administration of nicotine. This paradigm, which more closely parallels the situation of a nicotine-addicted smoker abstaining from nicotine by switching to a nAChR partial agonist, addresses the important question of whether nAChRs, once up-regulated by nicotine, would remain up-regulated during administration of a partial agonist, and thus leave the person abstaining from nicotine possibly more vulnerable to relapse once treatment with the partial agonist is stopped.
The results from our radioligand binding studies demonstrate that chronic nicotine treatment increases the number of nAChRs in the cerebral cortex by about 2-fold. This increase is reversible; thus, after switching from nicotine to saline treatment, the number of nAChRs returns toward control levels and is no longer significantly increased within 4 weeks of stopping nicotine treatment in rats and 1 week of stopping treatment in mice. In rats and mice switched from chronic nicotine to chronic varenicline, the nAChR density was maintained at a level similar to that in the animals maintained on nicotine throughout the two treatment periods. In contrast, in animals switched to saz-A, the receptor density returned to levels similar to that in the saline controls. The most likely explanation for this is that in the absence of nicotine (or varenicline) normal turnover mechanisms allow the steady-state levels of nAChRs to return to control levels.
Questions have been raised about whether the increased agonist binding sites represent an actual increase in receptor protein or a shift of α4β2* nAChRs to a confirmation with high affinity for agonists, or both (Vallejo et al., 2005; Govind et al., 2009; Govind et al. 2012). The increases in the nAChR subunit proteins seen in our western blot analyses closely parallel the results from our radioligand binding studies and are in good agreement with a previous western blot analyses by Moretti et al., (2010). Together, these data strongly support the view that the increase in receptor binding sites primarily reflects an increase in receptor protein. The mechanism for this up-regulation is not known with certainty, but evidence favors the hypothesis that nicotine acts as a protein chaperone and leads to an increase in maturation and assembly of the subunits in the endoplasmic reticulum (Sallette et al., 2004; 2005; Kuryatov et al., 2005; Srinivasan et al., 2011). Although this hypothesis is based on studies in transfected cells heterologously expressing nAChRs, a recent study in rat primary neurons is consistent with it (Lomazzo et al, 2011).
Nicotine, varenicline and saz-A have been reported to produce effects consistent with anxiolytic and/or antidepressant activity in rodents (Patterson et al. 2009; Kozikowski et al. 2009; Turner et al., 2010; 2011). Moreover, a recent study found that both low dose nicotine and the competitive antagonist DHβE produced anxiolytic effects in mice, suggesting that desensitization of nAChRs contributes to the anxiolytic effects of nicotine (Anderson and Brunzell, 2012). On the other hand, nicotine can also elicit an anxiogenic response in rats, and microinjection of the nAChR channel blocker mecamylamine into the medial septum attenuated this response (Zarrindast et al., 2013). To explore the anxiolytic/anxiogenic effects of these drugs during withdrawal from chronic nicotine, a condition that in humans usually provokes increased levels of anxiety (Picciotto et al., 2002; Pomerleau et al., 2005; Hogle et al., 2010; Piper et al., 2011), we compared the effects of continued administration of nicotine in mice with switching them to varenicline or saz-A after withdrawal from nicotine. Our assessment of anxiety with the NIH test is consistent with both varenicline and saz-A having an anxiolytic effect during withdrawal from nicotine, with saz-A showing an efficacy similar to continued nicotine and varenicline being slightly less efficacious at the doses used. These results suggest that both of these drugs have the potential to decrease anxiety during abstinence from nicotine. However, the exact brain areas where nicotinic drugs act to modulate anxiety and the nAChR subtypes that mediate these effects are not known.
It would be interesting to compare anxiety levels of the animals at various times after switching them from nicotine to saline (Nic/Sal group) as well as after terminating the other phase 2 treatments to determine if resensitization of nAChRs contributes to anxiety, and whether the treatment with saz-A, which allowed the receptors to return to control levels, attenuates that behavior. However, such a study will require, in addition to the rigorous behavioral measurements, assessments of the brain-specific pharmacodynamics and pharmacokinetics of saz-A, varenicline and nicotine to determine the appropriate window of time during withdrawal to carry out the behavior tests. Such studies are beyond the scope of this paper.
Weight gain may contribute to relapse in ex-smokers trying to abstain from smoking (Zoli and Picciotto, 2012). The mechanisms for this weight gain are not known, but nicotine decreases the rate of weight gain in normal rats (Grunberg et al., 1987), and recently an α3β4* nAChR in the hypothalamus was implicated in its actions in genetically obese mice (Mineur et al., 2011). In our studies, nicotine-treated rats gained weight at a somewhat slower rate than saline-treated controls, particularly during the first week of treatment. The rate of weight gain in rats that were treated with nicotine in phase 1 and then switched to saline in phase 2 increased rapidly during the first week after nicotine was stopped, and within about a week their weights reached the level of controls treated with saline throughout phase 1 and 2. Thereafter they continued to gain weight at the same rate as those saline controls. It is important to note that the weights of the rats switched from nicotine to saline (i.e., the rats most closely mimicking a smoker abstaining unaided from nicotine) never exceeded the weights of saline controls during the 4 week period of phase 2 treatment.
Throughout the 4 week phase 2 treatment period, the rats maintained on nicotine continued to gain weight at a slower rate than the saline controls, and at the end of that 4 week period, at which time they had received nicotine for a total of 6 weeks, their weights were approximately 8% lower than the saline-treated rats. The rats switched from nicotine in phase 1 to varenicline in phase 2 appeared to gain weight at approximately the same rate as the saline controls, and at the end of the 4 week phase 2 treatment period their weights appeared to be slightly, but not significantly, lower than the weights of the saline controls. In contrast, rats that were switched from nicotine in phase 1 to saz-A in phase 2 gained weight at a rate similar to the rate of the nicotine treated rats; thus their weights at the end of the 4 week period were approximately 11% lower than the saline controls. Although the weights of the rats treated with saz-A appeared to be slightly lower than the weights of the nicotine-treated rats, this difference was probably attributable to a fall in weight during the first few days on saz-A. Because saz-A is relatively selective nAChRs containing β2 subunits, these data suggest that these nAChR subtypes are also involved in the effects of nicotine and saz-A on body weight.
We have proposed that saz-A may affect behaviors by desensitizing the α4β2* nAChRs on the cell surface but doesn’t reach the intracellular concentration necessary to up-regulate these receptors or, as indicated here, maintain up-regulation after chronic treatment with nicotine. An alternative possibility is based on the observations that saz-A acts differently at the two different stoichiometries of the α4β2 nAChRs that have been studied in transfected oocytes. It is a very weak partial agonist at nAChRs with the α4(3)β2(2) stoichiometry, but nearly a full agonist at receptors with the α4(2)β2(3) stoichiometry (Zwart et al., 2008; Carbone et al. 2009; Mazzaferro et al., 2011). Both stoichiometric isoforms of this receptor are thought to be expressed in brain (Gotti et al., 2008); therefore, it is possible that the differences in the effects of saz-A are related to its differential effects on these two receptor isoforms.
Although we don’t yet know the mechanisms underlying the differences between saz-A and varenicline, if the differences seen here in rats and mice are also operative in human smokers, it is possible that saz-A or drugs with a similar pharmacological profile would allow the up-regulated nAChRs in smokers to return to pre-smoking levels and thus provide a better chance for long-term abstinence from nicotine.
Acknowledgments
Acknowledgements and Grant Support: Supported by the National Institute of Health Grant RO1-DA012976, RO1-NS11323, U19-DA027990, P50-CA143187, 1-K99-DA032681. We thank Rashmi Venkatesh, Nour Al-muhtasib and Thao Tran for their help in some of the experiments in this study.
Abbreviations
- [3H]EB
[3H]Epibatidine
- nAChR
neuronal nicotinic acetylcholine receptor
- Sal
Saline
- Nic
nicotine
- Saz-A
sazetidine-A
- Var
Varenicline
Footnotes
Conflicts of interest: Sazetidine-A was developed by KJK and YX and Georgetown University currently holds the patent on sazetidine-A.
References
- Anderson SM, Brunzell DH. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS One. 2012;7:e48665. doi: 10.1371/journal.pone.0048665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, Kwan M, Hanania T, Chellappan SK, Kozikowski AP, Olivier B, Picciotto MR, Ghavami A. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-I-A85380. Psychopharmacology (Berl) 2011;217:199–210. doi: 10.1007/s00213-011-2271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. Pentameric concatenated (α4)2(β2)3 and (α4)3(β2)2 nicotinic acetylcholine receptors: subunit arrangement determines functional expression. British J of Pharmacol. 2009;156:970–981. doi: 10.1111/j.1476-5381.2008.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. 2012 [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stikulus S, Krishnan-Sarin S, O’Malley S, Perry E, Tamganan G, Seibyl JP, Stalet JK. β2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Dávila-García MI, Musachio JL, Kellar KJ. Chronic nicotine administration does not increase nicotinic receptors labeled by [125I]epibatidine in adrenal gland, superior cervical ganglia, pineal or retina. J Neurochem. 2003;85:1237–1246. doi: 10.1046/j.1471-4159.2003.01774.x. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- Flores CM, Dávila-García MI, Ulrich YM, Kellar KJ. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J Neurochem. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 2011;213:715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo. Addiction. 2010;105:2002–2013. doi: 10.1111/j.1360-0443.2010.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor α4 or β2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of α4 and β2 subunits. Mol. Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced up-regulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. Nicotine-induced up-regulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J. Neurosci. 2012;32:2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Collins AC. Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem. 1994;62:1390–1398. doi: 10.1046/j.1471-4159.1994.62041390.x. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Popp KA. Sex differences in nicotine's effects on consummatory behavior and body weight in rats. Psychopharmacology (Berl) 1987;91:221–225. doi: 10.1007/BF00217067. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biol Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of a single injection of nicotine. J Pharmacol Exp Ther. 1990;252:15–20. [PubMed] [Google Scholar]
- Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, Levin ED, Blendy JA, Kellar KJ. Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharmacol Exp Ther. 2012;343:441–450. doi: 10.1124/jpet.112.198085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED. Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozikowski AP, Eaton JB, Bajjuri KM, Chellappan SK, Chen Y, Karadi S, He R, Caldarone B, Manzano M, Yuen PW, Lukas RJ. Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. ChemMedChem. 2009;4:1279–1291. doi: 10.1002/cmdc.200900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Xiao Y, Slade S, Cauley M, Wells C, Hampton D, Petro A, Rose JE, Brown ML, Paige MA, McDowell BE, Kellar KJ. Sazetidine-A, a selective α4β2 nicotinic receptor desensitizing agent and partial agonist, reduces nicotine self-administration in rats. J Pharmacol Exp Ther. 2010;332:933–939. doi: 10.1124/jpet.109.162073. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem. 1994;63:2125–2135. doi: 10.1046/j.1471-4159.1994.63062125.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, Collins AC, Lindstrom JM. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther. 2011;337:187–200. doi: 10.1124/jpet.110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Romm E, Gaffney DK, Collins AC. Nicotine-induced tolerance and receptor changes in four mouse strains. J Pharmacol Exp Ther. 1986a;237:809–819. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and α-bungarotoxin. Mol Pharmacol. 1986b;30:427–436. [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani L, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. Additional Acetylcholine (ACh) Binding Site at α4/α4 interface of (α4β2) 2α4 nicotinic receptor influences agonist sensitivity. J Biol Chem. 2011;286:31043–31054. doi: 10.1074/jbc.M111.262014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol. 2010;78:287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Ortells MO, Barrantes GE. Tobacco addiction: a biochemical model of nicotine dependence. Med Hypotheses. 2010;74:884–894. doi: 10.1016/j.mehy.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates α6- and β3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of α4β2, α3β2 and α3β4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106:418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Stereoselective nicotine-induced release of dopamine from striatal synaptosomes: concentration dependence and repetitive stimulation. J Neurochem. 1988;50:1123–1130. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Cauley M, Xiao Y, Kellar KJ, Levin ED. Effects of chronic sazetidine-A, a selective α4β2 neuronal nicotinic acetylcholine receptors desensitizing agent on pharmacologically-induced impaired attention in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2895-6. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, Li TK, Xiao Y, Brown ML, Paige MA, McDowell BE, Rose JE, Kellar KJ, Levin ED. Effects of sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology (Berl) 2010;211:161–174. doi: 10.1007/s00213-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell PP, Wonnacott S. Evidence for functional activity of up-regulated nicotine binding sites in rat striatal synaptosomes. J Neurochem. 1990;55:2105–2110. doi: 10.1111/j.1471-4159.1990.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Sallette J, Bohler S, Benoit P, Soudant M, Pons S, Le Novère N, Changeux JP, Corringer PJ. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J Biol Chem. 2004;279:18767–18775. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Lehmann J, Kellar KJ. Presynaptic nicotinic cholinergic receptors labeled by [3H]acetylcholine on catecholamine and serotonin axons in brain. J Neurochem. 1984;42:1495–1498. doi: 10.1111/j.1471-4159.1984.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther. 1986;238:486–491. [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. Nicotine up-regulates α4β2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O'Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of β2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AP, Moutinho LM, Bettler B, Balerio GN. Acute behavioural responses to nicotine and nicotine withdrawal syndrome are modified in GABA(B1) knockout mice. Neuropharmacology. 2012;63:863–872. doi: 10.1016/j.neuropharm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend. 2010;108:7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schütz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Tajik R, Ebrahimi-Ghiri M, Nasehi M, Rezayof A. Role of the medial septum cholinoceptors in anxiogenic-like effects of nicotine. Physiol Behav. 2013;119:103–109. doi: 10.1016/j.physbeh.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res. 2012;14:1270–1290. doi: 10.1093/ntr/nts159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine A is a potent and selective agonist at native and recombinant α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]