Abstract
Converging epidemiological studies indicate that cannabis abuse during adolescence increases the risk of developing psychosis and prefrontal cortex (PFC)-dependent cognitive impairments later in life. However, the mechanisms underlying the adolescent susceptibility to chronic cannabis exposure are poorly understood. Given that the psychoactive constituent of cannabis binds to the CB1 cannabinoid receptor, the present study was designed to determine the impact of a CB1 receptor agonist (WIN) during specific windows of adolescence on the functional maturation of the rat PFC. By means of local field potential (LFP) recordings and ventral hippocampal stimulation in vivo, we found that a history of WIN exposure during early (postnatal day -P- 35-40) or mid-(P40-45) adolescence, but not in late adolescence (P50-55) or adulthood (P75-80), is sufficient to yield a state of frequency-dependent prefrontal disinhibition in adulthood comparable to that seen in the juvenile PFC. Remarkably, this prefrontal disinhibition could be normalized following a single acute local infusion of the GABA-Aα1 positive allosteric modulator Indiplon, suggesting that adolescent exposure to WIN causes a functional downregulation of GABAergic transmission in the PFC. Accordingly, in vitro recordings from adult rats exposed to WIN during adolescence demonstrate that local prefrontal GABAergic transmission onto layer V pyramidal neurons is markedly reduced to the level seen in the P30-35 PFC. Together, these results indicate that early and mid-adolescence constitute a critical period during which repeated CB1 receptor stimulation is sufficient to elicit an enduring state of PFC network disinhibition resulting from a developmental impairment of local prefrontal GABAergic transmission.
Keywords: prefrontal cortex, CB1 receptor, adolescence, GABA, cannabinoid, ventral hippocampus
Introduction
The onset of schizophrenia and addiction-related syndromes often occurs during the periadolescent transition period1–3. Although the neurodevelopmental processes underlying this vulnerability remain largely unknown, recent epidemiological studies suggest that the endocannabinoid system is implicated. Relative to adults, adolescent cannabis abusers are more likely to develop psychosis and cognitive impairments later in life4–8, indicating an association between cannabis abuse during adolescence and increased risk of schizophrenia. Of particular interest is the negative impact of long-term cannabis use on cognitive functions within the working memory and decision making domains7,9–11, many of which are refined during adolescence and dependent on the functional maturation of the prefrontal cortex (PFC)12,13. Similar long-lasting deficits on PFC-dependent behaviors have been observed in rodent models of chronic cannabinoid exposure, in particular when CB1 receptor agonists were administered during adolescence14–18. From these animal studies, and despite the complex mixture of natural cannabinoids present in cannabis19, it has been proposed that a sustained activation of CB1 receptor signaling in the PFC by exogenous cannabinoids could contribute to the detrimental cognitive effects seen in chronic cannabis abusers20,21.
At the cellular level, CB1 receptor stimulation reduces neuronal network oscillations in the beta- and gamma-frequency ranges (20–100Hz)22, functions that are also impaired in schizophrenia and thought to be due to reduced transmission of GABAergic interneurons in cortical circuits23,24. Interestingly, the functional maturation of local prefrontal GABAergic circuits also occurs during adolescence25,26, suggesting a mechanistic link between the developmental window of PFC maturation and the adolescent liability to the effects of CB1 receptor stimulation resulting from cannabis exposure20. Thus, the goal of the present study is to directly test the hypothesis that excessive stimulation of the CB1 receptor during adolescence is sufficient to elicit an enduring disinhibited PFC state resulting from a developmental impairment of local GABAergic transmission. Here, the impact of adolescent exposure to the CB1 receptor agonist WIN55,212-2 on prefrontal inhibitory responses was assessed in adulthood by means of local field potential (LFP) recordings in vivo combined with pharmacological manipulations and in vitro patch-clamp recordings of GABAergic transmission in rats. Changes in the pattern of prefrontal LFP exerted by ventral hippocampal train stimulation at 10, 20 and 40 Hz were compared across age and treatment groups. This stimulation protocol was chosen because of its sensitivity in detecting functional changes in the PFC resulting from a developmental disruption of local GABAergic transmission during adolescence26,27.
Materials and Methods
All experimental procedures were approved by the Rosalind Franklin University IACUC in accordance with the USPHS Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats (Harlan, IN) were allowed to acclimate for at least 5 days before receiving any experimental manipulation. They were group housed (2–3 rats/cage) in a 12:12 hour light/dark cycle room with food and water available ad libitum at 21–23°C. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except for WIN, Indiplon, and AM251, which were obtained from Tocris Bioscience (Ellisville, MO).
Experimental groups
Adolescent rats (postnatal days -P- 35-40, P40-45, P50-55) were randomly assigned to receive daily non-contingent single i.p. injections of the CB1 receptor agonist WIN (2 mg/kg in 1% DMSO/saline) or vehicle (1% DMSO/saline) for 5 consecutive days. This protocol was chosen from studies showing that 5 days treatment with cocaine or MK-801 during adolescence is sufficient to cause long-lasting PFC impairments26,27. The dose of WIN was chosen from the 1.2 to 3 mg/kg range known to produce behavioral and neuronal effects when given chronically28,29. All electrophysiological recordings were conducted in adulthood (P65-95 age period) and >25 days from the last WIN or vehicle injection. To determine whether the effects of WIN are mediated by CB1 receptor activation, the inverse agonist AM-251 (1.5 mg/kg in 1% DMSO/saline) was administered 20 min prior to WIN injection. Finally, a cohort of adult-treated rats (P75-80) was included to determine whether the enduring effects of WIN are age dependent.
Medial PFC local field potential recordings of ventral hippocampal-evoked responses in vivo
All recordings were conducted as previously described26,27. Briefly, rats were deeply anesthetized with 8% chloral hydrate (400 mg/kg), placed in a stereotaxic apparatus (ASI instruments, MI), and maintained at 37–38°C (Physitemp Instruments, NJ). Prefrontal LFP recordings (B: +3.2 to +2.7; L: 0.8; V: −4.2)30 were conducted using a bipolar concentric electrode (SNE-100× 50 mm; Rhodes Medical Instruments Inc., CA), amplified and filtered (bandwidth 1–100 Hz; Cygnus Technology Inc., PA), and digitized at a sampling rate of 10 kHz (Digidata 1440A, Molecular Devices, CA). A second bipolar concentric electrode (NE-100× 50 mm) was placed in the ventral hippocampus (B: −6.1 to −6.3; L: 5.2; V: −4.5) to deliver different patterns of stimulation. Single and trains of square pulses of 300 μs duration were delivered every 15 s through a computer-controlled pulse generator Master 8 Stimulator (AMPI, Jerusalem, Israel). The stimulation intensity was determined from the input-output response curve (from 0.25 to 1.0 mA) and measuring the peak amplitude of the evoked LFP that falls within the 75–80% range of the intensity curve (typically ~0.75 mA). Here, 3 frequencies of ventral hippocampal train stimulation (10 pulses at 10, 20, and 40 Hz) were compared across age and treatment groups. At the end of the recording session, the recording and stimulating sites were anatomically determined as previously described26,27.
Local PFC microinfusions of Indiplon
All PFC microinfusions (1 μl, 0.1 μl/min) of aCSF-containing vehicle or the GABA-Aα1 receptor positive allosteric modulator Indiplon (5 μM/0.02% DMSO) were performed with a 28-gauge cannula (Plastics One Inc., VA) secured to the recording electrode, as previously described26,27. The effects of Indiplon on prefrontal LFP were determined within the 30-minute post-infusion period.
In vitro whole-cell patch-clamp recordings of inhibitory postsynaptic currents in the PFC
All experimental procedures were conducted as previously described31,32. Briefly, recordings from layer V pyramidal neurons of the medial PFC (infralimbic and prelimbic regions; 350μm-thick coronal slices) were conducted at 33–35°C using a cesium-based internal solution (in mM): 140 CsCl, 10 HEPES, 2 MgCl2, 5 NaATP, 0.6 NaGTP, and 3 QX-314 (pH: ~7.25, 280–282 mOsm). The recording aCSF contains 2 mM kynurenic acid and (in mM): 122.5 NaCl, 3.5 KCl, 25 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1 MgCl2, 20 glucose, 1 ascorbic acid (pH: ~7.42, 295–305 mOsm). Recordings were included for analyses only if the neuron remained stable for at least 20 min after obtaining the whole-cell configuration (voltage-clamp mode at −70 mV). The mean frequency of spontaneous inhibitory postsynaptic current (IPSC) events was calculated from at least 3 non-contiguous epochs of 60 s.
Statistical analysis
Differences among experimental groups were considered statistically significant at p<0.05 (Statistica 6.0, Tulsa, OK). Student’s t-test was used for two-group comparisons whereas ANOVA was used for comparing the effects along two or more variables. Data are presented as mean ± SEM.
Results
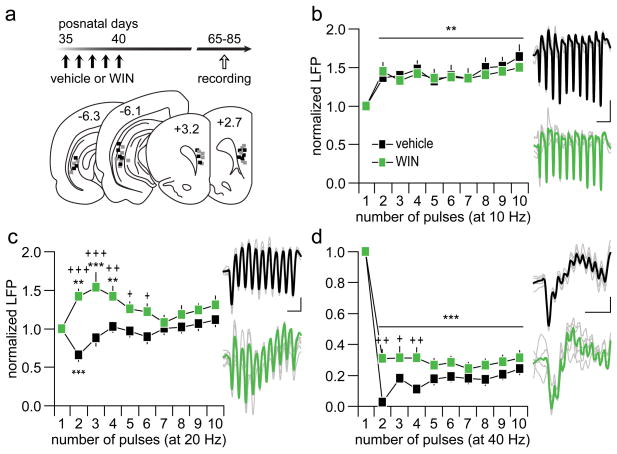
We first investigated how a history of repeated WIN treatment during early adolescence (P35-40) impacts the PFC network in adulthood by means of LFP recordings (Fig 1a). Results obtained from the input-output response curves indicate that PFC processing of single-evoked ventral hippocampal drive is not impaired by early adolescent WIN exposure (Supplementary Fig 1). However, the characteristic functional maturation of PFC response to ventral hippocampal train stimulation26 becomes impaired by early adolescent WIN exposure, in particular at 20 Hz and 40 Hz (Fig 1b–d). Typically, a facilitation of prefrontal LFP appears when a 10 Hz-train stimulation protocol is delivered into the ventral hippocampus (Fig 1b). This pattern of LFP facilitation was also found in the WIN-treated group, which is indistinguishable from the vehicle controls (Fig 1b). At 20 Hz, a transient attenuation of the LFP response was observed in the PFC of vehicle-treated rats (Fig 1c). Following early adolescent WIN exposure, a switch from transient attenuation to potentiation of the LFP emerges in the adult PFC at 20 Hz (Fig 1c). At 40 Hz, a distinct pattern of sustained suppression of LFP was observed in the adult PFC of vehicle- and WIN-treated rats (Fig 1d). However, the magnitude of this prefrontal LFP inhibition was significantly reduced in animals that received WIN treatment during early adolescence (Fig 1d). Interestingly, such frequency-dependent disruption of prefrontal LFP response was not observed when WIN exposure occurred in adulthood (P75-80, Supplementary Fig 2). Notably, the patterns of PFC response in early adolescent and adult vehicle-treated rats are indistinguishable despite the difference in age at the time of the recording (Fig 1 vs. Supplementary Fig 2). Together, these results show that a history of early adolescent WIN exposure selectively diminishes the frequency-dependent inhibitory response of prefrontal LFP to ventral hippocampal drive in an age-dependent manner.
Figure 1.
(a) Experimental design used to assess the impact of early adolescent (P35-40) exposure to repeated injections of the CB1 agonist WIN (2 mg/Kg, i.p.) or vehicle on prefrontal LFP responses in adulthood. (b) Adult rats (P65-85) that received vehicle (n=8) or WIN (n=9) treatment during P35-40 exhibited similar degrees of prefrontal LFP facilitation in response to hippocampal train stimulation at 10 Hz (main effect of pulse number, F(9,150)=3.3, **p<0.005, two-way ANOVA). (c) At 20 Hz, vehicle-treated rats responded with a transient LFP inhibition whereas a marked LFP facilitation was observed in the WIN-treated group (**p<0.005, ***p<0.0005 vs. first pulse, +p<0.05, ++p<0.005, +++p<0.0005 vs. vehicle, LSD post hoc test; main effect of treatment: F(1,150)=46.9, p<0.0005; treatment × pulse number interaction: F(9,150)=3.1, p<0.002, two-way ANOVA). (d) At 40 Hz, both vehicle and WIN-treated rats exhibited marked LFP depression in the PFC (main effect of pulse number: F(9,150)=74.3, ***p<0.0005, two-way ANOVA). However, a significant attenuation of the LFP inhibition was observed in the WIN-treated group (+p<0.05, ++p<0.005 vs. vehicle, LSD post-hoc test; main effect of treatment: F(1,150)=37.3, p<0.0005; treatment × pulse number interaction: F(9,150)=2, p<0.05, two-way ANOVA). Insets are example traces (vehicle: black; WIN: gray/green) of LFP recordings from the PFC during ventral hippocampal stimulation illustrating the effects shown in b, c and d (calibration bars: 3 μV/200 ms for b, 2 μV/100 ms for c and d).
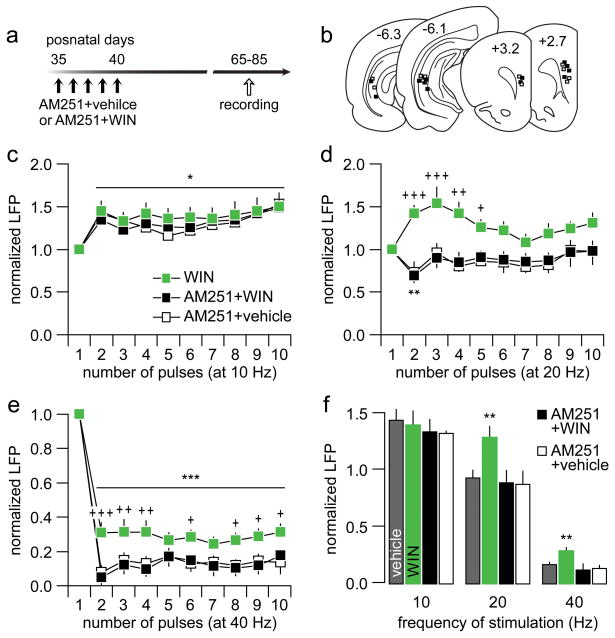
We next assessed whether activation of the CB1 receptor during early adolescence is responsible for the enduring effects of WIN on ventral hippocampal-evoked prefrontal LFP. To this end, P35-40 rats were pre-treated with the CB1 receptor inverse agonist AM251 20 min before the WIN injection to block the pharmacological action of WIN (Fig 2). As in the WIN-treated cohort, all LFP recordings were conducted in adulthood within the P65-85 age window (Fig 2a–b). Data show that the typical pattern of 10 Hz-induced prefrontal LFP facilitation remained unaltered following a history of AM251+WIN treatment (Fig 2c). At 20 Hz, the abnormal facilitation of prefrontal LFP observed in the early adolescent WIN-treated group was no longer detected following AM251 pre-treatment (Fig 2d). Instead, the normal pattern of 20 Hz-induced transient attenuation of the LFP response was observed in the PFC (Fig 2d). Similarly, AM251 treatment prior to WIN exposure prevented the attenuated 40Hz-induced prefrontal LFP observed in the WIN-treated group (Fig 2e). Interestingly, rats that received AM251 exposure alone (AM251+vehicle) exhibited normal patterns of LFP response to 20 and 40 Hz stimulation (Fig 2d–e). These data clearly demonstrate that AM251 effectively prevents the disrupting effects of early adolescent WIN treatment on prefrontal processing of ventral hippocampal inputs in adulthood (Fig 2f). Thus, the ability of early adolescent WIN exposure to interfere with the development of frequency-dependent inhibitory response in the PFC is attributable to repeated activation of the CB1 receptor.
Figure 2.
(a) Experimental design used to assess the impact of AM251 pretreatment on WIN exposure during early adolescence (i.e., P35-40). Data from the P35-40 WIN-treated group were included in c, d, e and f for comparison. (b) Schematic diagram of PFC and ventral hippocampal coronal sections summarizing all the recording and stimulating sites of the AM251 treatment cohort (AM251+vehicle: white; AM251+WIN: black). (c) The prefrontal LFP response to ventral hippocampal stimulation at 10 Hz remained unaffected by AM251 pretreatment (n=6; main effect of pulse number: F(9,130)=2.23, *p=0.024, two-way ANOVA). (d) In contrast, the abnormal 20 Hz-induced LFP facilitation found in the PFC of P35-40 WIN-treated rats was normalized by AM251 pretreatment (+p<0.05, ++p<0.005, +++p<0.0005 vs. AM251+WIN, LSD post-hoc test; main effect of treatment: F(1,130)=47.4, p<0.0005, two-way ANOVA). (e) Similarly, AM251 pretreatment prevented the attenuated 40 Hz-induced LFP inhibition observed in the PFC of P35-40 WIN-treated rats (+p<0.05, ++p<0.005, +++p<0.0005 vs. AM251+WIN, LSD post-hoc test; main effect of treatment: F(1,130)=41.1, p<0.0005; main effect of pulse number: F(9,130)=46.6, ***p<0.0005, two-way ANOVA). (f) Summary of the effects of AM251 pretreatment on WIN-induced disinhibition in the PFC. The average values were calculated from pulses 2 to 10. Note that the abnormal facilitation at 20 Hz was no longer observed in the AM251-pretreated group (**p<0.005 vs. vehicle, AM251+WIN or AM251+vehicle, LSD post hoc test; one-way ANOVA, F(3,25)=5.2, p=0.0065). At 40 Hz, AM251 pretreatment also normalized the magnitude of prefrontal LFP inhibition (**p<0.005 vs. vehicle, AM251+WIN or AM251+vehicle, LSD post hoc test; one-way ANOVA, F(3,25)=6.6, p=0.0017).
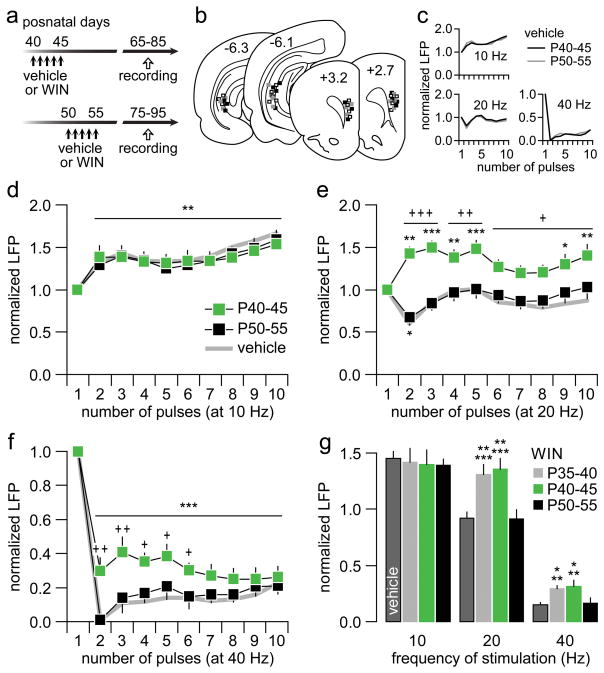
To further narrow the critical window of susceptibility within the P35-P55 adolescent period, two additional age groups of adolescent rats (P40-45 and P50-55) were subjected to repeated WIN treatment, and changes in prefrontal LFP responses to ventral hippocampal train stimulation were determined in adulthood (Fig 3a–c). These results show that the patterns of 10 Hz-induced prefontal LFP facilitation in the P40-45 and P50-55 age treatment groups are indistinguishable from each other and from the vehicle controls (Fig 3d). However, this was not the case at 20 Hz. A facilitation of the 20 Hz-evoked LFP emerged in the PFC of P40-45 WIN-treated rats whereas prefrontal recordings from the P50-55 WIN-treated group revealed a normal pattern of transient LFP inhibition similar to that observed in vehicle controls (Fig 3e). Likewise, only the P40-45 WIN-treated group exhibited the abnormal attenuation of prefrontal LFP suppression at 40 Hz as seen in P35-40 WIN-treated rats (Fig 3f). In summary, these results indicate that similar frequency-dependent deficits in prefrontal processing can be elicited when repeated WIN exposure occurs during early (P35-40) and mid-adolescence (P40-45), but not when WIN treatment is given after P50 (late adolescence; Fig 3g).
Figure 3.
(a) Experimental design for assessing the impact of WIN exposure at 2 age groups of adolescent rats. Note that all prefrontal LFP recordings were conducted in adulthood (white arrow). (b) Summary of the recording and stimulation sites as determined by means of histological analyses from Nissl-stained sections (vehicle: white; P40-45 WIN: back; P50-55 WIN: gray). (c) Both vehicle-treated groups exhibited comparable patterns of prefrontal LFP response to ventral hippocampal train stimulation (n=4 per age group). (d) As in the P35-40 age group (Fig 1), WIN treatment during the P40-45 (n=7) and P50-55 (n=7) adolescent periods did not alter the characteristic facilitation of prefrontal LFP response to ventral hippocampal stimulation at 10 Hz (main effect of pulse number, F(9,120)=4.5, p<0.0001, two-way ANOVA; **p<0.005 vs. first pulse, LSD post-hoc test). The thick gray line summarizes the pooled data obtained from both vehicle-treated groups (n=8). (e) At 20 Hz, two-way ANOVA revealed a significant main effect of age (F(1,120)=81, p<0.0001) and age × pulse number interaction (F(9,120)=2.1, p=0.034). Rats that received WIN treatment at the P40-45 period responded with a marked prefrontal LFP facilitation (*p<0.05, **p<0.005, ***p<0.0005 vs. first pulse, LSD post-hoc test) as seen in the P35-40 WIN-treated group (Fig 1). In contrast, rats that received WIN treatment after P50 exhibited the normal pattern of transient LFP inhibition (*p<0.03 vs. first pulse, +p<0.05, ++p<0.005, +++p<0.0005 vs. P40-45, LSD post-hoc test). (f) A robust inhibition of prefrontal LFP was observed in both P40-45 and P50-55 age groups at 40 Hz (main effect of pulse number: F(9,120)=33.5, ***p<0.0001, two-way ANOVA). However, the magnitude of inhibition in the P40-45 age treatment group was significantly less pronounced to that from the P50-55 WIN-treated group (main effect of age: F(1,120)=25.9, p<0.0001; +p<0.05, ++p<0.005, +++p<0.0005 vs. P50-55, LSD post-hoc test). (g) Summary of the LFP response recorded in the adult PFC of the 3 adolescent WIN-treated groups. The average values were calculated from pulses 2 to 10. Data from all vehicle-treated rats in the P35-40, P40-45, and P50-55 age groups were pooled. The abnormal facilitation of prefrontal LFP response at 20 Hz observed in P35-40- and P40-45-treated rats is absent in the P50-55 age treatment group (***p<0.0005 vs. vehicle, **p<0.005 vs. P50-55, LSD post-hoc test; one-way ANOVA, F(3,35)=10.9, p<0.0001). Similarly, the attenuation of prefrontal LFP at 40 Hz was observed only when WIN exposure occurred during P35-40 or P40-45, but not after P50 (**p<0.001 vs. vehicle, *p<0.01 vs. P50-55, LSD post-hoc test; one-way ANOVA, F(3,35)=8.1, p<0.0005).
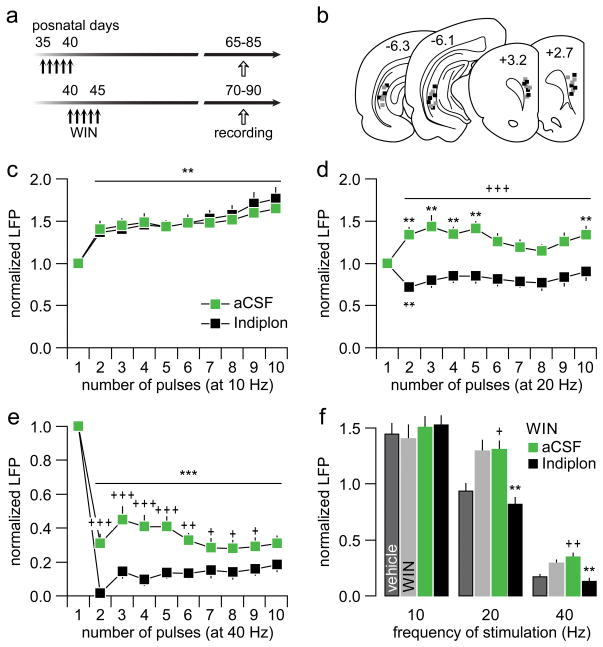
If the diminished suppression of prefrontal LFP response observed following a history of WIN exposure during early and mid-adolescence results from a downregulation of PFC GABAergic transmission26,27, we hypothesized that a local enhancement of GABA-A receptor function would be sufficient to restore the disinhibited/abnormal LFP response to normal control levels. To test this hypothesis, we infused the GABA-Aα1 receptor positive allosteric modulator Indiplon into the PFC of adult rats that were exposed to WIN treatment during P35-40 or P40-45 (Fig 4a–b). As expected, neither aCSF nor Indiplon administration into the PFC altered the characteristic 10 Hz-induced LFP facilitation (Fig 4c). However, data collected from the 20 Hz ventral hippocampal stimulation revealed that prefrontal infusion of Indiplon effectively reinstated the normal pattern of transient LFP attenuation as seen in the adult PFC of vehicle controls (Fig 4d). Similarly, the magnitude of the 40 Hz-induced prefrontal LFP suppression in the adolescent WIN-treated group was restored to normal levels following single PFC infusion of Indiplon (Fig 4e). This frequency-dependent reversal effect of Indiplon was specific to the changes induced by WIN since similar prefrontal infusions in the vehicle-treated group failed to alter the pattern or increase the degree of LFP inhibition (Supplementary Fig 3). Together, these results indicate that an upregulation of prefrontal GABA-Aα1 transmission is sufficient to overcome the enduring PFC disinhibitory state resulting from repeated WIN exposure during critical periods of adolescence (Fig 4f).
Figure 4.
(a) Impact of PFC delivery of the GABA-A α1 positive allosteric modulator Indiplon in adult rats that received WIN exposure during early (P35-40) or mid-(P40-45) adolescence. All aCSF solutions containing the same % vehicle used to dissolve Indiplon. Both age groups were chosen because WIN treatment during these two adolescent periods resulted in prefrontal LFP disinhibition (see Fig 1 and Fig 3). (b) Summary of the recording and stimulation sites for the aCSF (gray) and Indiplon (black) infusion groups. (c) Both aCSF (n=8)- and Indiplon (n=8)-treated PFC exhibited an identical pattern of LFP facilitation at 10 Hz (main effect of pulse number, F(9,140)=7.4, p<0.0001, two-way ANOVA; **p<0.005 vs. first pulse, LSD post-hoc test). (d) In contrast, the abnormal 20 Hz-induced LFP facilitation observed in the adult PFC of early and mid-adolescent WIN-treated rats is not longer present following local prefrontal infusion of Indiplon (main effect of treatment: F(1,140)=133.8, p<0.0001; treatment × pulse number interaction: F(9,140)=2.23, p=0.024, two-way ANOVA; *p<0.02, **p<0.005 vs. first pulse, +++p<0.0005 vs. Indiplon, LSD post-hoc test). Note that Indiplon completely restores the pattern of 20 Hz-induced LFP inhibition as seen in the normal control PFC (see Fig 1). (e) Similarly, the attenuated 40 Hz-induced LFP inhibition observed in the PFC of early and mid-adolescent WIN-treated rats is normalized following local infusion of Indiplon (+p<0.05, ++p<0.005, +++p<0.0005 vs. Indiplon, ***p<0.0005 vs. first pulse, LSD post-hoc test; main effect of pulse number: F(9,140)=58.1, p<0.0001; main effect of treatment: F(1,140)=87.7, p<0.0001; treatment × pulse number interaction: F(9,140)=2.6, p<0.01, two-way ANOVA). (f) Summary of the reversal effects of Indiplon. All average values were calculated from pulses 2 to 10. Data from the P35-40 age treatment group (Fig 1) were included for comparison. Note that the 20Hz-induced abnormal facilitation of prefrontal LFP (aCSF group) is not longer present following PFC infusion of Indiplon (**p<0.005 vs. aCSF or WIN, +p<0.05 vs. vehicle, LSD post-hoc test; one-way ANOVA, F(3,29)=10.3, p<0.0001). Similarly, the attenuated prefrontal LFP inhibition (aCSF group) at 40 Hz was normalized by local infusion of Indiplon (**p<0.005 vs. aCSF or WIN, ++p<0.005 vs. vehicle, LSD post-hoc test; one-way ANOVA, F(3,29)=11.7, p<0.0001).
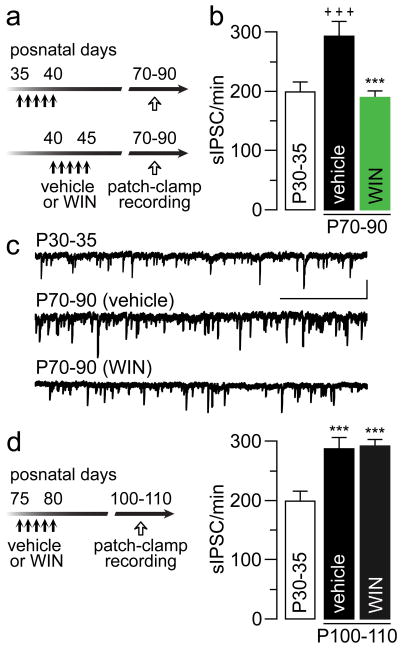
To determine whether GABAergic transmission in the adult PFC was indeed downregulated following repeated early/mid-adolescent WIN treatment, we conducted patch-clamp recordings in layer V pyramidal neurons to measure changes in spontaneous inhibitory postsynaptic current (IPSC) events (Fig 5a). Data obtained from naïve P30-35 rats were included to reveal that GABAergic transmission onto pyramidal neurons undergoes developmental upregulation in the PFC during the normal transition to adulthood (Fig 5b–c). Relative to the P30-35 age group, a significant 49% increase in prefrontal IPSC frequency was observed in the adult PFC of vehicle-treated rats. This facilitation was lacking after early/mid-adolescent WIN exposure (Fig 5b–c), despite the IPSC sensitivity to CB1 receptor stimulation remained unchanged (Supplementary Fig 4). In fact, the level of IPSC frequency recorded from the WIN-treated group was similar to that seen in the P30-35 PFC. In contrast, such downregulation in IPSC frequency was not observed in the PFC of adult-treated animals (Fig 5d). Thus, the attenuated IPSC transmission observed in the early/mid-adolescent WIN-treated group is due to an impairment of the normal facilitation of local GABAergic transmission occurring in the PFC during adolescence.
Figure 5.
(a) Experimental design used to determine the impact of WIN exposure on PFC GABAergic transmission. (b) Summary of the effects of adolescent WIN treatment on spontaneous GABAergic synaptic transmission (sIPSC/min) onto layer V pyramidal neurons of the medial PFC in adulthood. Data obtained from P30-35 naïve rats were included for comparison. A significant increase in the frequency of IPSC events was observed from P30-35 (n=21) to P70-90 (n=20, vehicle group; +++p<0.0005 vs. P30-40, LSD post-hoc test; one-way ANOVA, F(2,63)=12.1, p<0.0001). However, the mean IPSC frequency remains reduced in the WIN-treated group (n=23; ***p<0.0005 vs. vehicle, LSD post-hoc test). (c) Examples of spontaneous IPSC recordings from layer V pyramidal neurons illustrating the results shown in b (calibration bars: 50 pA/2.5 s). (d) Summary of the IPSC recordings from the adult-treated group (vehicle n=12; WIN n=12). Note that WIN exposure during adulthood failed to alter the frequency of IPSC as it did in the early/mid-adolescent group (***p<0.0005 vs. P30-35, LSD post-hoc; one-way ANOVA, F(2,42)=10.8, p<0.0002).
Discussion
The present study was designed to determine how repeated pharmacological stimulation of the CB1 receptor during adolescence impacts the functional maturation of the PFC network response to ventral hippocampal drive in adulthood. Using a non-contingent injection protocol and LFP recordings, we found that repeated activation of the CB1 receptor during early (P35-40) and mid- (P40-45) adolescence can trigger an enduring state of frequency-dependent prefrontal disinhibition in adulthood that resembles the response pattern seen in the juvenile PFC26. Such an impact on PFC function was not observed when WIN treatment occurred during late adolescence (P50-55) or adulthood (P75-80). Our data also indicate that the ability of early and mid-adolescent WIN exposure to interfere with the development of frequency-dependent inhibitory control is attributable to a downregulation of local prefrontal GABAergic transmission. Together, these results indicate that early and mid-adolescence constitute a critical period during which repeated CB1 receptor stimulation is sufficient to elicit an enduring state of PFC disinhibition resulting from a developmental impairment of local prefrontal GABAergic transmission.
Converging epidemiological data indicate that adolescent cannabis abusers are more likely to develop psychosis and PFC-related cognitive impairments later in life4–8. Studies in animal models also indicate that, relative to adults, adolescent rats are more susceptible to the chronic impact of cannabinoids (i.e., CB1 receptor agonists) as revealed by the development of persistent behavioral deficits in adulthood. These include cognitive deficits in the domains of working memory, object recognition, learning and memory as well as reduced social interactions and impaired sensorimotor gating14–18. However, little is known about the neural substrates accounting for the age-dependent, long-lasting behavioral effects of chronic cannabinoid exposure. It has been proposed that a disruption of PFC maturation resulting from excessive stimulation of CB1 receptors during sensitive periods of postnatal development plays a critical role in conferring such liability20. Accordingly, the frequency-dependent disinhibition observed in the adult PFC of WIN-treated rats resembles the immature state of input processing seen in juvenile animals 26. Our data also indicate that the effect of WIN on eliciting persistent PFC disinhibition is circumscribed to the early and mid-adolescent period of the rat (P35-45). Equivalent windows of susceptibility to cannabinoids that result in PFC-dependent cognitive deficits later in life have been reported in humans whose cannabis consumption started during adolescence. These include attention deficits, reduced executive functioning, and working memory, all of which are associated with an adolescent onset of cannabis abuse before age 1633–35. Thus, the deficits induced by exogenous cannabinoids are strictly age-dependent and delineate finite windows within early and mid-adolescence when overactivation of the CB1 receptor signaling may cause long-lasting PFC impairments. This is not entirely unexpected as all measurable variables of cellular, synaptic, and network functions in the PFC undergo dramatic remodeling during P35-50, but remain remarkably stable after P5025,26,31,32,36–40.
The pattern of PFC disinhibition observed in adolescent WIN-treated rats is indistinguishable from that elicited by local prefrontal infusion of the GABA-A receptor antagonist picrotoxin26,27. Although the use of chloral hydrate anesthesia may confound the interpretations of these findings, its known facilitatory action on brain’s GABA-A transmission48 points to an impaired GABAergic function underlying the long-lasting frequency-dependent PFC deficits observed following adolescent WIN exposure. The fact that strengthening prefrontal GABA-Aα1 function can normalize the enduring disinhibitory state further indicates that PFC GABAergic transmission is specifically compromised by insults received during early/mid-adolescence. Part of this susceptibility could be attributed to the functional maturation of specific GABAergic interneurons25 and the increase in GABAergic synaptic transmission (Fig 5) occurring in the PFC during this period. Although it remains unclear which GABAergic population mediates these prefrontal deficits, two interneuron subtypes, namely CCK-positive and parvalbumin (PV)-positive/fast-spiking interneurons, are known to be regulated by cannabinoids20. The abundant expression of CB1 receptors in CCK-positive cells would suggest that a disruption of this interneuronal subtype contributes to the prefrontal disinhibitory state observed following adolescent WIN exposure. However, this seems unlikely since the attenuated IPSC observed in the PFC of adolescent-treated rats appears to be attributable to the WIN-insensitive component of the GABAergic transmission (Supplementary Fig 4). Moreover, from the developmental perspective, PV/fast-spiking cells are the only GABAergic interneurons in the PFC that become functionally upregulated after P4525. One specific hallmark of this periadolescent maturation is the protracted facilitation of glutamatergic synaptic transmission onto PV/fast-spiking interneurons25. In this regard, we have proposed that excessive activation of CB1 receptors during critical periods of adolescent development (i.e., before P45) could reduce the level of glutamatergic drive needed for the functional maturation of prefrontal PV/fast-spiking interneurons. Both the reduced inhibitory tone onto pyramidal cells and the frequency-dependent disinhibition of the PFC network at 20 and 40 Hz observed here are likely due to a developmental impairment of local prefrontal GABAergic transmission.
While the goal of the present study was to isolate the effects of untimely CB1 receptor signaling in the development of prefrontal function, it is important to highlight that cannabis contains a numerous mixture of cannabinoids and flavanoids19, some of which have been shown to counteract the effects of Δ9-tetrahydrocannabinol (THC)41, the psychoactive constituent of cannabis and a partial agonist of the CB1 receptor42. In this regard, the recently reported benefits of cannabis use in schizophrenics’ cognitive functioning are intriguing43 considering that acute administration of THC alone induces a schizophrenia-like state in healthy controls44 and exacerbates cognitive and psychotic symptoms in schizophrenia patients45. Thus, we can only infer that such beneficial effects might be the result of other compounds in cannabis that act through a CB1-independent mechanism.
Our findings provide for the first time a potential mechanism for the detrimental, long-lasting effect of cannabis use during adolescence through a CB1-dependent disruption of GABAergic transmission in the PFC. Such an impairment is likely to contribute to the dramatic attenuation of prefrontal oscillations recently found in mice that received prolonged treatment of cannabinoids (i.e. WIN and THC) during adolescence15. Together with the epidemiological data associating cannabis use to schizophrenia, it is reasonable to conclude that altering the normative downregulation of CB1 receptor function in the PFC during adolescence37 through endogenous or exogenous cannabinoids is sufficient to elicit persistent deficits in local GABAergic transmission throughout adulthood. In this regard, any mechanism that interferes with the developmental regulation of CB1 receptor signaling could potentially alter the relationship between inhibitory and excitatory transmission in the PFC, and contribute to the development of cognitive deficits later in life.
In conclusion, the results of our present study have direct implications on the mechanisms that contribute to the long-lasting cognitive deficits resulting from early cannabis abuse, especially as the age of onset continues to decrease7. Furthermore, these findings should draw attention to the potential deleterious consequences of a new generation of synthetic, more potent CB1 receptor agonists (e.g., JWH-018, CP 47,497) currently available legally and illegally all over the world for which evidence in precipitating psychosis is accumulating46,47. Future studies aimed at determining the molecular mechanisms underlying the CB1 receptor-induced developmental deficits in prefrontal GABAergic transmission are warranted.
Supplementary Material
Acknowledgments
Supported by Rosalind Franklin University of Medicine and Science, the Brain Research Foundation and NIH Grant R01-MH086507 to KYT.
We thank Dr. Anthony West for helpful comments, and Ruvini Jayasinghe and Chanalee Hocharoen for technical assistance.
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
References
- 1.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 6.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 7.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakhsh MR, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440–447. doi: 10.1001/archgenpsychiatry.2010.6. [DOI] [PubMed] [Google Scholar]
- 9.Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176(3–4):239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- 10.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 12.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 14.O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18 (4):502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 15.Raver SM, Haughwout SP, Keller A. Adolescent Cannabinoid Exposure Permanently Suppresses Cortical Oscillations in Adult Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renard J, Krebs MO, Jay TM, Le Pen G. Long-term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: a strain comparison. Psychopharmacology (Berl) 2013;225(4):781–790. doi: 10.1007/s00213-012-2865-z. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28(10):1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 18.Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13(3–4):345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 19.Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Caballero A, Tseng KY. Association of Cannabis Use during Adolescence, Prefrontal CB1 Receptor Signaling, and Schizophrenia. Front Pharmacol. 2012;3:101. doi: 10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60(2):132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol Psychiatry. 2008;63(11):1075–1083. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26(31):8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0508-8. Feb 12, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomases DR, Cass DK, Tseng KY. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 2013;33(1):26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental Disruption of Gamma-Aminobutyric Acid Function in the Medial Prefrontal Cortex by Noncontingent Cocaine Exposure During Early Adolescence. Biol Psychiatry. 2013;74(7):490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagliaferro P, Javier Ramos A, Onaivi ES, Evrard SG, Lujilde J, Brusco A. Neuronal cytoskeleton and synaptic densities are altered after a chronic treatment with the cannabinoid receptor agonist WIN 55,212-2. Brain Res. 2006;1085(1):163–176. doi: 10.1016/j.brainres.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 29.Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Res. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- 31.Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic PKA and D1 dopamine receptor signaling. Biol Psychiatry. 2013 doi: 10.01016/j.biopsych.2013.07.033. Epub ahead of print Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heng LJ, Markham JA, Hu XT, Tseng KY. Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex. Neuropharmacology. 2011;60(6):953–962. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker B, Wagner D, Koester P, Bender K, Kabbasch C, Gouzoulis-Mayfrank E, et al. Memory-related hippocampal functioning in ecstasy and amphetamine users: a prospective fMRI study. Psychopharmacology (Berl) 2012;225(4):923–934. doi: 10.1007/s00213-012-2873-z. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 35.Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 36.Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY. Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3216-4. Aug 2, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65(4):278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15(1):49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 39.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17(5):1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61(10):843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loberg EM, Hugdahl K. Cannabis use and cognition in schizophrenia. Front Hum Neurosci. 2009;3:53. doi: 10.3389/neuro.09.053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 45.D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl) 2013;228(4):525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320–326. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 48.Lovinger DM, Zimmerman SA, Levitin M, Jones MV, Harrison NL. Trichloroethanol potentiates synaptic transmission mediated by gamma-aminobutyric acidA receptors in hippocampal neurons. J Pharmacol Exp Ther. 1993;264(3):1097–1103. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.