Abstract
This paper provides a review of the current literature addressing substance abuse treatment in persons living with HIV/AIDS. Clinical management of HIV must account for the “triple diagnosis” of HIV, psychiatric diagnosis, and substance use disorders and requires integrated treatment services that focus beyond just mitigation of substance use and psychiatric and medical symptoms but also address other health behaviors. Because clinical management of HIV/AIDS has shifted significantly with the advent of highly active antiretroviral therapies (HAART) in the mid 1990's, a literature review focusing on literature published since 2000, and using relevant key words was conducted using a wide range of literature search databases. This literature review was complemented by studies to expand on specific treatment modalities for which there was a dearth of literature addressing HIV infected cohorts and to provide discussion of issues around substance abuse treatment as an HIV prevention tool. Existing models of substance abuse treatment including cognitive behavioral therapy and motivational interviewing have proven to be useful for enhancing adherence and reducing substance use in outpatient populations, while methadone maintenance and directly observed treatment have been useful with specific subgroups of users. Contextualization of services heightens the likelihood of successful outcomes and relapse prevention.
Keywords: substance abuse, HIV, psychiatric disorder, triple diagnosis, treatment
Substance use and abuse are common among HIV positive individuals, with nearly 50% of persons living with HIV/AIDS reporting current or past histories of drug or alcohol disorders. 1,2 Substance use is associated with key health behaviors and outcomes including non-adherence, immunosuppression, increased sexual risk behaviors, and increased burdens on health care systems. 2-6 In general, HIV seropositive drug users have higher age matched morbidity and mortality when compared to non-drug using HIV seropositive cohorts. 7 In addition, drug use represents both a direct and indirect vector of transmission. Direct vectors of transmission occur through sharing contaminated injection equipment, while indirect pathways of risk and transmission largely result from increased likelihood of engaging in riskier sexual behaviors secondary to decreased inhibitions, poorer affect regulation, increased sexual arousal, and in some cases erectile dysfunction which may result in men being receptive partners. 8,9
Persons living with HIV/AIDS tend to underutilize substance use treatment 10,11, and this can be more pronounced in certain HIV seropositive subgroups including LGBT individuals. 11 Integrated care, particularly substance abuse treatment, is important in identifying and monitoring patients within this high-risk population presenting with scaffolded or ‘triply’ diagnosed conditions of HIV, substance abuse, and psychiatric comorbidities. 7,12-14 The triply diagnosed condition severely impacts patients' quality of life and exacerbates preexisting circumstances. 15 In addition, the portal of substance abuse treatment has ramifications for both HIV care and prevention,16 as help-seeking for a substance abuse diagnosis may represent an opportunity for testing, education, and connection to primary care. Despite awareness about the clinical needs of the triply diagnosed, 8,17 knowledge is still lacking on optimal modes of substance abuse treatment and outcome research in persons living with HIV/AIDS. 6
This paper provides a review of the current literature addressing substance abuse treatment in persons living with HIV/AIDS. The primary aim of this paper is to provide an overview of studies that highlight treatment outcomes in samples of HIV seropositive men and women over the age of 18 years, summarize the findings, and generate both clinical recommendations as well as suggesting areas that require further empirical study. In order to achieve this goal, the paper also addresses the epidemiology of drug and alcohol use in persons living with HIV/AIDS, and contextualizes the issue of substance use treatment within a framework of health outcomes, comorbid psychiatric disorder and symptomatology, and sexual risk. Because substance abuse treatment can impact and be impacted by these concomitant health issues, it is hoped that embedding these issues within that context will highlight the issue of integrated care.
The literature review was conducted using multiple literature databases to ensure that we covered research deriving from multiple disciplines related to this topic. Specifically, the search databases included AccessMedicine, PILOTS Database, Google Scholar, ProQuest Psychology Database, PsycArticles, PsychINFO, PubMed (includes MEDLINE) and employed keywords and various combinations of search terms as follows: substance abuse, substance dependence, substance use, drug abuse, drug dependence, drug use, alcohol abuse, alcohol dependence and alcohol use, HIV, AIDS, and treatment. The decision to choose articles published 2000 and later was largely driven by the fact that treatment, health outcomes, and survival times in persons living with HIV/AIDS shifted significantly after the mid-1990's with the advent of highly active antiretroviral therapies, and this choice of publication date was meant to draw from literature that is congruent with the contemporary treatment environment for HIV/AIDS. Additional literature was then utilized to build upon points raised by these treatment-focused studies, and to address the contextual factors listed above. Epidemiology statistics were derived from CDC publications and data. When literature prior to 2000 is cited, it was selected because it addressed populations who were underrepresented in the literature or provided subtext for substance abuse treatment issues raised by our initial review of the literature. Finally, a subset of articles that did not focus on substance abuse treatment in individuals already infected with HIV but rather on samples at risk for HIV by dint of substance use behaviors, psychopathology or sexual risk was utilized to complement the treatment literature as well as to address the utility of substance abuse treatment as an HIV prevention tool. After ruling out articles that were deemed inappropriate for our focus (e.g. focus on international samples, participants under the age of 18, examination of substance use treatment, but not substance use outcomes) we selected roughly eighty articles to use for this paper.
Rates of Drug and Alcohol Use in Persons Living with HIV/AIDS
Rates of lifetime and/or current substance use, abuse, and dependence are high in HIV seropositive cohorts when contrasted with comparable HIV seronegative cohorts, and significantly higher than observed in community samples. HIV seropositivity is observed in 30-40% of injection drug users.18 Data from the HIV Cost Services and Utilization Survey (HCSUS) reveal that among persons living with HIV who drank, 8% were heavy drinkers, and levels of drinking were higher in those abusing other substances such as cocaine and heroin. 19 Consistent with most large studies, in our cohort of 287 HIV+ adults enrolled in a study of psychopathology, substance use, sexual risk and adherence, we obtained lifetime rates of substance abuse/dependence of 84%, current rates of 13% with cannabis, alcohol, and cocaine most prevalent, and 13% of the sample currently met criteria for a dual diagnosis. 20
Substance Use and Health Outcomes
Substance use is directly associated with poorer health outcomes in individuals with HIV. Carrico et al 21 observed five-fold higher HIV viral load among those endorsing regular stimulant use compared to those who did not report stimulant use. A history of substance use also has implications for care, with HIV infected individuals with substance use histories less likely to receive highly active antiretroviral therapies (HAART) 22, and other clinical assessments such as viral load testing 23. Hepatitis C (HCV) is observed in 60-90% of HIV infected drug users. HCV/HIV co-infection can accelerate hepatitis C infection to end stage liver disease 7, and HCV is often undertreated in drug users with HIV. 24 In addition, bacterial infections can cause increased morbidity and mortality in injection drug users with HIV. 7
Substance abuse among HIV positive persons has been associated with nonadherence to HAART and other medications. 25 Alcohol use and drug use disorders are consistently associated with poorer HAART adherence, decreased health care utilization, and poorer immunologic and virologic outcomes. 6,26 Factors associated with non-adherence include poor health, comorbid psychiatric conditions, cognitive impairment, psychosocial factors, demographic factors, substance use and abuse, and health beliefs, as well as interactions between these variables. 27,28 Hinkin et al29 found current substance users were over four times more likely to not adhere to medication than those not using illicit substances. This suggests that substance abuse treatment programs allow a platform for care providers to openly address not only substance abuse but also adherence to HAART,30 and suggest that substance abuse treatment is an essential part of HIV related primary care. When care is not integrated, triply diagnosed patients often drop out of treatment due to fear, stigma, and denial resulting in continued and escalating substance abuse, escalation of psychiatric symptomatology, reduced HAART adherence, increased immunosuppression, and viral replication.31
Substance Use and Risk
Increased sexual risk taking is frequently observed in HIV seropositive individuals engaged in substance use. Stimulant use has been consistently associated with increased sexual risk behavior,32,33 especially among heavy users. 34 This may in part be probabilistic given the high rates of HIV infection in methamphetamine using cohorts (estimates as high as 61% in some cohorts), and the likelihood of users drawing partners from these cohorts. 34 Overall, the empirical research has suggested that drug use, psychiatric symptomatology, and sexual risk behaviors operate synergistically. In addition, drug use patterns vary across groups as a function of ethnicity, gender and sexual orientation which can place different groups at differential risk35,36 (refer to Meade et al37 for a more comprehensive review of HIV risk and mental illness). From a prevention perspective, standardized integration of HIV testing into substance abuse treatment is an essential prevention tool, and while outpatient substance use treatment programs are increasingly offering testing, there have not been significant increases in community outreach which provides a key educational and prevention tool among drug users. 38 Substance abuse treatment services represent a rich opportunity to reduce incident infections by addressing injection drug use as well as other HIV risk behaviors. 38
Uptake of Substance Abuse Treatment
Factors associated with the utilization of outpatient substance use treatment among persons with HIV include lower income levels, lower educational levels, ethnicity, sexual orientation, composition of social networks, psychiatric symptomatology, homelessness, trauma history and lower CD4 count.11,39,40 In one sample of 951 HIV seropositive adults, while 71% reported substance use, only 24% reported receiving substance abuse treatment, and fewer than 50% reported discussing substance use with HIV medical providers (and this was pronounced in older adults), despite patient-provider discussions about substance use associated with a greater likelihood of entering treatment. 41 As such, training of providers to initiate such discussions may represent a bridge to facilitate the passage of HIV infected drug users into substance abuse treatment.
Inpatient services for substance abuse are often the least accessible for clients seeking substance abuse treatment services. This is largely due to the prohibitive cost of inpatient substance use services. HIV disproportionately impacts individuals with limited economic resources and who lack health insurance or benefits that would cover the cost of inpatient treatment. In general, studies addressing substance abuse treatment in persons living with HIV/AIDS have found that low-cost and no-cost options such as 12-step and self-help programs are most commonly used.11,41 Practical barriers to treatment initiation and retention such as transportation, can be ameliorated by simply providing transportation assistance. 41 One review revealed that in a subset of women, a diagnosis of HIV was the impetus for attempts at abstaining from drugs or initiation of treatment, though stressors such as parenting and economic stressors would often contribute to relapse after initial attempts at abstaining from drug use. 36 More frequently observed was an intensification of drug use in the face of an HIV diagnosis. In addition, threats of custody loss often motivated women to initiate treatment programs and avoid relapse. 36 Treatment services for women in particular must be responsive to caregiving issues, and strive to provide support structures to assist with caregiving duties while also harnessing the motherhood role as a reinforcer of sobriety and abstinence.
The Role of Psychopathology
Among HIV-positive individuals 10-28% have co-occurring psychiatric and substance abuse disorders. 6 Recurring psychiatric comorbidities presented by HIV-positive persons include: depression, mood disorders, psychotic disorders, anxiety disorders, adjustment disorders, personality disorders, sleep disorders, and other somatic complaints, with estimates of psychiatric disorders in infected cohorts at 50% and higher 1,34,42, and 10-25% of persons living with HIV/AIDS dually diagnosed. 19,43 Mood disorders are most common in HIV infected cohorts with overall prevalence rates suggesting that 10% of adults with HIV present with major depressive disorder and overall are at elevated risk for developing major depressive disorder. 44 HIV is typically not the direct contributor to the epigenesis of psychiatric symptoms 2, but rather the burdens of illness, medical symptoms, and psychosocial factors such as stigma and grief can result in the development of symptoms or the exacerbation of pre-existing psychiatric conditions.
The present of triple diagnosis can impede engagement in substance abuse treatment, magnify the deleterious consequences of drug and alcohol use, and increase the likelihood of relapse and/or treatment withdrawal. 6 The intersection of HIV, mood disorders, and substance use is associated with lower medication adherence and increased sexual risk behaviors. 45 Treatment of mental health issues combined with substance abuse treatment not only is key in mitigating or ending substance use/abuse, but can also assist with adherence to ART and address risk behaviors. Carrico et al21 suggest that the mechanism of increased affect regulation is crucial as it enhances positive affect and subsequently enhances the efficacy of adherence interventions. Because the presence of depressive symptoms is associated with risk, nonadherence and higher levels of alcohol consumption 46, addressing these symptoms becomes a key clinical and prevention tool.
Farley et al 47 highlight the central role of trauma in substance abuse treatment with nearly 90% of individuals seeking treatment for substance use disorders reporting a history of trauma. Factors associated with trauma such as higher rates of mental disorders, and increased rate of relapse can further complicate substance abuse treatment. Trauma is often cited as a precipitating event for the initiation of drug use, particularly in women. 36 Rates of PTSD in HIV seropositive samples, while variable, remain high with estimates ranging from 5-50% depending on the composition of the cohort. 48 Machtinger et al 49 report rates of PTSD in their sample of HIV seropositive women at 30% compared to population prevalence rates of 5%. In our community sample of 288 HIV seropositive men and women, we observed a lifetime rate of PTSD of 16% but among those who met lifetime criteria for PTSD, 91% also had a history of substance abuse or dependence. 20 Given that a high proportion of persons with HIV report trauma – the addition of trauma to the triple diagnostic picture necessitates awareness of trauma in an integrated treatment model.8 Recognition that clients with trauma histories may require additional support, more protracted periods of trust building with systems and practitioners, longer term therapy, and higher risk of relapse must inform integrated treatment models.
Social support has been found to be a protective factor among those who are triply diagnosed – mediating relationships between psychopathology and substance use. Social support may be a critical treatment variable given the high levels of stigma that persons with HIV face in addition to other marginalized statuses including racial/ethnic minority status, sexual orientation and psychiatric diagnosis. To the degree social support is a buffer against substance use, then shoring up and developing support networks remains a key element of substance use treatment,50 especially since dispensing with former substance using networks is associated with better outcomes. Substance use may serve as a short term coping tool for individuals with depressive symptoms in HIV, which can result in increased depressive symptoms, poor affect regulation, and faster disease progression.21 In addition, substance use may escalate in persons recently diagnosed with HIV.42
The neuropsychiatric portrait of HIV and specifically HIV Associated Neurocognitive Disorder (HAND) exists on a continuum ranging from mild cognitive impairment to full-blown dementia. Triple diagnosis can interact with these central nervous system (CNS) changes to result in greater functional impairment, poorer adherence, and poorer health outcomes.29,51 HAND may be pronounced in persons not receiving HAART, and given that substance users are often underprescribed HAART, they may be more vulnerable to cognitive symptoms, especially in light of premorbid CNS impacts from a history of substance use as well as other risk factors including head injury, preexisting neurologic conditions, and environmental issues such as poor education and poverty.
The chronic nature of personality disorders makes assessment and recognition of these disorders an integral element in providing efficacious treatment of substance use disorders in persons with HIV/AIDS. Widely varying prevalence estimates of personality disorder in HIV infected samples have been noted with studies employing structured diagnostic interviews reporting prevalence rates of approximately 19-33%.52,53 Disorders characterized by behavioral, interpersonal and affective instability (Cluster B) are most prevalent in HIV + samples, with borderline (BPD) and antisocial (ASPD) personality disorder most common.53-56 In our sample of 288 HIV+ adults, antisocial (17%), avoidant (6.3%) and borderline (5.6%) PD were most common. 20
These disorders are often characterized by behavioral patterns that are associated with HIV risk, including impulsive sexual behavior and substance abuse. Thus, a PD may represent a risk factor for acquisition of HIV. After infection, those with PD are likely to continue engaging in risky behaviors that increase their risk for sexually transmitted infections, exposure to other infectious diseases, and other health problems associated with substance abuse as well as the risk of infecting others. HIV seropositive persons with antisocial personality traits showed a greater propensity to engage in injection drug use. 57 Clinicians traditionally believe that patients with PD and substance use disorders are likely to have poorer outcomes, though studies suggest that while pre-treatment and post treatment problem severity may be greater, improvement in treatment is not impacted by the presence of PD. 58 In a cohort of persons with HIV, narcissistic personality was associated with a greater likelihood of sex following substance use, higher numbers of partners, more unprotected sex acts, unprotected sex with seronegative partners, and lower condom intentions. 59 Because narcissism as a trait may impact engagement in treatment, it may be important to target key behavioral issues such as impulsivity, cognitive errors such as inaccurate probability estimates about transmission, and maladaptive attitudes such as entitlement. 59 As noted above, trauma is endemic in HIV infected populations, and is often a risk factor for development of personality disorders such as borderline personality which has also been associated with a greater risk of behavioral impulsivity as observed in substance use and sexual risk taking.60
Substance Abuse Treatment: Overview
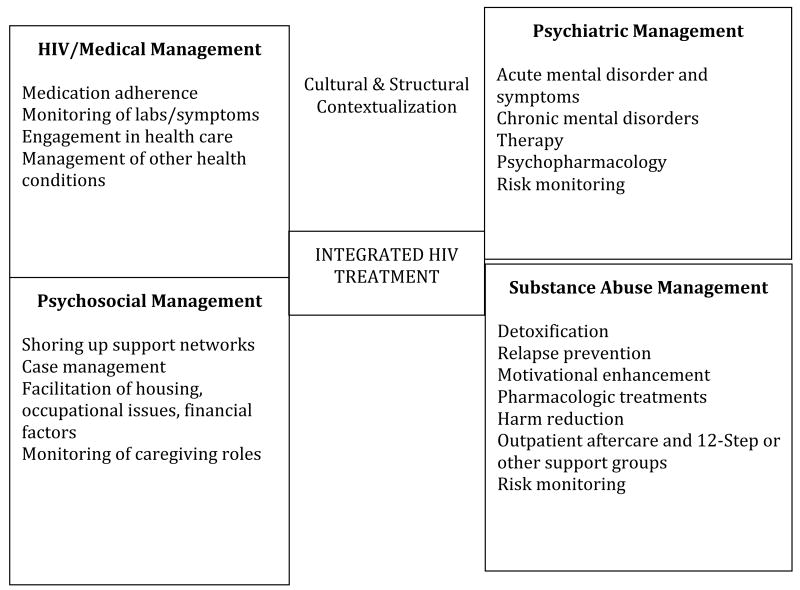
Halkitis61 notes that “HIV cannot be considered in isolation. The HIV epidemic is inextricably linked to other health and social conditions including, but not limited to psychological comorbidities, substance abuse, poverty, and discrimination.”61 Substance abuse treatment in HIV requires intersectional conceptualization and reliance on models such as the biopsychosocial framework to contextualize and treat. 61 Effective substance abuse treatment of persons with HIV requires illuminating the interconnectivity of the triple diagnosis of HIV, substance abuse, mental health disorders and syndromes62 and embedding substance use treatment in an integrated model of treatment (SEE FIGURE 1).
Figure 1. Model of Integrated HIV Treatment.
Substance abuse treatment of HIV positive persons occurs on a continuum of (1) individuals becoming aware of their HIV status, (2) engaging in HIV, psychiatric and substance abuse related care, and (3) avoidance of relapse and continued adherence to treatment; with the recognition that these steps can be bidirectional.25 Lapses and setbacks in psychiatric symptomatology, drug use and adherence behaviors should not only be expected but also planned for. Health care providers administering substance abuse treatment, psychiatric care, and HIV services vary in education, training, opinion, attitude, and experience, which often allows for varied treatment approaches, continuum specificity, and outcomes. Furthermore, lack of acceptance and communication among providers often causes patients to receive contradictory explanations of their conditions and disorders which lowers hope and confidence in their care providers. 63
Substance Abuse Treatment Techniques and Outcomes in the Triply Diagnosed
The body of literature directly addressing the provision of substance abuse treatment to HIV infected individuals is limited. The research is often less focused on the efficacy of treatment vis-à-vis substance use outcomes and instead focuses on other outcomes such as adherence. Because substance abuse treatment is often embedded in larger systems of integrated care, teasing out the impact of substance abuse treatment per se is often a challenge. With these limitations in mind, the literature revealed that the primary modalities of substance abuse treatment models in persons living with HIV included cognitive behavioral therapy, social support/support group models, motivational enhancement/motivational interviewing, transtheoretical frameworks, and directly observed therapy.
Methadone maintenance treatment programs remain central to the management of drug use in HIV given the high proportion of seropositive IDUs. Methadone maintenance programs have been shown to be effective treatments for opioid dependent persons living with HIV/AIDS, and have shown additional utility in providing medical management, psychiatric care, slow disease progression, and mitigate risk behaviors 64. Other pharmacological treatments that have been considered for use in individuals with HIV include the use of lamotrigine in HIV seropositive persons using crack cocaine 65. The use of pharmacologic treatments for opioid, alcohol and tobacco use and abuse including methadone, buprenorphine, naltrexone, disulfiram, varenicline, buproprion, and nicotine replacement products have not been consistently studied in HIV, and there are numerous potential interactions between pharmacologic products used to manage substance use, psychiatric medications and HIV medications. As such, the use of pharmacologic management of substance use in triply diagnosed patients raises additional challenges. 7
Models such as methadone maintenance also highlight the role of Directly Observed Therapy (DOT) in triply diagnosed individuals. DOT is typically employed in settings such as methadone maintenance programs, day treatment, and incarceration. It has provided variable outcomes in terms of improved health outcomes such as increased CD4 count and decreased HIV viral load,66 and improved medication adherence, but the findings have been context dependent with less consistency and efficacy with incarcerated populations.17 While DOT is not a practicable solution for most clients, the key components of DOT – structure, support, and routine, are suggested as the ingredients that may facilitate its success as an intervention, and to the degree possible should be built into all integrated care.
Cognitive behavioral treatment (CBT) models for substance abuse treatment in HIV infected samples have revealed reductions in alcohol use, withdrawal and dependence symptoms, functional impairment, depressive symptoms and maladaptive behaviors such as delinquency in selected cohorts. 67,68 However results are often variable, and may not necessarily generalize across drugs of abuse. These models typically relied on traditional cognitive behavioral techniques in conjunction with behavioral tools such as contingency management and behavioral activation. Shoptaw et al69 compared Gay Specific Cognitive Behavioral Therapy and Gay Specific Social Support Therapy. This CBT model focused on building skills such as identifying relapse triggers, interrupting cravings for drug use, and return to abstinence, while contextualizing the treatment in a framework that remained responsive to the societal and interpersonal experiences of gay men. Participants in both conditions showed a two-fold decrease in substance use and sexual risk behaviors, but among those using methamphetamine, CBT was more useful in sustaining reduction in use.
Cognitive behavioral interventions with HIV infected substance using cohorts have also been found to have differential impacts on behaviors such as adherence 17 with some studies revealing improvements in depression, but not adherence. 70 Substance use treatment itself has been suggested to be a useful adherence intervention tool, with the transition from using to non-use of drugs being associated with improved adherence. 71,72 Again, this has not been consistent across studies, with participation in substance use treatment associated with greater access to HAART but not necessarily greater adherence,30 and others noting that it was the receipt of psychiatric care, and not necessarily substance abuse treatment that enhanced adherence. 17
An intervention based on the transtheoretical model 73 used a continuum of care which targeted clients' differential state of readiness for change. 74 The model focused on establishment of the therapeutic relationship, individual client assessment, client education about triple diagnosis, motivational enhancement and goal setting. The intermediate phase of treatment included shoring up coping skills, building social support, engaging in meaningful activities, developing a consistent sense of self, and addressing grief and loss issues. The final phase of treatment was centered on maintenance of change and preparing for setbacks. Both individual and group treatment were employed, as well as psychopharmacology as needed. This program proved to have good outcomes, with decreases in substance use and psychiatric symptoms,
The extant literature has highlighted the utility of motivational interviewing as an evidence based tool for substance abuse treatment across populations. 75 Motivational interviewing (MI) and skills training tailored for HIV have shown good outcomes both for drug use behaviors and adherence. Studies with small samples have demonstrated MI to be a feasible treatment model with HIV seropositive men and women and to demonstrate reductions in substance use and improvements in medication adherence. 76 Ingersoll et al77 developed a 6 session motivational interviewing and feedback and skills building program which they compared to video information plus debriefing intervention (which was largely educational in nature) – in a sample of HIV seropositive crack cocaine users who reported poor medication adherence. Both interventions increased adherence and decreased problems due to drug use and number of days of crack cocaine use, and highlighted the potential utility of lower-cost interventions such as video training which may work well with subgroups of HIV seropositive drug users. 77 MI has also been integrated into methadone maintenance programs and has resulted in less risky sex in these cohorts.
Contextualized treatment programs such as the Structural Ecosystems Therapy employed by Feaster et al78 focus on the social environment, facilitation of adaptive interactions, and reduction of maladaptive interactions between HIV seropositive women and systems including families, health care, and community organizations. This intervention, which was conducted with 126 HIV+ women in recovery resulted in decreases in the proportion of women living with an active substance user and greater drug treatment utilization, though significant gains were not made with medication adherence. These findings suggest that addressing structural and contextual factors in any substance abuse intervention, especially with triply diagnosed individuals, may foster relapse prevention, bolster social networks, and motivate ongoing health promotion.
Given the higher rates of personality disorders including borderline personality disorder (BPD) in HIV infected samples, treatment models such as dialectical behavior therapy (DBT) that have shown utility for symptom management in BPD may prove efficacious for management of triply diagnosed individuals with BPD. 60 The adaptation employed by these investigators involves opening up the framework of DBT to specifically address targets such as adherence, and mindfulness in HIV specific contexts. DBT uses a 4-stage system that entails pretreatment targets and commitment to therapy, first stage targets of stability, connection and safety, second stage targets of exposure and emotional processing of the past, and third stage targets of individual goals. While little outcome data exists for the use of DBT in substance using HIV seropositive cohorts, the use of this technique has resulted in promising outcomes for a group that is likely at high risk for triple diagnosis.
Particularly in the triply diagnosed, integrated care models 12 may provide the best means of simultaneously addressing multiple clinical issues (Figure 1). In one study examining an integrated care model that coordinated AIDS service agencies, case management, primary care and substance abuse treatment, the authors found that the participants in the program had more medical visits than those not in the program, though they also found that those in the program had lower CD4 counts (which was believes to be an artifact of the medical acuity of this sample). In general, in non-HIV infected samples, research on integrated care models for dually diagnosed patients suggest that such programs yield better outcomes because both psychiatric and substance use treatment are brought together simultaneously, minimizing duplication of services and contradictory messages79 and this becomes even more critical in the triply diagnosed where coordination of HIV management and primary care is essential to clinical management.
Cultural Factors and Intersectionality
Disparities in the prevalence of HIV/AIDS as a function of ethnicity, sexual orientation, socioeconomic status and gender highlight the issue that treatment programs must be culturally responsive. The intersectionality of these variables can raise even greater challenges, with ethnic minority women requiring very different treatment models than gay men. Issues such as stigma differentially impact various groups, and as such, may need to be addressed differentially in treatment. Focus group studies with African American men highlight the utility of extant community structures such as Black churches, fostering open dialogue about sexual behavior and drug use, and capacity building for families.15 Halkitis35 also cites the interaction between person and environment when confronting sensitive issues such as bathhouses as a venue for prevention messages in gay men. Because many interventions, particularly adherence interventions, are individually targeted, they may also be less useful with inner city ethnic minority group individuals as these models often overlook systemic issues such as poverty, community and family. 80 HIV/AIDS remains a global health crisis with triple diagnosis endemic in settings where psychiatric and substance use treatment is not often available. Internationally, additional treatment barriers include human rights violations against those who are infected, abusive and criminal treatment of IDUs and LGBT individuals, and lower availability of HIV care. 81
Summary
The complexity of clinical needs among triply diagnosed individuals and persons living with HIV/AIDS who require substance abuse treatment mandates treatment services that are comprehensive, integrated, continuous, and culturally responsive. Integrated care systems must focus beyond just mitigation of substance use and psychiatric symptoms but also serve as a portal to address issues such as sexual risk, self-care, and adherence. Existing models of psychotherapy and substance abuse treatment including cognitive behavioral therapy and motivational interviewing have proven to be useful for both enhancing adherence as well as reducing substance use in outpatient populations, while methadone maintenance and other directly observed treatment models have been useful with more acute populations or those already in daily treatment settings. Contextualization of such services in a way that accounts for structures including social networks, communities, and family likely augment successful outcomes and relapse prevention.
There is a relative dearth of literature specifically focusing on substance abuse treatment in persons living with HIV/AIDS, a notable deficit given the high rates of substance abuse in this population. Comorbid medical and psychiatric conditions and complex cofactors such as poverty, access to care, and psychosocial variables make assessment and interpretation of results challenging and make it difficult to disentangle treatment effects. Nonetheless, randomized clinical trials, and outcome studies of existing treatment programs focused on persons with HIV/AIDS, particularly in high risk groups and those using substances that augment sexual risk (e.g. methamphetamine). A large proportion of substance abuse treatment research focuses not just on drug use, but also other health behaviors such as adherence to other treatments. Ongoing research that looks at integrated care models is also necessary to achieve empirically validated recommendations on best practices for working with substance using clients living with HIV/AIDS. In addition, as technology becomes more available to a wider range of clients and adaptable to a variety of treatment paradigms, it is strongly suggested that research agendas focusing on the use of technology as a tool to mitigate risk, minimize relapse, and foster health behaviors.
Treatment programs that provide services to both HIV seropositive and seronegative cohorts can serve as portals of prevention, and HIV testing and prevention messages should be standard of care in substance abuse treatment programs. The goal of fast track abstinence in these populations may be untenable. Myriad other clinical issues including physical/medical symptoms, mood symptoms, psychosocial factors, and burdens due to economic factors mean that this is a marathon and not a sprint, and triage of clinical goals will be driven by biological, psychological, and social exigencies.
The global burden of disease due to mental and substance use disorders pose significant challenges to health care systems globally. Too often, mental health and substance use issues have been set apart from “medicalized” health care – and this false dichotomy can result in undertreatment of persons with mental health issues, substance abuse disorders, and chronic illnesses such as HIV/AIDS 82 or greater burden of disease as a result of “dis”-integrated care. Globally, management of mental illness and substance use disorders are emerging as key elements of risk reduction, primary care, and amelioration in quality of life and overall health in persons living with HIV/AIDS.
Acknowledgments
This work was supposed in part by NIMH Grant 1SC1MH093181-01A1.
References
- 1.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the united states. Arch Gen Psychiatry. 2001;58(8):721. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Rabkin JG, McElhiney MC, Ferrando SJ. Mood and substance use disorders in older adults with HIV/AIDS: Methodological issues and preliminary evidence. AIDS. 2004;18:43–48. [PubMed] [Google Scholar]
- 3.Masson C, Sorensen J, Phibbs C, Okin R. Predictors of medical service utilization among individuals with co-occurring HIV infection and substance abuse disorders. AIDS Care. 2004;16(6):744–755. doi: 10.1080/09540120412331269585. [DOI] [PubMed] [Google Scholar]
- 4.Conigliaro J, Madenwald T, Bryant K, et al. The veterans aging cohort study: Observational studies of alcohol use, abuse, and outcomes among human immunodeficiency Virus–Infected veterans. Alcoholism: Clinical and Experimental Research. 2004;28(2):313–321. doi: 10.1097/01.alc.0000113414.73220.21. [DOI] [PubMed] [Google Scholar]
- 5.Kelley JL, Petry NM. HIV risk behaviors in male substance abusers with and without antisocial personality disorder. J Subst Abuse Treat. 2000;19(1):59–66. doi: 10.1016/s0740-5472(99)00100-2. [DOI] [PubMed] [Google Scholar]
- 6.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients. Drugs. 2006;66(6):769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 7.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. The Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinkenberg W, Sacks S, for the Hiv/aids Treatment Adherence, Health Outcomes and Cost Study Group Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care. 2004;16(sup1):22–42. doi: 10.1080/09540120412331315303. [DOI] [PubMed] [Google Scholar]
- 9.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: Crystal methamphetamine drug use in relation to HIV transmission. J Homosex. 2001;41(2):17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RB, Rotheram-Borus MJ, Johnson MO, et al. Insurance coverage, usual source of care, and receipt of clinically indicated care for comorbid conditions among adults living with human immunodeficiency virus. Med Care. 2005;43(4):401–410. doi: 10.1097/01.mlr.0000156850.86917.f8. [DOI] [PubMed] [Google Scholar]
- 11.Burnam MA, Bing EG, Morton SC, et al. Use of mental health and substance abuse treatment services among adults with HIV in the united states. Arch Gen Psychiatry. 2001;58(8):729. doi: 10.1001/archpsyc.58.8.729. [DOI] [PubMed] [Google Scholar]
- 12.Zaller N, Gillani F, Rich J. A model of integrated primary care for HIV-positive patients with underlying substance use and mental illness. AIDS Care. 2007;19(9):1128–1133. doi: 10.1080/09540120701335196. [DOI] [PubMed] [Google Scholar]
- 13.Douaihy A, Daley D, Stowell K, Park T. Relapse prevention: Clinical strategies for substance use disorders. Therapist's guide to evidence-based relapse prevention. 2007:37–73. [Google Scholar]
- 14.Gupta M, Kumar K, Garg P. Dual diagnosis vs. triple diagnosis in HIV: A comparative study to evaluate the differences in psychopathology and suicidal risk in HIV positive male subjects. Asian Journal of Psychiatry. 2013 doi: 10.1016/j.ajp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Buseh AG, Stevens PE, McManus P, Addison RJ, Morgan S, Millon-Underwood S. Challenges and opportunities for HIV prevention and care: Insights from focus groups of HIV-infected african american men. Journal of the Association of Nurses in AIDS Care. 2006;17(4):3–15. doi: 10.1016/j.jana.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Brown LS, Jr, Kritz SA, Goldsmith RJ, et al. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: The national drug abuse treatment clinical trials network. J Subst Abuse Treat. 2006;30(4):315–321. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uldall K, Palmer N, Whetten K, Mellins C. HIV/AIDS treatment adherence, health outcomes and cost study group. adherence in people living with HIV/AIDS, mental illness, and chemical dependency: A review of the literature. AIDS Care. 2004;16(1):S71–S96. doi: 10.1080/09540120412331315277. [DOI] [PubMed] [Google Scholar]
- 18.Francis H. Substance abuse and HIV infection. Top HIV Med. 2003;11(1):20–24. [PubMed] [Google Scholar]
- 19.Galvan FH, Burnam MA, Bing EG. Co-occurring psychiatric symptoms and drug dependence or heavy drinking among HIV-positive people. J Psychoactive Drugs. 2003;35(sup1):153–160. doi: 10.1080/02791072.2003.10400510. [DOI] [PubMed] [Google Scholar]
- 20.Durvasula RS. Progress report: Psychopathology, decision making and sexual risk. 2013. [Google Scholar]
- 21.Carrico AW, Johnson MO, Moskowitz JT, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007;69(8):785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 22.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. JAIDS J Acquired Immune Defic Syndromes. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 23.Raboud JM, Abdurrahman ZB, Major C, et al. Nonfinancial factors associated with decreased plasma viral load testing in ontario, canada. JAIDS J Acquired Immune Defic Syndromes. 2005;39(3):327–332. doi: 10.1097/01.qai.0000143603.94728.b2. [DOI] [PubMed] [Google Scholar]
- 24.Scott JD, Wald A, Kitahata M, et al. Hepatitis C virus is infrequently evaluated and treated in an urban HIV clinic population. AIDS Patient Care STDS. 2009;23(11):925–929. doi: 10.1089/apc.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas GM. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 2011;88(21):948–952. doi: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112(3):178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grierson J, Koelmeyer R, Smith A, Pitts M. Adherence to antiretroviral therapy: Factors independently associated with reported difficulty taking antiretroviral therapy in a national sample of HIV-positive australians. HIV medicine. 2011;12(9):562–569. doi: 10.1111/j.1468-1293.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 28.Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Educ Couns. 2002;46(2):93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- 29.Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: The impact of substance abuse treatment. Addiction. 2004;99(3):361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 31.Lucas GM, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care STDS. 2007;21(8):564–574. doi: 10.1089/apc.2006.0192. [DOI] [PubMed] [Google Scholar]
- 32.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 33.Colfax G. The methamphetamine epidemic: Implications for HIV prevention and treatment. Current HIV/AIDS Reports. 2005;2(4):194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 34.Peck JA, Shoptaw S, Rotheram-Fuller E, Reback CJ, Bierman B. HIV-associated medical, behavioral, and psychiatric characteristics of treatment-seeking, methamphetamine-dependent men who have sex with men. Journal of addictive diseases. 2005;24(3):115–132. doi: 10.1300/J069v24n03_10. [DOI] [PubMed] [Google Scholar]
- 35.Halkitis PN. Reframing HIV prevention for gay men in the united states. Am Psychol. 2010;65(8):752. doi: 10.1037/0003-066X.65.8.752. [DOI] [PubMed] [Google Scholar]
- 36.Barroso J, Sandelowski M. Substance abuse in HIV-positive women. Journal of the Association of Nurses in AIDS Care. 2004;15(5):48–59. doi: 10.1177/1055329004269086. [DOI] [PubMed] [Google Scholar]
- 37.Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: A systematic review. Clin Psychol Rev. 2005;25(4):433–457. doi: 10.1016/j.cpr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Pollack HA, D'Aunno T, Lamar B. Outpatient substance abuse treatment and HIV prevention: An update. J Subst Abuse Treat. 2006;30(1):39–47. doi: 10.1016/j.jsat.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Orwat J, Saitz R, Tompkins CP, Cheng DM, Dentato MP, Samet JH. Substance abuse treatment utilization among adults living with HIV/AIDS and alcohol or drug problems. J Subst Abuse Treat. 2011;41(3):233–242. doi: 10.1016/j.jsat.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundgren LM, Delgado M. HIV outreach and substance abuse treatment for latino drug users: Implications for program planning. Eval Program Plann. 2008;31(1):61–63. doi: 10.1016/j.evalprogplan.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Korthuis PT, Josephs JS, Fleishman JA, et al. Substance abuse treatment in human immunodeficiency virus: The role of patient–provider discussions. J Subst Abuse Treat. 2008;35(3):294–303. doi: 10.1016/j.jsat.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubenstein D, Sorrentino D. Psychotherapy with HIV/AIDS patients: Assessment and treatment plan development. Am J Psychother. 2008;62(4):365–375. doi: 10.1176/appi.psychotherapy.2008.62.4.365. [DOI] [PubMed] [Google Scholar]
- 43.Dausey DJ, Desai RA. Psychiatric comorbidity and the prevalence of HIV infection in a sample of patients in treatment for substance abuse. J Nerv Ment Dis. 2003;191(1):10–17. doi: 10.1097/00005053-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 45.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. Journal of health psychology. 2007;12(2):357–370. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosek SG, Harper GW, Domanico R. Predictors of medication adherence among HIV-infected youth. Psychol, Health Med. 2005;10(2):166–179. doi: 10.1080/1354350042000326584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farley M, Golding JM, Young G, Mulligan M, Minkoff JR. Trauma history and relapse probability among patients seeking substance abuse treatment. J Subst Abuse Treat. 2004;27(2):161–167. doi: 10.1016/j.jsat.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Martin L, Kagee A. Lifetime and HIV-related PTSD among persons recently diagnosed with HIV. AIDS and Behavior. 2011;15(1):125–131. doi: 10.1007/s10461-008-9498-6. [DOI] [PubMed] [Google Scholar]
- 49.Machtinger E, Wilson T, Haberer J, Weiss D. Psychological trauma and PTSD in HIV-positive women: A meta-analysis. AIDS and Behavior. 2012;16(8):2091–2100. doi: 10.1007/s10461-011-0127-4. [DOI] [PubMed] [Google Scholar]
- 50.Hansen NB, Cavanaugh CE, Vaughan EL, Connell CM, Tate DC, Sikkema KJ. The influence of personality disorder indication, social support, and grief on alcohol and cocaine use among HIV-positive adults coping with AIDS-related bereavement. AIDS and Behavior. 2009;13(2):375–384. doi: 10.1007/s10461-007-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lovejoy TI, Suhr JA. The relationship between neuropsychological functioning and HAART adherence in HIV-positive adults: A systematic review. J Behav Med. 2009;32(5):389–405. doi: 10.1007/s10865-009-9212-9. [DOI] [PubMed] [Google Scholar]
- 52.Johnson JG, Williams JB, Goetz RR, et al. Personality disorders predict onset of axis I disorders and impaired functioning among homosexual men with and at risk of HIV infection. Arch Gen Psychiatry. 1996;53(4):350–357. doi: 10.1001/archpsyc.1996.01830040086013. [DOI] [PubMed] [Google Scholar]
- 53.Perkins DO, Davidson EJ, Leserman J, Liao D. Personality disorder in patients infected with HIV: A controlled study with implications for clinical care. Am J Psychiatry. 1993 doi: 10.1176/ajp.150.2.309. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsberg L, Frances A, Perry S. Axis II diagnoses among volunteers for HIV testing and counseling. Am J Psychiatry. 1995;152(8):1222–1224. doi: 10.1176/ajp.152.8.1222. [DOI] [PubMed] [Google Scholar]
- 55.James ME, Rubin CP, Willis SE. Drug abuse and psychiatric findings in HIV-seropositive pregnant patients. Gen Hosp Psychiatry. 1991;13(1):4–8. doi: 10.1016/0163-8343(91)90003-f. [DOI] [PubMed] [Google Scholar]
- 56.Golding M, Perkins DO. Personality disorder in HIV infection. International Review of Psychiatry. 1996;8(2-3):253–258. [Google Scholar]
- 57.Newville H, Haller DL. Relationship of axis II pathology to sex-and drug-related risk behaviors among patients in HIV primary care. AIDS Care. 2012;24(6):763–768. doi: 10.1080/09540121.2011.630367. [DOI] [PubMed] [Google Scholar]
- 58.Welch S. Substance use and personality disorders. Psychiatry. 2007;6(1):27–29. [Google Scholar]
- 59.Martin AM, Benotsch EG, Perschbacher Lance S, Green M. Transmission risk behaviors in a subset of HIV-positive individuals: The role of narcissistic personality features. Personality and Individual Differences. 2012 [Google Scholar]
- 60.Wagner EE, Miller AL, Greene LI, Winiarski MG. Dialectical behavior therapy for substance abusers adapted for persons living with HIV/AIDS with substance use diagnoses and borderline personality disorder. Cognitive and Behavioral Practice. 2004;11(2):202–212. [Google Scholar]
- 61.Halkitis PN, Wolitski RJ, Millett GA. A holistic approach to addressing HIV infection disparities in gay, bisexual, and other men who have sex with men. Am Psychol. 2013;68(4):261–273. doi: 10.1037/a0032746. [DOI] [PubMed] [Google Scholar]
- 62.Ferrando SJ. Features-substance abuse and HIV infection-this article reviews substance use, comorbid psychiatric disorders, and risk behaviors for HIV; the prevalence of substance use disorders among. Psychiatric Annals. 2001;31(1):57–62. [Google Scholar]
- 63.Timko C, Dixon K, Moos RH. Treatment for dual diagnosis patients in the psychiatric and substance abuse systems. Ment Health Serv Res. 2005;7(4):229–242. doi: 10.1007/s11020-005-7455-9. [DOI] [PubMed] [Google Scholar]
- 64.Ferrando SJ, Batki SL. Substance abuse and HIV infection. New Dir Ment Health Serv. 2000;(87):57–67. (87) [PubMed] [Google Scholar]
- 65.Margolin A, Avants SK, DePhilippis D, Kosten TR. A preliminary investigation of lamotrigine for cocaine abuse in HIV-seropositive patients. Am J Drug Alcohol Abuse. 1998;24(1):85–101. doi: 10.3109/00952999809001700. [DOI] [PubMed] [Google Scholar]
- 66.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: A randomized controlled trial. Drug Alcohol Depend. 2011;113(2):192–199. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esposito-Smythers C, Brown LK, Wolff J, et al. Substance abuse treatment for HIV infected young people: An open pilot trial. J Subst Abuse Treat. 2013 doi: 10.1016/j.jsat.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT HEALTHY: A combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cognitive and behavioral practice. 2010;17(3):309–321. doi: 10.1016/j.cbpra.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shoptaw S, Reback CJ, Larkins S, et al. Outcomes using two tailored behavioral treatments for substance abuse in urban gay and bisexual men. J Subst Abuse Treat. 2008;35(3):285–293. doi: 10.1016/j.jsat.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Safren SA, O'Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. J Consult Clin Psychol. 2012;80(3):404. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. JAIDS J Acquired Immune Defic Syndromes. 2001;27(3):251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 72.Clarke S, Delamere S, McCullough L, Hopkins S, Bergin C, Mulcahy F. Assessing limiting factors to the acceptance of antiretroviral therapy in a large cohort of injecting drug users. HIV medicine. 2003;4(1):33–37. doi: 10.1046/j.1468-1293.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 73.Prochaska JO, DiClemente CC. The transtheoretical approach. Handbook of psychotherapy integration. 1992;2:147–171. [Google Scholar]
- 74.Bouis S, Reif S, Whetten K, Scovil J, Murray A, Swartz M. An integrated, multidimensional treatment model for individuals living with HIV, mental illness, and substance abuse. Health Soc Work. 2007;32(4):268–278. doi: 10.1093/hsw/32.4.268. [DOI] [PubMed] [Google Scholar]
- 75.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction. 2001;96(12):1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 76.Parsons JT, Rosof E, Punzalan JC, Maria LD. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: Results of a pilot project. AIDS Patient Care & STDs. 2005;19(1):31–39. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- 77.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV crack cocaine users. Drug Alcohol Depend. 2011;116(1):177–187. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feaster DJ, Mitrani VB, Burns MJ, et al. A randomized controlled trial of structural ecosystems therapy for HIV medication adherence and substance abuse relapse prevention. Drug Alcohol Depend. 2010;111(3):227–234. doi: 10.1016/j.drugalcdep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Granholm E, Anthenelli R, Monteiro R, Sevcik J, Stoler M. Brief integrated outpatient dual diagnosis treatment reduces psychiatric hospitalizations. The American journal on addictions. 2003;12(4):306–313. [PubMed] [Google Scholar]
- 80.Markowitz JC, Spielman LA, Sullivan M, Fishman B. An exploratory study of ethnicity and psychotherapy outcome among HIV-positive patients with depressive symptoms. J Psychother Pract Res. 2000;9(4):226. [PMC free article] [PubMed] [Google Scholar]
- 81.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. The Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 82.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: Findings from the global burden of disease study 2010. The Lancet. 2013 doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]