Abstract
Background:
Antiretroviral therapy (ART) is associated with incomplete restoration of resting memory B (RMB) cell percentages in adults infected with human immunodeficiency virus (HIV), but the effects on RMB cells in children are less well defined, in part because changes in RMB cell percentages are confounded by development and maturation of the RMB cell pool. The objective of this study was to assess the effect of age at ART initiation on RMB cell percentages over time in HIV-infected Zambian children.
Methods:
RMB cell percentages (CD19+CD21+CD27+) were measured by flow cytometry in 146 HIV-infected Zambian children (9 to 120 months old) at baseline and at 3-month intervals after ART initiation and in 34 control children at a single study visit.
Results:
RMB cell percentages among untreated HIV-infected children younger than 24 months did not differ from those of control children (p=0.97). Among HIV-infected children older than 24 months of age, however, each 12-month increase in age at ART initiation was associated with a 1.8% decrease in RMB cell percentage. In contrast, RMB cell percentages in control children up to 48 months old increased 4.4% with each 12-month increase in age. After 12 months of ART, children 24-60 months old had a significant increase in RMB cell percentages that no longer differed from those of control children.
Conclusions:
Initiation of ART in two- to five-year-old HIV-infected children resulted in reconstitution of RMB cell percentages to levels similar to control children and may help restore normal development and maintenance of B cell immunity.
Keywords: resting memory B cell, antiretroviral therapy, pediatric HIV, immune reconstitution
Background
Approximately 330,000 children were infected with human immunodeficiency virus (HIV) in 2011, 90% of whom reside in sub-Saharan Africa.1 Without antiretroviral treatment (ART), half of all perinatally-infected children die by their second birthday.2 ART vastly reduces morbidity and mortality of HIV-infected children, with earlier ART initiation producing better clinical and immunological outcomes.3-5 The 2013 guidelines from the World Health Organization recommend initiating ART for all HIV-infected children younger than five years of age, in contrast to prior recommendations to treat all children younger than two years, and emphasize initiating ART in the first year of life.6
Memory B cells are responsible for antibody responses to previously encountered antigens. Increased cellular activation by HIV was associated with the loss of resting memory B (RMB) cells7-12 and RMB cell deficits were not fully restored by ART in older children and adults.7;13-16 In a cross-sectional study of HIV-infected children in Italy, however, those who began ART before one year of age did not have significant differences in RMB cells compared with uninfected children, whereas children who began ART after one year of age had significantly lower RMB cell percentages compared to uninfected children.17 Given the absence of longitudinal data on B cell subsets in HIV-infected children receiving ART, particularly in sub-Saharan Africa, we characterized RMB cells before and after ART initiation in HIV-infected Zambian children to assess whether earlier ART initiation may mitigate the detrimental effects of HIV infection on RMB cell levels.
Methods
We conducted a prospective, observational cohort study between January 2009 and February 2012 to assess general and measles virus-specific immune reconstitution in HIV-infected Zambian children initiating ART at two public clinics in Lusaka, Zambia. Children nine to 120 months old were eligible for enrollment on the day of ART initiation if they had a documented history of measles vaccination. Study visits occurred every three months in concert with routine clinical care. At each visit, 3-5 mL of blood were collected and a questionnaire was administered to the parent or guardian. Children whose HIV infection status was unknown but presumed negative based on clinical assessment were enrolled for a single study visit during a routine clinic visit and were considered population controls. Informed consent was obtained from the accompanying parent or guardian, and assent was obtained from children older than seven years.
Immunophenotyping of total and RMB cells was performed by flow cytometry using previously described methods.18 Briefly, lymphocytes were gated based on side- and forward-scatter, from which CD19+ B cells were detected using monocloncal antibody to CD19 conjugated to peridinin chlorophyll A protein (PerCP)-Cy5.5. Among CD19+ B cells, RMB cells were identified by the expression of CD21 and CD27,12;19 as detected by monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC) and phycoerythrin (PE), respectively. In separate aliquots, T cells were detected with antibody to the pan-T cell marker CD3 conjugated to PerCP, among the lymphocyte population. Subsets of T cells were detected using monoclonal antibodies against CD4 or CD8 conjugated to allophycocyanin (APC). Isotype controls were used as negative controls to establish gating boundaries. All monoclonal antibodies were obtained from BD Biosciences (Franklin Lakes, NJ, USA) except CD21, which was from Beckman Coulter (Fullerton, CA, USA). Data were collected on a FACSCalibur flow cytometer using CellQuest Pro software (BD Biosciences) and analyzed in FlowJo v7.5 (Treestar, Ashland, OR, USA).
Characteristics of the study population were compared using Χ2 tests for ordinal variables, Fisher’s exact tests for binomial variables and Student’s t tests for continuous variables. Cell populations at enrollment were compared between HIV-infected and control children at enrollment using Student’s t test and between 12-month age intervals using linear regression. To account for repeated measures among HIV-infected children, generalized estimating equations were used to assess the effects of age at ART initiation on the change in RMB cell percentage at each 3-month interval after ART initiation. Results from this model were used to adjust for age in reporting the change in RMB cell percentages after ART initiation for HIV-infected children. To evaluate age-specific changes in RMB cell percentages after 12 months of ART, small sample sizes necessitated collapsing age categories to <24 months, 24-60 months and >60 months and restricting the analysis to data collected at the study visits before and 12 months after ART initiation. Data are presented as the mean (95% confidence interval) unless otherwise noted and all p-values are two-sided. Statistical analyses were performed using Stata v10.1 (College Station, TX) and R v2.15.
Results
The study population consisted of 146 HIV-infected children and 34 control children (Table 1). At enrollment, HIV-infected and control children did not differ significantly with respect to age (p=0.24) and half were female (Table 1). HIV-infected children had significantly lower CD4+ and higher CD8+ T cell percentages than control children (Table 1).
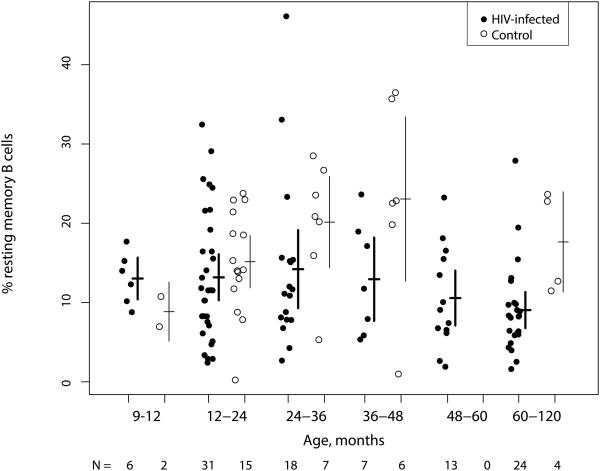
Among the 99 HIV-infected children who had B cell data before ART initiation, the unadjusted mean RMB cell percentage was 12.0% (10.4, 13.5), significantly lower than the mean of 17.5% (14.6, 20.4) for the 34 control children (p<0.001 by Student’s t test). Mean RMB cell percentages did not differ by 12 months of age among HIV-infected children younger than 24 months at ART initiation (p=0.97 by linear regression) but were 1.8% (0.21, 3.3) lower with each 12-month increase in age among HIV-infected children older than 24 months (p=0.03) (Figure 1). In contrast, the mean RMB percentage among control children was 4.4% (1.0, 7.8) higher for each 12-month increase in age up to 48 months (p=0.01) (Figure 1), after which time there were insufficient data to assess trends in RMB cells. Mean RMB cell percentages did not differ between HIV-infected children with nadir CD4+ T cell percentages less than or greater than 15% (p=0.51 by Student’s t test) (Supplemental Figure 1).
Figure 1. Resting memory B cell percentages among total B cells in HIV-infected Zambian children before ART initiation and control children stratified by 12-month increases in age at enrollment.
Lines represent means and 95% confidence intervals.
ART = highly active antiretroviral therapy
Changes in RMB cell percentages after ART
Measurements of RMB cells were available from 556 person-visits (Table 2). Sixty-seven (68%) of the 99 HIV-infected children with B cell data prior to ART initiation contributed RMB cell data from an additional 218 person-visits after ART initiation. The 47 HIV-infected children without pre-ART B cell measurements had B cell data at a total of 239 person-visits, and 43 of these children (91%) contributed B cell data at two or more post-ART study visits. The 99 children with B cell data before ART initiation were significantly older at ART initiation than the 47 children with B cell data after ART (p<0.01), but did not differ in nadir CD4+ or CD8+ T cell percentages (Supplemental Table 1). The mean percentages of resting memory B cells among the 33 children with data both before and at 12 months of ART did not differ from children who had B cell data either before (n=66) or after 12 months of ART (n=32) (p=0.333 and p=0.954, data not shown).
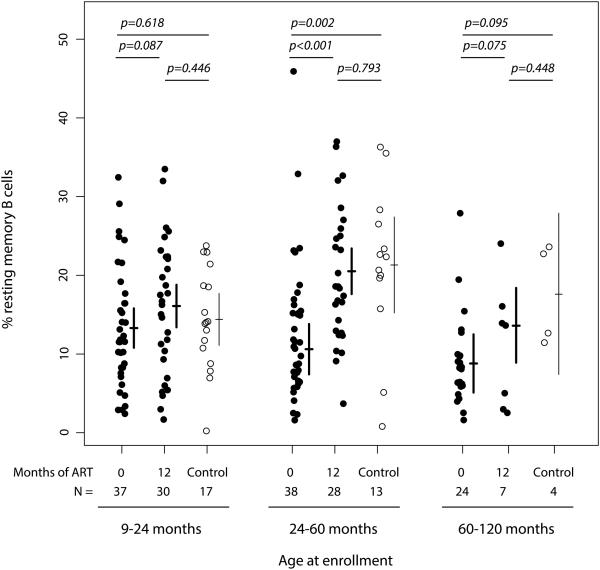
Twelve months of ART restored RMB cell percentages of HIV-infected children to levels of control children (Figure 2). While HIV-infected children younger than 24 months had a marginal increase in RMB cells of 3.5% (−0.5, 7.5) after 12 months of ART, their mean RMB cell percentage before ART did not differ from that of control children. In contrast, HIV-infected children 24 to 60 months of age had a 10.6% (6.2, 15.0) increase in RMB cell percentage, reaching a mean of 20.7% (17.8, 23.6) that no longer differed from that of control children (p=0.793). HIV-infected children older than 60 months had a marginal RMB cell increase of 5.5% (−0.6, 11.6), achieving a mean of 13.6% (8.9, 18.4). While this percentage did not differ significantly from control children (p=0.448), the sample sizes in this age category were small (Figure 2).
Figure 2. Resting memory B cell percentages among total B cells in HIV-infected Zambian children before and 12-months after ART initiation compared to control children stratified by age at enrollment.
Lines represent means and 95% confidence intervals.
ART = antiretroviral therapy
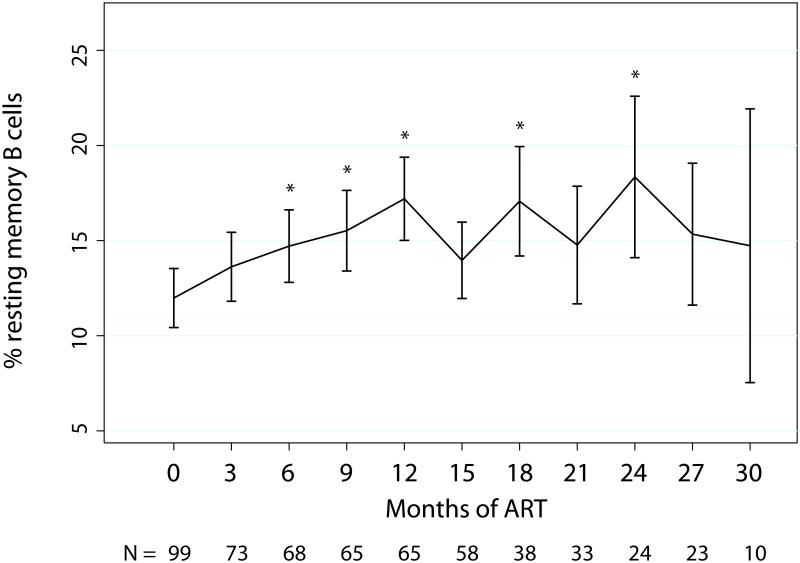
Among all HIV-infected children, the age-adjusted mean RMB cell percentage increased by 3.0% (0.6, 5.3) within 6 months of ART initiation and continued to increase through 12 months of ART, with an overall mean change of 5.0% (2.6, 7.4). After 12 months of ART, the age-adjusted mean percentage of RMB cells leveled off at 17.2% (15.0, 19.4) and no statistically significant changes occurred for the remainder of study follow-up (Figure 3, Supplemental Table 2).
Figure 3. Mean resting memory B cell percentages among total B cells in HIV-infected Zambian children before ART and in 3-month intervals after ART initiation.
Data were adjusted for age at enrollment using generalized estimating equations. Error bars represent 95% confidence interval.
*p<0.05 compared to baseline.
ART = highly active antiretroviral therapy
Figure corresponds with data presented in Supplemental Table 2.
Nadir CD4+ T cell percentage was not associated with the percentage of RMB cells after 12 months of ART for the 63 HIV-infected children with data on both RMB cells and CD4+ T cells. There was no statistically significant difference in the RMB cell percentages after 12-months of ART between children with nadir CD4+ T cells less than or greater than 15% (p=0.34), suggesting severe immunosuppression as measured by CD4+ T cell percentage did not affect RMB cell reconstitution.
Discussion
During normal immunologic development, the proportion of RMB cells among circulating B lymphocytes increases with age and exposure to pathogens, plateauing in early adulthood at approximately 40% of the total CD19+ B cell population.19 Infection with HIV, however, activates RMB cells and promotes differentiation into antibody-secreting plasmablasts, 13 resulting in a non-specific depletion of the RMB cell pool, an over-abundance of antibody-secreting plasmablasts, and high levels of immunoglobulins.14;20;21 HIV-infected Zambian children had decreasing RMB cell percentages with increasing age before ART initiation, evidence of a progressive loss of RMB cells in untreated HIV-infected children. These results are consistent with prior studies showing a negative correlation between age and CD27+ memory B cells in HIV-infected children 5 to 16 years old,22 as well as studies in HIV-infected adults showing decreased percentages of RMB cells with increased duration of untreated HIV infection.23
Early initiation of ART may result in preservation or at least partial restoration of RMB cell percentages. RMB cell percentages between HIV-infected and control Zambian children younger than 24 months did not differ significantly and 12 months of ART resulted in only a marginal increase in RMB cell percentages in this age group. In contrast, HIV-infected children who began ART between 24 and 60 months of age achieved near-normal levels of RMB cells within 12-months of ART despite evidence of a reduction in RMB cell percentage with increasing age. These findings suggest relatively minimal damage to the RMB cell compartment before two years of age but reversible damage between 2 and 5 years of age following ART. In adults, ART initiation within 6 months of HIV infection resulted in restoration of RMB cell percentages after 12 months of therapy that did not differ from those of uninfected adults, while chronically infected individuals were unable to restore normal RMB cell levels.24 Together, these findings suggest early initiation of ART in both children and adults may enable restoration of the RMB cells lost during untreated HIV infection to levels observed in healthy individuals.
The ability of HIV-infected individuals to reconstitute the memory component of the humoral immune response following ART initiation has implications for previously acquired immunity from both natural infection and vaccination. Multiple studies demonstrated that HIV-infected children have lower antibody-secreting cells and antibody concentrations to other pathogens compared to uninfected children.17;25-27 While we did not measure pathogen-specific B cells in this cohort, circulating antibody concentrations against measles virus were not restored in these children with ART alone.28 If untreated HIV infection results in the loss of vaccine-specific RMB cells, immunologic memory acquired prior to ART initiation may be lost and children beginning ART after routine immunization would require revaccination.
The lack of B cell measurements at all study visits from HIV-infected children was a limitation of this study. However, sensitivity analyses showed few differences between HIV-infected children with and without baseline data (Supplemental Table 1). The increase in RMB cell percentages until 48 months of age in control children followed by lower RMB cell percentages in children older than 60 months was likely due to small sample size. This also limited the power to detect changes in RMB cell percentages among children older than 48 months in response to ART initiation. While restoration of RMB cell percentages is promising, we did not directly assess B cell function in this cohort and the ability of these cells to respond appropriately to antigens is critical. Additionally, these children were enrolled from urban public clinics in Lusaka, Zambia that primarily serve individuals of low socioeconomic status and results may not be generalizable to all HIV-infected children.
Immunological development is profoundly altered in HIV-infected children and RMB cells are important to the development of protective antibody responses as these cells differentiate into antibody-secreting plasma cells. The finding that ART initiation in HIV-infected children younger than five years of age restores age-appropriate RMB cell levels support recent guidelines for ART initiation in all HIV-infected children younger than 5 years of age.2-5;29 Reducing the duration of HIV-associated immune destruction with early initiation of therapy may allow for near-normal development of the RMB cell repertoire as well as maintenance of pathogen- and vaccine-specific immunity into later childhood and adolescence.
Supplementary Material
Acknowledgements
We thank the participants of this study and the clinicians and study staff who collected data and cared for the participating children.
Footnotes
Presented in part at the Keystone Symposium “Immunological Mechanisms of Vaccination” in Seattle, Washington, October 27th – November 1st, 2010.
Conflicts of Interest and Sources of Funding: We declare that we have no conflicts of interest. This study was funded by the U.S. National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R01AI070018].
References
- 1.UNAIDS . Global report: UNAIDS report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2012. [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, McCarthy N, Morris L, Walker BD, Goulder P. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–43. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 4.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, Sinkala M, Kankasa C, Wilson CM, Wilfert CM, Mwango A, Levy J, Abrams EJ, Bulterys M, Stringer JS. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 7-1-2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html. [PubMed]
- 7.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–58. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Feyen O, Jebran AF, Huck K, Jetzek-Zader M, Bas M, Niehues T. Memory B cell function in HIV-infected children-decreased memory B cells despite ART. Pediatr Res. 2009;66:185–90. doi: 10.1203/PDR.0b013e3181aa057d. [DOI] [PubMed] [Google Scholar]
- 9.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15:957–64. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 11.Buckner CM, Moir S, Ho J, Wang W, Posada JG, Kardava L, Funk EK, Nelson AK, Li Y, Chun TW, Fauci AS. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol. 2013;87:5800–5811. doi: 10.1128/JVI.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir S, Fauci AS. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12–19. doi: 10.1016/j.jaci.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagase H, Agematsu K, Kitano K, Takamoto M, Okubo Y, Komiyama A, Sugane K. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin Immunol. 2001;100:250–259. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]
- 14.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, narita M, Grutzmeier S, Sonnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 15.Titanji K, Chiodi F, Bellocco R, Schepis D, Osorio L, Tassandin C, Tambussi G, Grutzmeier S, Lopalco L, De MA. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS. 2005;19:1947–55. doi: 10.1097/01.aids.0000191231.54170.89. [DOI] [PubMed] [Google Scholar]
- 16.Moir S, Malaspina A, Ho J, Wang W, DiPoto AC, O'Shea MA, Roby G, Mican JM, Kottilil S, Chun TW, Proschan MA, Fauci AS. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–79. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 17.Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, Bernardi S, Thorstensson R, Chiodi F, Rossi P. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A. 2009;106:7939–44. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainwater-Lovett K, Nkamba HC, Mubiana-Mbewe M, Moore CB, Moss WJ. Immunologic risk factors for early mortality after starting antiretroviral therapy in HIV-infected Zambian children. AIDS Res Hum Retroviruses. 2013;29:479–87. doi: 10.1089/aid.2012.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong Y, Ikematsu H, Kikuchi K, Yamamoto M, Murata M, Nishimura M, Nabeshima S, Kashiwagi S, Hayashi J. Selective CD27+ (memory) B cell reduction and characteristic B cell alteration in drug-naive and HAART-treated HIV type 1-infected patients. AIDS Res Hum Retroviruses. 2004;20:219–26. doi: 10.1089/088922204773004941. [DOI] [PubMed] [Google Scholar]
- 21.D'Orsogna LJ, Krueger RG, McKinnon EJ, French MA. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS. 2007;21:1747–52. doi: 10.1097/QAD.0b013e32828642c7. [DOI] [PubMed] [Google Scholar]
- 22.Cagigi A, Palma P, Nilsson A, Di CS, Pensieroso S, Kakoulidou M, Bernardi S, Rossi P, Chiodi F. The impact of active HIV-1 replication on the physiological age-related decline of immature-transitional B-cells in HIV-1 infected children. AIDS. 2010;24:2075–80. doi: 10.1097/QAD.0b013e32833c3298. [DOI] [PubMed] [Google Scholar]
- 23.Titanji K, De MA, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, Chiodi F. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 24.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O'Shea MA, Kottilil S, Chun TW, Proschan MA, Fauci AS. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116:5571–79. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss WJ, Scott S, Mugala N, Ndhlovu Z, Beeler JA, Audet SA, Ngala M, Mwangala S, Nkonga-Mwangilwa C, Ryon JJ, Monze M, Kasolo F, Quinn TC, Cousens S, Griffin DE, Cutts FT. Immunogenicity of standard-titer measles vaccine in HIV-1-infected and uninfected Zambian children: an observational study. J Infect Dis. 2007;196:347–55. doi: 10.1086/519169. [DOI] [PubMed] [Google Scholar]
- 26.Bekker V, Scherpbier H, Pajkrt D, Jurriaans S, Zaaijer H, Kuijpers TW. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118:e315–e322. doi: 10.1542/peds.2005-2616. [DOI] [PubMed] [Google Scholar]
- 27.Iwajomo OH, Finn A, Moons P, Nkhata R, Sepako E, Ogunniyi AD, Williams NA, Heyderman RS. Deteriorating pneumococcal-specific B-cell memory in minimally symptomatic African children with HIV infection. J Infect Dis. 2011;204:534–43. doi: 10.1093/infdis/jir316. [DOI] [PubMed] [Google Scholar]
- 28.Rainwater-Lovett K, Nkamba HC, Mubiana-Mbewe M, Bolton-Moore C, Moss WJ. Changes in Measles Serostatus Among HIV-Infected Zambian Children Initiating Antiretroviral Therapy Before and After the 2010 Measles Outbreak and Supplemental Immunization Activities. J Infect Dis. 2013 doi: 10.1093/infdis/jit404. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Recommendations for a public health approach. World Health Organization; Geneva, Switzerland: 2010. Antiretroviral therapy of HIV infection in infants and children: Towards universal access. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.