Abstract
Background
Alcohol and nicotine are the most commonly abused drugs. The frequent co-morbidity of alcohol and nicotine addiction has led to the hypothesis that they may act via a common substrates: the nicotinic acetylcholine receptors (nAChRs) especially α4β2 and α7 subtypes, the most prevalent nAChRs in the brain. Compelling evidence suggests that alcohol enhances the function of α4β2 subtype. The FDA approved smoking cessation drug, varenicline (“Chantix”), a partial agonist of α4β2 nAChR subtype, reduces alcohol self-administration and alcohol craving in humans and rodents. The cholinergic basal forebrain (BF) controls various functions including arousal, attention and cognition and there is a predominance of α4β2 and α7 subtypes. We have shown that the BF has an important role in mediating the effects of alcohol and local infusion of nicotine in the BF activates nucleus accumbens. Does BF have any role in mediating the effect of nicotine on alcohol consumption? This study was designed to address this question.
Methods
Under standard surgical procedure, C57BL/6J mice were stereotaxically implanted with bilateral stainless steel guide cannula above the BF. Following post-operative recovery and habituation, the animals were exposed to the “drinking-in-the-dark” paradigm whereby alcohol (20%) was presented for 2 hours daily for three days. On fourth day, nicotine or artificial cerebrospinal fluid (ACSF) was microinjected bilaterally in the BF. After one hour, mice were exposed to alcohol and allowed to self-administer for four hours. The effect of BF nicotine infusion on sucrose consumption was also examined. On completion, mice were euthanized, brain removed and processed to localize the BF injection sites.
Results
As compared to the ACSF, bilateral nicotine injections into the BF significantly (p<0.05; N=5/group) increased alcohol consumption. Sucrose consumption remained unaffected.
Conclusions
Based on our results, we believe that the BF may have an important role in nicotine-alcohol co-use.
Keywords: Nicotine, Basal Forebrain, Microinjection, Mice, Alcohol
INTRODUCTION
Alcohol and nicotine, an addictive substance in tobacco smoking, are amongst the most common co-abused drugs. Approximately, 90% of alcoholics smoke, whereas approximately 60% of smokers binge drink or consume substantial amount of alcohol (DiFranza and Guerrera, 1990; Batel et al., 1995). Early onset of smoking in adolescents predicts the development of alcohol-related problems in later life (Grant, 1998; Riala et al., 2004). The amount of alcohol consumed and the degree of alcohol dependence is positively correlated with the amount of tobacco consumed (Barrett et al., 2006). In contrast, smoking cessation reduces alcohol consumption (Mitchell et al., 2012; McKee et al., 2009; Fucito et al., 2011). While nicotine is believed to be one of the greatest risk factor for the development of alcoholism, little is known about underlying neurobiological mechanisms responsible for nicotine and alcohol co-abuse (Davis and de Fiebre, 2006).
It has been suggested that nicotine and alcohol may act via a common neurological pathway: the nicotinic acetylcholine receptors (nAChRs) (Davis and de Fiebre, 2006). The nACRs are ligand-gated ion channels that are activated by the neurotransmitter, acetylcholine and nicotine. The nAChRs in the CNS are pentameric combinations, formed from a portfolio of genetically distinct α- and β-subunits (α2–α10 and β2–β4). Differential association of various subunits confers distinct functional and structural properties to the resultant nAChRs subtype. The most abundant nAChR subtypes in the brain are the heteromeric α4β2 and homomeric α7 (Millar and Gotti, 2009).
Human genetic association studies implicate CHRNA5-CHRNA3-CHRNB4 gene cluster, encoding for the α3, α5, and β4 nAChRs subunits, in nicotine and alcohol dependency (Joslyn et al., 2008; Wang et al., 2009; Hong et al., 2010). However, compelling clinical and preclinical evidence suggest that alcohol enhances the function of α4β2 subtype, inhibits α7 subtype and has minimal effects on α3β2 subtype (Cardoso et al., 1999; Taslim and Dar, 2010; Taslim et al., 2008; Secko, 2005; de Fiebre and de Fiebre, 2005; Narahashi et al., 2001; Davis and de Fiebre, 2006; Blomqvist et al., 1992; Butt et al., 2004; Butt et al., 2003). Furthermore, varenicline, a partial agonist of α4β2 containing nAChRs reduces alcohol seeking and consumption in rodents (Steensland et al., 2007; Chatterjee and Bartlett, 2010). Thus, while there is compelling evidence implicating nAChRs in alcohol and nicotine co-abuse, it is yet unclear as to where in the brain nicotine acts to affects alcohol consumption.
Recently we have demonstrated that the cholinergic basal forebrain (BF) has an important role in mediating the behavioral effects of alcohol (Sharma et al., 2010a; Lodhi et al., 2011; Sharma et al., 2010b; Thakkar et al., 2010). Moreover, the BF neurons exhibit high expressions of α4β2 and α7 nACHRs subtypes (Azam et al., 2003) and systemic administration of nicotinic receptor agonist activates BF neurons (Biton et al., 2007; Thomsen et al., 2010). The BF has strong connections with several brain regions implicated in alcohol and nicotine addiction. For example, the BF receives strong dopaminergic projections from the VTA and GABAergic projections from the nucleus accumbens and is the major source of cholinergic inputs to several brain regions including the cortex and amygdala (Zaborszky et al., 2012). Furthermore, bilateral BF infusion of nicotine coupled with systemic alcohol activates nucleus accumbens (Dumontier et al., 2012).
Does nicotine act via the BF to promote alcohol consumption? To address this issue, we examined the effects of local administration of nicotine into the BF on alcohol consumption in C57BL/6J mice.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice [(7–8 weeks old; 22–26 g; Jackson Laboratories (Bar Harbor, ME, USA)] were housed under ambient room temperature (25 ± 2°C), reverse 12–12 hour light/dark cycle [light onset = 10.00 PM] and with ad libitum food and water for one week before experiments were begun. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Harry S. Truman Memorial Veterans’ Hospital.
Drugs
Alcohol solution (20% v/v) was prepared from ethanol (200 proof; Fisher Scientific, Pittsburgh, PA, USA) in tap water. Sucrose (D-sucrose; Fisher Scientific) solution (10%, w/v) was prepared in tap water. 1mM stock solution of (−)- Nicotine hydrogen tartrate (Sigma-Aldrich Co. LLC, St Louis, MO) was prepared with artificial cerebrospinal fluid (ACSF =147mM NaCl, 3mM, KCl, 1.2mM CaCl2 and 1.0mM MgCl2; pH=7.0), aliquoted and stored at −20°C. All working solutions were prepared fresh on the day of the experiment.
Surgery
Under isoflurane anesthesia and sterile conditions, mice (N=20) were stereotaxically implanted with bilateral stainless steel guide cannula (27 G), 2.0 mm above the target site [target co-ordinates: Anterior = 0.0 mm; Lateral = ±1.5 mm and Ventral = 5.3 mm, relative to the bregma and skull surface (Franklin and Paxinos, 2008)]. Two anchor screws were also implanted and the entire assembly was fixed onto the skull with dental cement. A 31-guage stainless steel stylet was inserted in order to maintain the patency. Subcutaneous flunixin (25mg/kg/12 hours for one day) was used as a post-operative analgesic. The animals were observed until ambulatory and then each mouse was singly housed in the experimental cage (similar to normal mouse cage with one grommeted hole on the shorter side for dispensing water/alcohol) and allowed to recover for 5–7 days.
Alcohol consumption
We used the drinking-in-the-dark (DID) procedure as described previously (Rhodes et al., 2005; Rhodes et al., 2007). In brief: 2.5 hours after dark onset, water bottles were removed from animal cages. A single pre-weighed 15 ml plastic bottle with metal sipper tubes containing 20% alcohol was introduced into each mouse cage after 30 min. Mice were allowed to consume alcohol for 2 hours after which the alcohol bottles were replaced with original water bottles. Subsequently, alcohol bottles and animals were weighed to calculate the amount of alcohol consumed (g/Kg of the body weight). The same procedure was repeated on days 2, 3 and 4, except on day 4, mice had access to 20% alcohol for 4 hours.
Drug infusions
In order to reduce stress, all mice were habituated to the infusion procedures by performing sham-injections on days 2 and 3. The sham-injection protocol was identical to the microinjection protocol and performed at the same time (one hour before alcohol exposure) except that the sham-injector was shorter (remained 1.5 mm above the target site; to avoid damaging target sites) and no fluid was infused.
On Day 4, one hour before the onset of alcohol exposure, mice were randomly divided into two groups. The ACSF group (N=5) was bilaterally infused with ACSF (100 nL/side). The Nicotine group (N=5) was bilaterally infused with nicotine [(10 pmol (equivalent to 1.6 ng of nicotine free base)/100 nL/side] into the BF. The 10 pmol dose of nicotine was selected because this dose has been shown to produce maximal effects when infused locally in select brain region (Taslim et al., 2011; Smith and Dar, 2007). The infusion protocol was as described previously (Thakkar et al., 2010). In brief: Mouse was removed out of his cage and gently held to remove the stylus and insert the injector cannula [connected to a 0.5 μL Hamilton syringe (Hamilton, Reno, NV, USA) via FEP connector (Eicom, San Diego, CA)] into the ipsilateral guide cannula. Once the injector was in place, ACSF or nicotine was slowly microinjected. On completion, the injector cannula was left in place for an additional 1 min before retracting. The same protocol was repeated to infuse ACSF or nicotine on the contralateral side. On completion, the animal was returned back to his cage. The entire infusion protocol was completed in approximately 5 min.
Blood alcohol concentration (BAC)
BAC was measured as described previously (Sharma et al., 2013). In brief: On day 4, immediately after the measurement of alcohol consumption, mice were removed from their cages and a small amount (~25 μl) of blood was removed from the tail vein and centrifuged to separate plasma. The plasma was used for BAC analysis by using ethanol measurement kit as per manufacturer’s instructions (Genzyme, Framingham, MA).
Sucrose Consumption
To validate the specificity of the effect on alcohol consumption, we examined the effects of bilateral BF nicotine infusion on sucrose (10%) consumption (DID protocol) in separate groups (ACSF and Nicotine; 5/group) of 10 mice.
Localization of injection sites
On completion of the experiments, mice were euthanized by sequential transcardiac perfusion with 40 mL of 0.9% cold saline and 10% formalin (Fisher Scientific). The brains were removed, post-fixed, equilibrated [20% sucrose in 0.1 M phosphate buffered saline (PBS, pH 7.4)] and serially sectioned (40 μm; 3 series) with freezing microtome. One series was used for choline acetyl transferase (ChAT) immunohistochemistry to localize the injection site in the BF (Sharma et al., 2010a).
Statistics
Unpaired t-test (Prism; Graphpad Software, La Jolla, CA) was used to examine the effects of BF nicotine infusion on alcohol/sucrose consumption (α = 0.05). Pearson’s correlation was used to examine the dependence of BAC on alcohol consumption.
RESULTS
Localization of injection sites
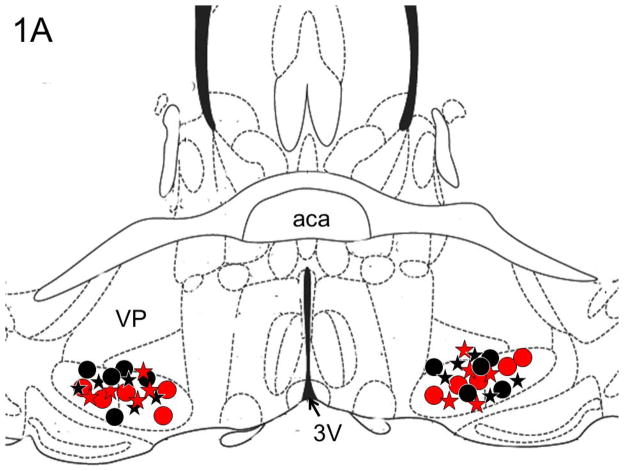
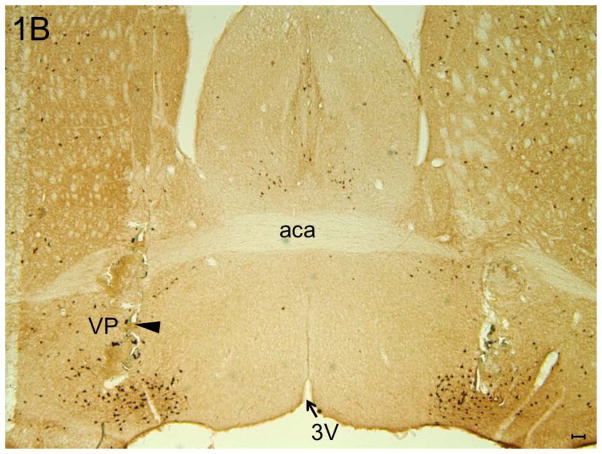
All injections sites (N=20) were localized in the BF region (between AP = 0.26 to AP = −0.10) and are described in one coronal schematic (AP = 0.14) in Figure 1A, [adapted from Figure 30; see (Franklin and Paxinos, 2008)]. A representative photomicrograph depicting the bilateral injection sites in the midst of cholinergic neurons in the BF is shown in Figure 1B.
Figure 1. Localization of injections sites.
Panel A: Schematic representation of all injections sites (N=20) mapped on one coronal schematic (AP = 0.14) [adapted from Figure 30; see (Franklin and Paxinos, 2008)] is described All injection sites were localized in the cholinergic BF (between AP = 0.26 to AP = −0.10). Circles denote alcohol consumption (Black circle = ACSF infusion; Red circle = nicotine infusion); Stars denote sucrose consumption (Black star = ACSF infusion; Red star = nicotine infusion).
Panel B: A representative photomicrograph depicting the bilateral lesions caused due to microinjections in the BF (large arrowhead) in the midst of ChAT “+ve” cholinergic neurons. Abbreviations: aca = anterior commissure, anterior part, VP = Ventral Pallidum and 3V = third ventricle. Calibration bar = 100um.
Alcohol consumption
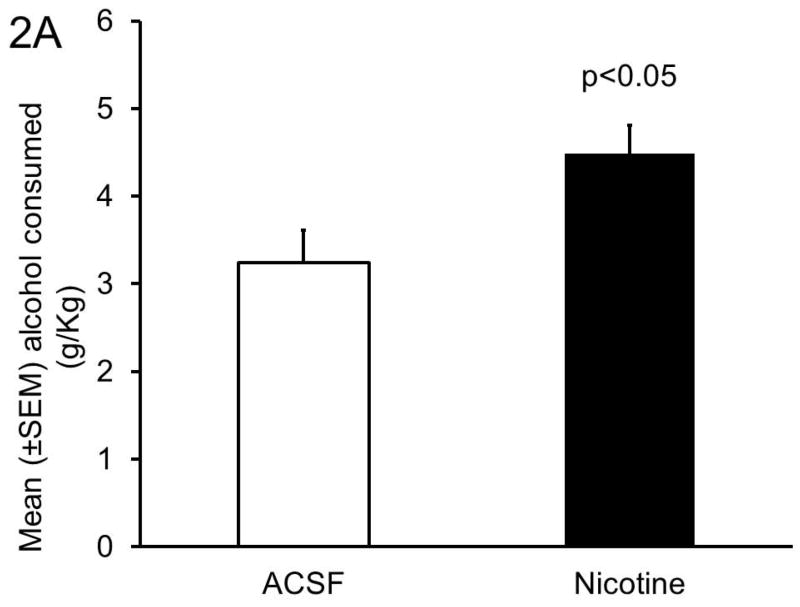
As compared to the ACSF infusions (3.2 ± 0.4 g/Kg), bilateral BF nicotine infusion significantly (t=2.6, df=8, p<0.05; unpaired t-test) increased the amount of alcohol consumed (4.5 ± 0.3 g/Kg) during 4 hours of alcohol access on Day 4 (Figure 2A).
Figure 2. Effect of BF nicotine infusion on alcohol consumption.
Panel A: Bilateral microinjections of nicotine (10 pmol) into the BF significantly increased alcohol consumption.
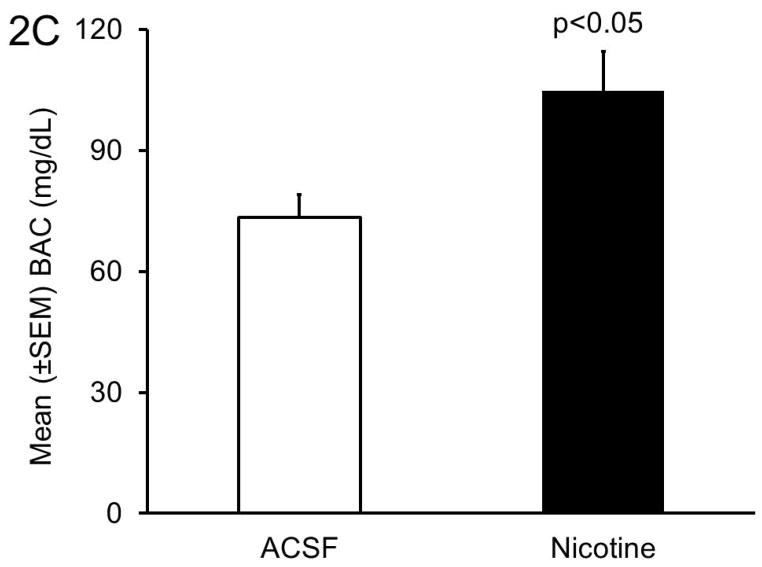
Panel B: The BAC paralleled alcohol consumption. Mice infused with nicotine in the BF displayed significantly higher BAC as compared to mice infused with ACSF.
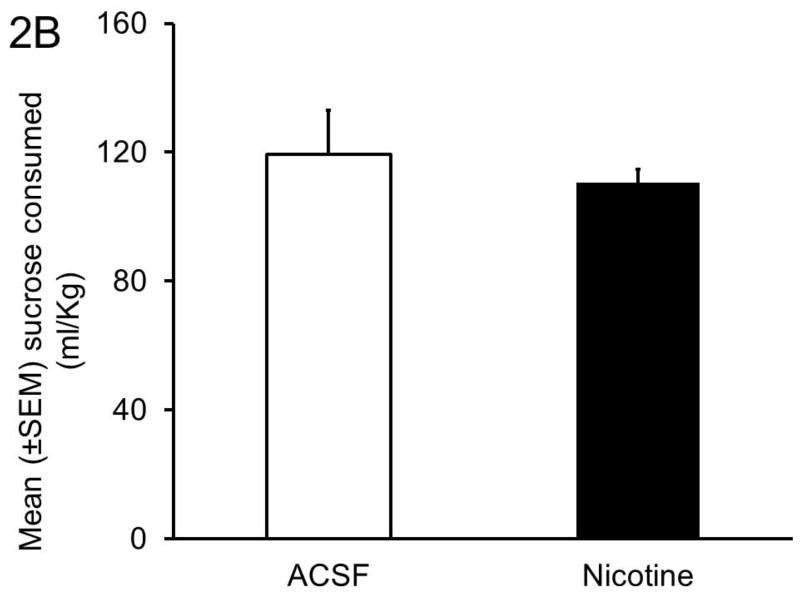
Panel C: Nicotine (10 pmol) infusion in the BF had no effect on sucrose consumption.
BAC
The BAC levels were significantly (Pearson r=0.95; p<0.0001; N=10) correlated with the amount of alcohol consumption on day 4. As compared to bilateral BF infusion of ACSF (73.4 ± 5.7 mg/dL) BAC was significantly (t=2.8, df=8, p<0.05; unpaired t-test) higher in mice infused with nicotine (105 ± 9.8 mg/dL) (Figure 2B).
Sucrose consumption
In contrast to alcohol concentration, bilateral BF nicotine infusions did not alter sucrose consumption. Mice infused with ACSF consumed 119 ± 13.6 ml/Kg and mice infused with nicotine consumed 110 ± 4.3 ml/Kg during 4 hours of sucrose exposure on Day 4 (Figure 2C).
DISCUSSION
The results of our study suggest that nicotine infusion into the cholinergic BF increases alcohol consumption implicating the role of BF in alcohol and nicotine co-abuse. Infusion of nicotine in the BF had no effect on sucrose consumption further validating the specificity of the effect on alcohol consumption.
In the present study, we have used “drinking-in-the-dark” (DID) paradigm to examine the alcohol consumption following BF nicotine infusion. Originally designed to examine binge-like alcohol consumption, the DID model utilizes the predisposition of C57BL/6J mice to voluntarily consume large quantities of alcohol within a two to four hours, resulting in a pharmacologically relevant BAC (Rhodes et al., 2005; Rhodes et al., 2007). Importantly, no prior training is required to habituate the animals to high concentration of alcohol (Sprow and Thiele, 2012). The DID paradigm has been extensively used to examine the central effects of several neurotransmitters/neuropeptides on alcohol consumption (Moore and Boehm, 2009; Sparrow et al., 2012; Sparta et al., 2013; Lowery et al., 2010; Lowery-Gionta et al., 2012; Linsenbardt and Boehm, 2009). Ample evidences suggest that the dopaminergic ventral tegmental area (VTA) has a critical role in mediating the effects of nicotine and alcohol. For example, infusion of nicotine into the VTA enhances alcohol induced increase in accumbal dopamine (Tizabi et al., 2002). This effect is blocked by infusion of nicotinic receptors antagonists in the VTA (Erricson et al., 2003). Furthermore, the VTA of alcohol preferring P rats shows enhanced sensitivity to the reinforcing effects of nicotine suggesting a genetic linkage between high alcohol preference and the sensitivity of the VTA toward reinforcing actions of nicotine. Finally, Roguski et al (2013) have recently demonstrated that concurrent gestational exposure to alcohol and nicotine disrupts the dopaminergic circuitry in the VTA resulting in increased nicotine self-administration in adolescents.
Although the mechanism of how nicotine infusion in the BF increases alcohol consumptions is unclear and under investigation, it is interesting to note that the BF has a predominance of α4β2 and α7 subtypes of nAChRs and both these receptors play a pivotal role in mediating alcohol consumption (Davis and de Fiebre, 2006). Varenicline, a partial agonist of α4β2 containing nAChRs reduces alcohol consumption in humans and rodents (Steensland et al., 2007; Fucito et al., 2011; Chatterjee and Bartlett, 2010; McKee et al., 2009; Mitchell et al., 2012). Systemic injection of cytisine, a partial agonist of α4β2 containing nAChRs nicotinic receptor also reduces alcohol consumption (Hendrickson et al., 2009). Local BF infusion of nicotine coupled with systemic alcohol administration activates nucleus accumbens, an essential brain region in the mesolimbic dopaminergic pathway associated with rewarding and reinforcing effects of both alcohol and nicotine (Dumontier et al., 2012; Erricson et al., 2003; Tizabi et al., 2002).
Ongoing studies in our laboratory are investigating the effects of selective nicotinic antagonists such as methyllycaconitine (selective antagonist for α7 containing nAChRs) and dihydro β-erythroidine (a selective antagonist for α4β2 containing nAChRs) on alcohol consumption to examine the basal and/or tonic involvement of BF nicotinic receptors in nicotine and alcohol co-abuse.
In conclusion, the results of our study suggest that the nicotine infusion into the cholinergic BF increases alcohol consumption suggesting that the BF may have a role in nicotine and alcohol co-abuse.
Acknowledgments
We thank Shafi Lodhi, Kevin Bradshaw and David DeRoode for helping us with microinjection and immunohistochemistry and Carrie Harris for animal care. This work was supported by the Harry S. Truman Memorial Veterans Hospital, and funding from National Institute of Alcohol Abuse and Alcoholism (AA020334 & AA0174720).
References
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Biton B, et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacol. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A polymorphism in the alpha4 nicotinic receptor gene (Chrna4) modulates enhancement of nicotinic receptor function by ethanol. Alcohol Clin Exp Res. 2003;27:733–742. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- Butt CM, King NM, Stitzel JA, Collins AC. Interaction of the nicotinic cholinergic system with ethanol withdrawal. J Pharmacol Exp Ther. 2004;308:591–599. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Fiebre NC, de Fiebre CM. alpha7 Nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not beta-amyloid-induced neurotoxicity. Neurosci Lett. 2005;373:42–47. doi: 10.1016/j.neulet.2004.09.054. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Dumontier S, Sharma R, DeRoodes D, Sahota P, Thakkar M. A putative role of cholinergic basal forebrain in alcohol and nicotine co-abuse. Alcoholism: Clinical and Experimental Research. 2012;36:36A. [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. New York, NY: Academic Press; 2008. [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA, Jr, Toalston JE, Ding ZM, Truitt WA, Bell RL, McBride WJ, Rodd ZA. Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol. 2013 doi: 10.1111/adb.12048. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi S, Sharma R, deRoode D, Thakkar MM. Epigentic modifications in the wake-promoting basal forebrain may contribute to insomnia during alcohol withdrawal. 2011. p. 149. [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacol. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Boehm SL. Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123:555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Soderpalm B, Ericson M, Olausson P, Engel JA, Zhang X, Nordberg A, Marszalec W, Aistrup GL, Schmidt LG, Kalouti U, Smolka AM, Hedlund L. Mechanisms of alcohol-nicotine interactions: alcoholics versus smokers. Alcohol Clin Exp Res. 2001;25:152S–156S. doi: 10.1097/00000374-200105051-00026. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Riala K, Hakko H, Isohanni M, Jarvelin MR, Rasanen P. Teenage smoking and substance use as predictors of severe alcohol problems in late adolescence and in young adulthood. J Adolesc Health. 2004;35:245–254. doi: 10.1016/j.jadohealth.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Roguski EE, Sharp BM, Chen H, Matta SG. Full-gestational exposure to nicotine and ethanol augments nicotine self-administration by altering ventral tegmental dopaminergic function due to NMDA receptors in adolescent rats. J Neurochem. 2013 doi: 10.1111/jnc.12504. (In Press) [DOI] [PubMed] [Google Scholar]
- Secko D. Craving nicotine: It’s in the genes. CMAJ. 2005;172:175–176. doi: 10.1503/cmaj.045235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar M. Rapid tolerance development to the NREM sleep promoting effect of alcohol. Sleep. 2013 doi: 10.5665/sleep.3598. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem. 2010a;115:782–794. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann SC, Sahota P, Thakkar MM. Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res. 2010b;34:813–818. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Dar MS. Behavioral cross-tolerance between repeated intracerebellar nicotine and acute Delta(9)-tetrahydrocannabinol-induced cerebellar ataxia: role of cerebellar nitric oxide. J Pharmacol Exp Ther. 2007;322:243–253. doi: 10.1124/jpet.107.120634. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Pena J, Navarro M, Kash TL, Thiele TE. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacol. 2012;37:1409–1421. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hopf FW, Gibb SL, Cho SL, Stuber GD, Messing RO, Ron D, Bonci A. Binge ethanol-drinking potentiates corticotropin releasing factor r1 receptor activity in the ventral tegmental area. Alcohol Clin Exp Res. 2013;37:1680–1687. doi: 10.1111/acer.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol Behav. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslim N, Al-Rejaie S, Dar MS. Attenuation of ethanol-induced ataxia by alpha(4)beta(2) nicotinic acetylcholine receptor subtype in mouse cerebellum: a functional interaction. Neuroscience. 2008;157:204–213. doi: 10.1016/j.neuroscience.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Taslim N, Dar MS. The Role of Nicotinic Acetylcholine Receptor (nAChR) alpha(7) Subtype in the Functional Interaction Between Nicotine and Ethanol in Mouse Cerebellum. Alcohol Clin Exp Res. 2010;35(3):540–9. doi: 10.1111/j.1530-0277.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- Taslim N, Soderstrom K, Dar MS. Role of mouse cerebellar nicotinic acetylcholine receptor (nAChR) alpha(4)beta(2)- and alpha(7) subtypes in the behavioral cross-tolerance between nicotine and ethanol-induced ataxia. Behav Brain Res. 2011;217:282–292. doi: 10.1016/j.bbr.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Hay-Schmidt A, Hansen HH, Mikkelsen JD. Distinct neural pathways mediate alpha7 nicotinic acetylcholine receptor-dependent activation of the forebrain. Cereb Cortex. 2010;20:2092–2102. doi: 10.1093/cercor/bhp283. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Louis VA, Taylor RE. Effects of Combined Systemic Alcohol and Central Nicotine Administration into Ventral Tegmental Area on Dopamine Release in the Nucleus Accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Wang JC, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The Basal Forebrain Cholinergic Projection System in Mice. In: Charles W, Paxinos G, Puelles L, editors. The Mouse Nervous System. San Diego: Academic Press; 2012. pp. 684–718. [Google Scholar]