Abstract
Problem
Cytomegalovirus (CMV) infection was previously reported in pregnancy complications. However, its seroprevalence and associated Toll-like receptor (TLR) expression in early onset preeclampsia (EOPE) with Hemolysis, Elevated Liver enzyme and Low Platelets syndrome (HELLPs) are unexplored.
Method of study
A case-control study was performed to examine maternal CMV antibodies, neutrophil Toll like receptor (TLR)-2 and -4 expression as well as the cytokine profile in EOPE with HELLPs (EOPE-HELLPs) (n=10), late onset preeclampsia (LOPE) (n=20), normal pregnancy (n=60), and non-pregnancy (n=20) controls.
Results
EOPE-HELLPs had significantly increased CMV IgG sero-positivity, up-regulated TLR-2/-4 mRNA expression, elevated serum IL-6 and TNF-α, and reduced IL-10 compared with matched normal and non-pregnancy controls. No significant difference was observed between LOPE and normal pregnancy controls.
Conclusion
We observed a significant association of CMV infection and innate immune response in EOPE-HELLPs. Our data suggest CMV infection may be a risk factor for this disorder.
Introduction
Preeclampsia (PE), characterized by hypertension and proteinuria during the second half of pregnancy, is a consequence of diverse pathophysiological processes involving impaired implantation, endothelial dysfunction and systemic inflammation.1, 2 It is a major cause of maternal and fetal mortality and morbidity.3 Preeclampsia is a heterogeneous disorder, and has been suggested to subdivide into early onset preeclampsia (EOPE) defined as preeclampsia that develops before 34 weeks of gestation, and late onset preeclampsia (LOPE) defined as preeclampsia that develops at or after 34 weeks of gestation.4 HELLPs (Hemolysis, Elevated Liver enzymes, Low Platelet syndrome), which is regarded as a variant of severe pregnancy complication, occurs in 0.5 to 0.9% of all pregnancies and in 10–20% of cases with severe preeclampsia.5 Not only is HELLPs a serious obstetric complication, but it also places the mother at increased risk for lipoprotein metabolism dysfunction later in life, 6 and is associated with severe fetal outcomes, such as prematurity, low birth-weight and still birth.7, 8
Human cytomegalovirus (CMV), a ubiquitous intracellular pathogen, is correlated with immune-compromised conditions.9, 10 During pregnancy, the maternal immune system tends to tolerate fetal derived paternal antigens by suppressing cell-mediated immunity, which may induce a state of increased susceptibility to intracellular pathogen reactivation, such as CMV.11 Previously, CMV infection was reported in either preeclampsia or HELLPs cases,12, 13 and has been reported to initiate Toll-like receptor (TLR) signalling.14
In CMV-seropositive persons, the monocyte appears to be the major cell type harbouring the virus in blood, presumably in a latent state.15, 16 However, there are numerous reports that the neutrophil is a major reservoir of infectious CMV. 17 In viremic patients, infectious virus was more often associated with the neutrophil fraction than the monocyte fraction of peripheral blood. The quantity of CMV DNA was significantly greater in poly-morphonuclear cells than in mononuclear leukocytes. Neutrophils have also been shown to stain positively for the CMV structural virion phosphoproteins pp65 (UL83) and pp150 (UL32).18, 19 Further, evidence suggests that the extent of neutrophil activation correlates with the severity of preeclampsia.20,21
TLRs comprise the major family of pattern recognition receptors that are involved in innate immune response to infectious agents.22, 23 They are expressed in various cell types, including circulating immune cells and maternal-fetal interface trophoblasts; their expression pattern may vary according to the different stage of pregnancy.23 TLR-2 and TLR-4 are expressed on peripheral neutrophils.24 They were known as bacterial sensors, but also reported to sense virus infection.25, 26 Although TLR-3 is recognized as a sensor for virus expression, it is not expressed on neutrophils.27 A previous study showed that CMV activates inflammatory cytokine responses via CD14 and TLR-2.28 CMV also can induce TLR-4 signalling and alter downstream cytokine production.25 Polymorphisms in TLR-2 associate with congenital CMV infection.29 Accumulating evidence also suggest that TLR-2 and TLR-4 may play important roles in preeclampsia and their expression is unregulated in this condition.30
The aim of this study was to examine maternal CMV seroprevalence, neutrophil TLR-2/-4 expression, and inflammatory cytokines (interleukin [IL]-6, tumour necrosis factor [TNF]-α, interferon [IFN]-γ and IL-10) in EOPE with HELLPs (EOPE-HELLPs) and LOPE, compared with matched normal pregnancy and non-pregnancy controls.
Methods
Blood was collected from women following informed consent. Ethics approval was granted by the University of British Columbia and the Children's and Women's Health Centre of British Columbia (C&W).
HELLPs was defined as hemolysis (defined as serum lactate dehydrogenase (LDH) >600 U/L and/or haptoglobin ≤0.3 g/L), elevated liver enzymes (serum aspartate aminotransferase (AST) >70 U/L or serum alanine aminotransferase (ALT) >70 U/L), and a low platelet count (<100×109/L).31 Preeclampsia was diagnosed as hypertension (blood pressure [BP] ≥140/90 mmHg, taken twice a day more than 4 hours apart after 20 weeks of gestation, and proteinuria (≥0.3 g/day, ≥2+ dipstick reading for proteinuria, or ≥30mg protein/mmol creatinine)20. BPs were taken in a semi-recumbent position, with a supported arm and appropriately-sized cuff, using a manual mercury sphygmomanometer, with Korotkoff V used to determine diastolic BP 20.
Study subjects were 30 preeclampsia cases including 10 EOPE-HELLPs (<34 weeks) and 20 LOPE (≥34 weeks). 60 normal pregnancy controls and 20 non-pregnancy controls were matched with cases for maternal age (± 5 years), gestation age (± 2 weeks), and parity (± 1).
Clinical specimen
venous blood was taken antenatally. Serum was prepared by centrifugation and specimens were frozen at -80°C for case-control pair analysis. (See method supplement)
Detection anti-CMV IgG, IgM and IgA serology
Enzyme-linked immunosorbent assays (ELISA) were used to detect anti-CMV IgG, IgM (Calbiotech, Sprint Valley, CA, USA), and IgA (Diagnostic Automation Inc, CA, USA). (See method supplement)
Neutrophil isolation
Neutrophil-enriched cell pellet were isolated and saved for RNA preparation and cDNA synthesis (Qiagen RNeasy mini kit and ThermoScript RT-PCR System, Invitrogen, CA, USA). (See method supplement)
Relative quantitative SYBR Green real-time polymerase chain reaction (PCR)
The sequences of the primer pairs for TLR-2 and TLR4 are listed as follows:
human TLR-2-sense: 5′-GAATCCTCCAATCAGGCTTCTCT-3′
human TLR-2-antisense: 5′-CCTGAGCTGCCCTTGCA-3′
human TLR-4-sense: 5′-GGCATGCCTGTGCTGAGTT-3′
human TLR-4-antisense: 5′-GGACCGACACACCAATGATG-3′
TLR-2 and TLR-4 mRNA levels were measured with SYBR Green real-time PCR on a Sequence Detection System (ABI Prism 7300, Applied Biosystems), and normalised to an endogenous control 18s rRNA. For each clinical sample, the TLR-2/TLR-4 CT value was normalized as ΔC = CT (TLR-2/ TLR-4) - CT (18s). This relative expression was measured as: ΔΔCT = ΔCT (1) sample-ΔCT(1) calibrator. The value used to plot relative TLR-2/TLR-4 expression was calculated using the expression 2-ΔΔCT. (See method supplement)
Flow cytometry
Neutrophil surface TLR-2 and TLR-4 protein expression was examined with flow cytometry (BD FACS Calibur System, Mississauga, ON, Canada). (See method supplement)
Cytokine measurements using multiplex immunoassays
Serum cytokine profile was measured with a multiplexed fluorescent microsphere immunoassay by Luminex 100 System (Luminex Corporation, Austin, TX, USA). (See method supplement)
Statistics
Data were analysed with Prism 4.0 software (GraphPad, San Diego, CA, USA). Chi-square (χ2), Kruskal-Wallis ANOVA (KW), and Mann-Whitney U (MWu) tests were used, as appropriate. p <0.05 was considered statistically significant.
Results
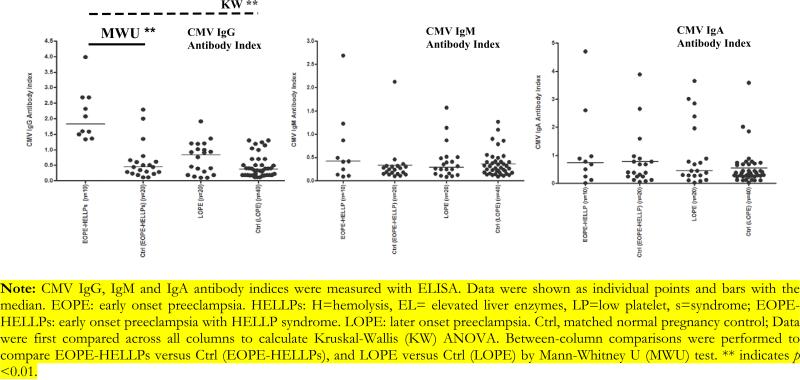
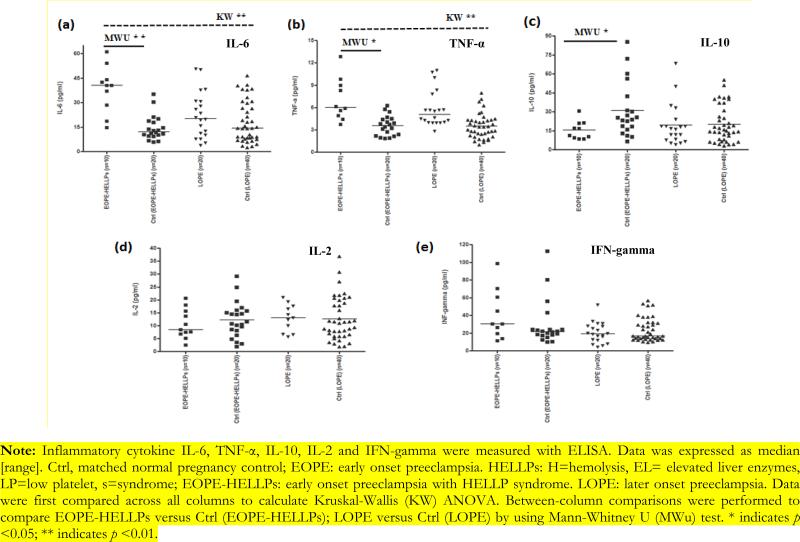
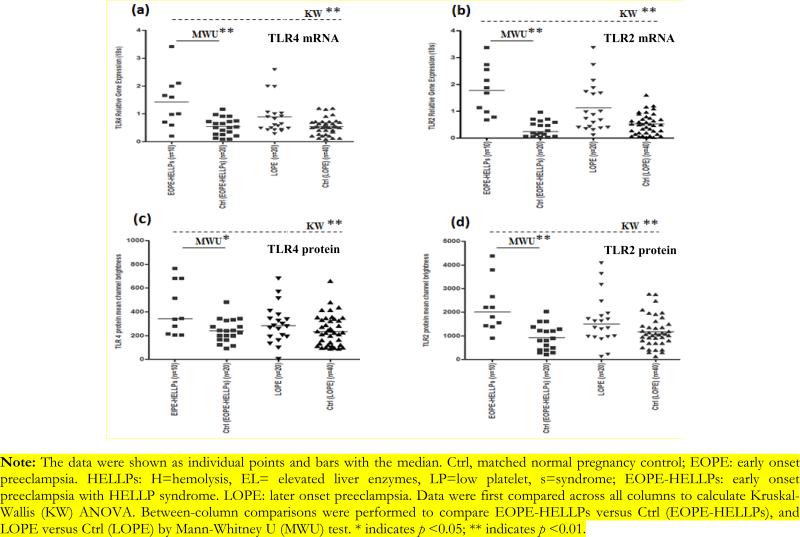
Study population characteristics were described in Table 1. Mean maternal age in patients with EOPE-HELLPs and the matched controls was 34 and 33 years respectively. EOPE-HELLPs more likely delivered premature babies and low birth weight babies than matched normal pregnancy controls (gestational age 25.9 weeks [24.1, 32.0] versus 37.5 weeks [37, 39.6], MWu p<0.05; baby birth weight: 1738 gram [395, 3500] versus 3380 gram [3000, 4615], MWu p<0.05). EOPE-HELLPs had higher CMV IgG seropositive rate (70% versus 30%, MWu p<0.01) (Table 2) and CMV IgG antibody index level than matched normal pregnancy controls (median [range], 1.83 [1.34, 3.99] versus 0.44 [0.11, 2.33], MWu p<0.01) (Figure 1). In addition, compared with matched pregnancy controls, EOPE-HELLPs was associated with increased TLR-4 (median [interquartile range] (IQR), 1.30 [0.23, 3.42] versus 0.51 [0.08, 1.16], MWu p<0.01) and TLR-2 mRNA expression (median IQR, 1.78 [0.68, 3.38] versus 0.23 [0.02, 0.97], MWu p<0.01). Also, we observed TLR-2 protein expression elevated in EOPE-HELLPs compared with normal pregnancies (median IQR, 2007.5 [900, 4380] versus 845 [200, 2014], MWu p<0.01). Moreover, EOPE-HELLPs had elevated serum cytokine IL-6 (median [IQR] 40.5 pg/ml [14.7, 61.2] versus 13.5 pg/ml [5.7, 35.0], MWu p<0.05), increased TNF-Δ (median [IQR] 5.9 pg/ml [4.4, 12.8] versus 4.5 [2.1, 9.4], MWu p<0.05), and reduced IL-10 (median [IQR] 16.6 pg/ml [8.5, 30.6] versus 30.9pg/ml [6.5, 85.2], MWu p<0.05) concentration compared with matched pregnancy controls (Figure 2). No significant difference was detected between LOPE and matched pregnancy controls (Figure 1, 2 and 3, Table 2). CMV IgG antibody index is correlated with inflammatory cytokine IL-6, TNF-α production, TLR-2 and TLR-4 gene expression (See data supplement). The association is more likely happened in the same individual (See data supplement).
Table 1.
Maternal and perinatal clinical characteristics
| Contrast Groups | Contrast Groups | ||||||
|---|---|---|---|---|---|---|---|
| Clinical Characteristic | EOPE-HELLPs (n=10) | Vs | Ctrl (EOPE-HELLPs) (n=20) | LOPE (n=20) | Vs | Ctrl (LOPE) (n=40) | p value (χ2 or KW) |
| Maternal | |||||||
| Maternal age (year) | 34 [20, 42] | 33 [24, 40] | 34 [23, 41] | 33 [29, 42] | >0.05 | ||
| Gestational age at delivery (weeks) | 25.9 * [24.1, 32.0] | 37.5 [37, 39.6] | 37.6 [34.4, 40.1] | 39.9 [37.9, 40.9] | <0.05 | ||
| MAP (mmHg) | 126 * [86, 139] | 82 [80, 102] | 118 [84, 139] | 83 [81, 103] | <0.0001 | ||
| Uric acid (uM) | 328 [236, 436] | - | 384 [298, 464] | - | N/A | ||
| Platelets (× 109/L) | 102 * [77, 150] | 200 [136, 268] | 176 [77, 277] | 215 [151, 269] | <0.05 | ||
| AST (uM) | 32.5 [27, 133] | - | 29 [20, 237] | - | N/A | ||
| Proteinuria (>= ‘++’) | 10 ** (100) | 0 (0) | 20 (100) | 0 (0) | <0.0001 | ||
| Perinatal | |||||||
| Birth weight (g) | 1738 * [395, 3500] | 3380 [3000, 4615] | 2702 [1480, 4400] | 3448 [2885, 4270] | <0.0001 | ||
| SGA (<5th centile) | 7 ** (70) | 0 (0) | 7 (35) | 0 (0) | <0.0001 | ||
Note: Data were expressed as median [range] or n (n %). Ctrl, matched normal pregnancy control; EOPE: early onset preeclampsia; HELLPs: Hemolysis Elevated Liver enzyme and Low Platelets syndrome. EOPE-HELLPs: EOPE with HELLPs; LOPE: late onset preeclampsia; KW, Kruskal-Wallis analysis of variance; MAP, mean arterial pressure = diastolic blood pressure + (pulse pressure/3); AST, aspartate transaminase; SGA, small for gestational age. For SGA, the pregnancy is deemed to have achieved the outcome if any one fetus was born less than fifth percentile for gestational age and gender using multiethnic Canadian birth weight charts. Data were first compared across all columns to calculate Kruskal-Wallis (KW) ANOVA or χ2 p-value. Between-column comparisons were performed to compare EOPE-HELLPs versus Ctrl (EOPE-HELLPs); LOPE versus Ctrl (LOPE) by using Mann-Whitney U (MWu) test or Chi-square test. Vs: versus.
indicates p <0.05
indicates p <0.01.
Table 2.
CMV Seroprevalence in cases and controls.
| Contrast Groups | Contrast Groups | ||||||
|---|---|---|---|---|---|---|---|
| CMV Seroprevalence | EOPE-HELLPs (n=10) | Vs | Ctrl (EOPE-HELLPs) (n=20) | LOPE (n=20) | Vs | Ctrl (LOPE) (n=40) | p value (χ2) |
| IgG seropositive rate | 7 (70) ** | 6 (30) | 10 (50) | 16 (40) | p<0.01 | ||
| IgM seropositive rate | 2 (20) | 1 (5) | 2 (10) | 2 (5) | p>0.05 | ||
| IgA seropositive rate | 2 (20) | 3 (15) | 5 (25) | 3 (15) | p>0.05 | ||
Note: Data were expressed as n (n %). Ctrl, matched normal pregnancy control; EOPE: early onset preeclampsia. HELLPs: H=hemolysis, EL= elevated liver enzymes, LP=low platelet, s=syndrome; EOPE-HELLPs: early onset preeclampsia with HELLP syndrome. LOPE: later onset preeclampsia; CMV: cytomegalovirus. Data were first compared across all columns to calculate χ2 p-value. Vs: versus. Between-column comparisons were performed to compare EOPE-HELLPs versus Ctrl (EOPE-HELLPs); LOPE versus Ctrl (LOPE) by using Chi-square (χ2) test.
indicates p<0.01.
Figure 1.
CMV Antibody Index in cases and controls.
Note: CMV IgG, IgM and IgA antibody indices were measured with ELISA. Data were shown as individual points and bars with the median. EOPE: early onset preeclampsia. HELLPs: H=hemolysis, EL= elevated liver enzymes, LP=low platelet, s=syndrome; EOPEHELLPs: early onset preeclampsia with HELLP syndrome. LOPE: later onset preeclampsia. Ctrl, matched normal pregnancy control; Data were first compared across all columns to calculate Kruskal-Wallis (KW) ANOVA. Between-column comparisons were performed to compare EOPE-HELLPs versus Ctrl (EOPE-HELLPs), and LOPE versus Ctrl (LOPE) by Mann-Whitney U (MWU) test. ** indicates p <0.01.
Figure 2.
Inflammatory cytokine profile in cases and controls.
Note: Inflammatory cytokine IL-6, TNF-α, IL-10, IL-2 and IFN-gamma were measured with ELISA. Data was expressed as median [range]. Ctrl, matched normal pregnancy control; EOPE: early onset preeclampsia. HELLPs: H=hemolysis, EL= elevated liver enzymes, LP=low platelet, s=syndrome; EOPE-HELLPs: early onset preeclampsia with HELLP syndrome. LOPE: later onset preeclampsia. Data were first compared across all columns to calculate Kruskal-Wallis (KW) ANOVA. Between-column comparisons were performed to compare EOPE-HELLPs versus Ctrl (EOPE-HELLPs); LOPE versus Ctrl (LOPE) by using Mann-Whitney U (MWu) test. * indicates p <0.05; ** indicates p <0.01.
Figure 3.
TLR-4 and TLR-2 mRNA and protein expression in cases and controls.
Note: The data were shown as individual points and bars with the median. Ctrl, matched normal pregnancy control; EOPE: early onset preeclampsia. HELLPs: H=hemolysis, EL= elevated liver enzymes, LP=low platelet, s=syndrome; EOPE-HELLPs: early onset preeclampsia with HELLP syndrome. LOPE: later onset preeclampsia. Data were first compared across all columns to calculate Kruskal-Wallis (KW) ANOVA. Between-column comparisons were performed to compare EOPE-HELLPs versus Ctrl (EOPE-HELLPs), and LOPE versus Ctrl (LOPE) by Mann-Whitney U (MWU) test. * indicates p <0.05; ** indicates p <0.01.
Discussion
This study is the first to show an association of CMV infection, TLR-2/-4 up-regulation, and pro-inflammatory phenotype in EOPE-HELLPs, but not in LOPE, compared with matched pregnancy controls. A previous study reported the odds ratio for perinatal death and severe neonatal morbidity was significantly increased in EOPE compared with LOPE.32 We observed EOPE cases more frequently delivered premature babies, and more likely developed HELLPs, which was similar to published findings.30,33 EOPE and LOPE may share some etiologic features, but differ with regard to several risk factors, and lead to different outcomes.32 The two preeclampsia types should therefore be treated as distinct entities from an etiologic and prognostic standpoint.32
In the present study, we examined three classes of CMV-specific serum antibodies IgG, IgM and IgA, which were measured among EOPE with HELLP syndrome, LOPE, and matched normal pregnancy controls. IgG indicates previous infection; anti-CMV IgM suggests acute or primary CMV infection; and anti-CMV IgA is associated with primary, chronic, or recurrent infections 34, but it is not a specific indicator for CMV infection. In chronic or recurrent infection, detectable IgM is uncommon, while IgG levels may rise quickly 34. Previously, we have observed increased anti-CMV IgG antibody level in women who later developed EOPE 35. To confirm CMV primary infection or reactivation, CMV avidity testing should be applied, since it is a useful method to distinguish CMV primary infection from non-primary infection and reactivation.36
Several mechanisms may explain the relation between CMV infection and EOPE-HELLPs. CMV infection has direct effects on the arterial wall, including endothelial-platelet dysfunction 37 and acute atherosis, which can result in relative uteroplacental ischemia, a key pathogenic feature in EOPE.38 CMV infection also has indirect effects, via immune CD14, TLR-2 and TLR-4, to induce pro-inflammatory cytokines, as an initiator of placental, fetal injury and liver dysfunction.26, 39 Moreover, increasing evidence suggests that up-regulated TLR expression plays an important role in the apoptosis in first trimester trophoblast cells; 40 and elevated circulating IL-6 and TNF-α contribute to endothelial dysfunction, which may link with the pathogenesis of EOPE and HELLPs 33, 41,42
The primary limitation of this work was the relatively small sample size. Additionally, it was designed for primiparous subjects, and may not be extrapolated to multiparous women. Given the finding concerning CMV infection in the present study, to attain sufficient statistical power, our data needs to be confirmed in a larger obstetric population followed longitudinally.
Supplementary Material
Acknowledgment
This work was supported by investigator pilot grant from the Canadian Institutes of Health Research (CIHR) Institute to Dr. Peter von Dadelszen and National Institute of Health (DP1HDO75624) to Dr. Joe Nadeau.
Details of ethics approval This study was approved by the University of British Columbia Clinical Research Ethics Board and the BC Children's and Women's Health Centre Review Board. Reference certification number: CW01-0035.
Footnotes
Disclosure of interest None
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 4.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2003;22(2):143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 5.Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC pregnancy and childbirth. 2009;9:8. doi: 10.1186/1471-2393-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman TM, Wendon J. Severe hepatic dysfunction in pregnancy. QJM : monthly journal of the Association of Physicians. 2002;95(6):343–57. doi: 10.1093/qjmed/95.6.343. [DOI] [PubMed] [Google Scholar]
- 7.Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18(16):2975–88. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CM, Chang SD, Cheng PJ, Chao AS. Comparisons of maternal and perinatal outcomes in Taiwanese women with complete and partial HELLP syndrome and women with severe pre eclampsia without HELLP. The journal of obstetrics and gynaecology research. 2006;32(6):550–8. doi: 10.1111/j.1447-0756.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 9.Colomba C, Lalicata F, Siracusa L, Saporito L, Di Bona D, Giammanco G, et al. [Cytomegalovirus infection in immunocompetent patients. Clinical and immunological considerations]. Le infezioni in medicina : rivista periodica di eziologia, epidemiologia, diagnostica, clinica e terapia delle patologie infettive. 2012;20(1):12–5. [PubMed] [Google Scholar]
- 10.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(11):1439–47. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerging infectious diseases. 2006;12(11):1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie F, Hu Y, Magee LA, Money DM, Patrick DM, Krajden M, et al. An association between cytomegalovirus infection and pre-eclampsia: a case-control study and data synthesis. Acta Obstet Gynecol Scand. 2010;89(9):1162–7. doi: 10.3109/00016349.2010.499449. [DOI] [PubMed] [Google Scholar]
- 13.Ohkuchi A, Minakami H, Suzuki I. Ayustawati, Izumi A, Sato I. Liver dysfunction in late pregnancy: cytomegalovirus-induced hepatitis or the HELLP syndrome? The journal of obstetrics and gynaecology research. 2001;27(6):319–23. doi: 10.1111/j.1447-0756.2001.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie F, Hu Y, Turvey SE, Magee LA, Brunham RM, Choi KC, et al. Toll-like receptors 2 and 4 and the cryopyrin inflammasome in normal pregnancy and pre-eclampsia. BJOG. 2010;117(1):99–108. doi: 10.1111/j.1471-0528.2009.02428.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–64. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 16.Craigen JL, Yong KL, Jordan NJ, MacCormac LP, Westwick J, Akbar AN, et al. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997;92(1):138–45. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saltzman RL, Quirk MR, Jordan MC. Disseminated cytomegalovirus infection. Molecular analysis of virus and leukocyte interactions in viremia. J Clin Invest. 1988;81(1):75–81. doi: 10.1172/JCI113313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revello MG, Percivalle E, Di Matteo A, Morini F, Gerna G. Nuclear expression of the lower matrix protein of human cytomegalovirus in peripheral blood leukocytes of immunocompromised viraemic patients. J Gen Virol. 1992;73(Pt 2):437–42. doi: 10.1099/0022-1317-73-2-437. [DOI] [PubMed] [Google Scholar]
- 19.Grefte A, Harmsen MC, van der Giessen M, Knollema S, van Son WJ, The TH. Presence of human cytomegalovirus (HCMV) immediate early mRNA but not ppUL83 (lower matrix protein pp65) mRNA in polymorphonuclear and mononuclear leukocytes during active HCMV infection. J Gen Virol. 1994;75(Pt 8):1989–98. doi: 10.1099/0022-1317-75-8-1989. [DOI] [PubMed] [Google Scholar]
- 20.Belo L, Santos-Silva A, Caslake M, Cooney J, Pereira-Leite L, Quintanilha A, et al. Neutrophil activation and C-reactive protein concentration in preeclampsia. Hypertens Pregnancy. 2003;22(2):129–41. doi: 10.1081/PRG-120021059. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol. 2007;29(2):163–7. doi: 10.1007/s00281-007-0073-4. [DOI] [PubMed] [Google Scholar]
- 22.Xie F, Turvey SE, Williams MA, Mor G, von Dadelszen P. Toll-like receptor signaling and pre-eclampsia. Am J Reprod Immunol. 2010;63(1):7–16. doi: 10.1111/j.1600-0897.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 23.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, et al. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100(5):1860–8. [PubMed] [Google Scholar]
- 25.Yew KH, Carpenter C, Duncan RS, Harrison CJ. Human cytomegalovirus induces TLR4 signaling components in monocytes altering TIRAP, TRAM and downstream interferon-beta and TNF-alpha expression. PLoS One. 2012;7(9):e44500. doi: 10.1371/journal.pone.0044500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102(7):2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 28.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi R, Koyano S, Suzutani T, Goishi K, Ito Y, Morioka I, et al. Polymorphisms in TLR-2 are associated with congenital cytomegalovirus (CMV) infection but not with congenital CMV disease. Int J Infect Dis. 2013 doi: 10.1016/j.ijid.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Xie F, Hu Y, Turvey SE, Magee LA, Brunham RM, Choi KC, et al. Toll-like receptors 2 and 4 and the cryopyrin inflammasome in normal pregnancy and pre-eclampsia. BJOG. 2010;117(1):99–108. doi: 10.1111/j.1471-0528.2009.02428.x. [DOI] [PubMed] [Google Scholar]
- 31.Sibai BM, Taslimi MM, el-Nazer A, Amon E, Mabie BC, Ryan GM. Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia-eclampsia. Am J Obstet Gynecol. 1986;155(3):501–9. doi: 10.1016/0002-9378(86)90266-8. [DOI] [PubMed] [Google Scholar]
- 32.Lisonkova S, Joseph KS. Incidence of pre-eclampsia: risk factors and outcomes associated with early- versus late-onset disease. American journal of obstetrics and gynecology. 2013 doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 33.van Rijn BB, Franx A, Steegers EA, de Groot CJ, Bertina RM, Pasterkamp G, et al. Maternal TLR4 and NOD2 gene variants, pro-inflammatory phenotype and susceptibility to early-onset preeclampsia and HELLP syndrome. PLoS One. 2008;3(4):e1865. doi: 10.1371/journal.pone.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman MG aE, A.W. The use of serum IgA as a diagnostic marker in viral infections. Immunol Infect Dis. 1991;1:223–34. [Google Scholar]
- 35.Xie F, Hu Y, Magee LA, Money DM, Patrick DM, Krajden M, et al. An association between cytomegalovirus infection and pre-eclampsia: a case-control study and data synthesis. Acta Obstet Gynecol Scand. 2010;89(9):1162–7. doi: 10.3109/00016349.2010.499449. [DOI] [PubMed] [Google Scholar]
- 36.Vauloup-Fellous C, Berth M, Heskia F, Dugua JM, Grangeot-Keros L. Re-evaluation of the VIDAS((R)) cytomegalovirus (CMV) IgG avidity assay: determination of new cut-off values based on the study of kinetics of CMV-IgG maturation. J Clin Virol. 2013;56(2):118–23. doi: 10.1016/j.jcv.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Reyna-Villasmil E, Mejia-Montilla J, Reyna-Villasmil N, Torres-Cepeda D, Pena-Paredes E, Santos-Bolivar J, et al. [Endothelial microparticles in preeclampsia and eclampsia]. Medicina clinica. 2011;136(12):522–6. doi: 10.1016/j.medcli.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 38.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta obstetricia et gynecologica Scandinavica. 2002;81(7):642–8. [PubMed] [Google Scholar]
- 39.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171(8):4320–8. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 40.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26(7):540–7. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Bamberg C, Fotopoulou C, Thiem D, Roehr CC, Dudenhausen JW, Kalache KD. Correlation of midtrimester amniotic fluid cytokine concentrations with adverse pregnancy outcome in terms of spontaneous abortion, preterm birth, and preeclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(6):812–7. doi: 10.3109/14767058.2011.587918. [DOI] [PubMed] [Google Scholar]
- 42.Buying sex, safely. Lancet. 1996;348(9024):347. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.