Abstract
The decline in sperm count rates over the last 50 years appears to parallel the rising prevalence of obesity. As lipids levels are strongly associated with obesity, high lipids levels or hyperlipidemia may thus play an important role in the decline in fertility in addition to other environmental or lifestyle factors. The objective of this population based cohort study was to evaluate the association between men’s serum lipid concentrations and semen quality parameters among 501 male partners of couples desiring pregnancy and discontinuing contraception. Each participant provided prospectively up to two semen samples (94% of men provided one or more semen samples, and 77% of men provided a second sample approximately one month later). Linear mixed effects models were used to estimate the associations between baseline lipid concentrations and semen quality parameters, adjusted for age, body mass index, and race. We found that higher levels of serum total cholesterol, free cholesterol and phospholipids were associated with a significantly lower percentage of sperm with intact acrosome and smaller sperm head area and perimeter. Our results suggest that lipid concentrations may affect semen parameters, specifically sperm head morphology, highlighting the importance of cholesterol and lipid homeostasis for male fecundity.
Keywords: epidemiology, fecundity, lipoprotein metabolism, semen quality
INTRODUCTION
There is great controversy over the decline in male fertility and semen quality around the globe (Jensen, Carlsen et al., 2002;Joffe, 2000;Joffe, Key et al., 2006;Sallmen, Weinberg et al., 2005). Some data have shown that sperm count rates have declined over the last 50 years with current reference ranges of approximately 15 million/mL (Swan, Elkin et al., 2000; Swan and Elkin, 1999; Swan, Elkin et al., 1997; Bonde, Kold et al., 1998; Rolland, Le Moal et al., 2103; Cooper, Noonan et al., 2010; Fisch, Goluboffe et al., 1996). However, other data are equivocal with regard to global semen quality parameters (Fisch 2013). This potential decline in semen parameters appears to parallel the rising prevalence of obesity. The prevalence of obesity has also increased over the same time period. In fact, the prevalence of obesity (defined as a body mass index ≥30) has increased from 12.8% in 1976-1980 to 35.5% by 2009–2010 (Ogden, Carroll et al., 2012; Flegal, Carroll et al., 1998). A global hyperlipidemia epidemic paralleled obesity rates during this time period, though of late hyperlipidemia rates are declining in some developed countries (Carroll, 2012). As lipids levels are strongly associated with obesity, high lipids levels or hyperlipidemia may thus play an important role in the decline in fertility in addition to other environmental or lifestyle factors (Crawford, Cote et al., 2010; Charlton 2009).
A potential link between lipids and human fecundity is plausible given that cholesterol is the main substrate for steroid synthesis, and also has been shown to play a crucial role in steroidogenesis and associated downstream effects including spermatogenesis (Gwynne and Strauss, III, 1982). Moreover, there is ample evidence from animal studies linking cholesterolemia, steroidogenesis and male fertility. Hypercholesterolemia in rabbits lowers Leydig and sertoli cell secretory function and lowers serum testosterone response to hCG (Yamamoto, Shimamoto et al., 1999). Cholesterol fed rats and rabbits have reductions in spermatid cell population, seminiferous tubules, and Leydig’s cell nuclear dimensions (Gupta and Dixit 1988). Even mild hyperlipidemia induced by cholesterol feeding significantly reduced sperm motility and density in the cauda epididymides and testis (Purohit and Daradka 1999; Bataineh and Nusier 2005). However, there are a lack of population level studies evaluating lipid concentrations and semen quality among men of couples seeking pregnancy without fertility treatment.
The purpose of this study is to evaluate the link between lipid concentrations and semen quality parameters independent of obesity in a population based prospective cohort study. We designed the recently completed Longitudinal Investigation of Fertility and the Environment (LIFE) Study to address environmental influences on human fecundity. These hypotheses are of great interest as there is a need to identify modifiable risk factors to improve male fecundity, particularly at the population level.
METHODS
Design and Study Population
The LIFE Study was a prospective cohort study designed to investigate environmental influences on human fecundity and fertility, and its design and methods have been described previously in detail (Buck Louis, Schisterman et al., 2011). In brief, 501 male partners of couples discontinuing contraception for the purposes of becoming pregnant were recruited from 16 counties in Michigan and Texas from 2005-2009 using sampling frameworks tailored for each State allowing for the identification of couples planning pregnancy in the near future. Eligible men were aged 18-51 years in a committed relationship; were able to communicate in English or Spanish; and were not surgically or medically sterile. Full human subjects’ approval was granted prior to obtaining informed consent from all participants.
Data Collection
Upon enrollment, in-person interviews were conducted with each male partner to ascertain health, demographic, and reproductive histories, as well as physical activity, and medication and supplement use (including lipid lowering drugs use). All data and biospecimens were collected in the home, and baseline interviews were followed by a standardized anthropometric assessment for determination of body mass index (BMI) as conducted by research nurses (Lohman TG, Roche AF, et al., 1988). The research nurse obtained non-fasting blood (~2 mL) for quantification of serum lipids. Samples were transported on ice to the site laboratories for processing where they were centrifuged for 15 minutes after approximately 2 hours and aliquoted according to the protocol. Samples were frozen at −20° or colder until shipment on ice to the CDC laboratory for analysis of serum lipids.
Laboratory Analysis of Serum Lipids
All assays were completed using a Hitachi Model 912 clinical analyzer at the Centers for Disease Control and Prevention Environmental Health Laboratory. Total cholesterol was analyzed with the Roche Cholesterol/HP method (Roche Diagnostics, Indianapolis, IN), an enzymatic colorimetric determination using cholesterol esterase and cholesterol oxidase. Free cholesterol used the Wako Free Cholesterol C method (Wako Chemicals USA, Inc., Richmond, VA), an enzymatic colorimetric assay which uses cholesterol oxidase and peroxidase but omits cholesterol esterase. Triglycerides were analyzed with the Roche Triglycerides/GPO method without blanking, and phospholipids were measured by using the Wako Phospholipids B enzymatic colorimetric method. The Wako phospholipids method uses phospholipase D and choline oxidase for the analysis, so it measures specifically the major choline-containing phospholipids including lecithin, lysolecithin, and sphingomyelin (Takayama, Itoh et al., 1977). All analyses were performed according to the directions of each kit. All unknown samples were analyzed concurrently with quality control (QC) materials with known concentrations. QC materials were evaluated using published QC rules (Caudill, Schleicher et al., 2008). All reported results were from runs found to be in control by standard statistical methods. Overall, the coefficients of variation range between 3-9%.
Semen Collection and Analysis
A semen sample was obtained at baseline followed by a second sample approximately one month apart (to identify azoospermia) irrespective of couples’ pregnancy status. Men collected semen samples through masturbation without the use of any lubricant following a recommended two days of abstinence using home collection kits (actual abstinence time: median 3 days, mean 4.12 days)(Royster, Lobdell et al., 2000;Turner TW and Schrader, 2006). At collection, a glass capillary tube was placed into the semen, and each subject recorded the duration of abstinence, time of semen collection and any information regarding sample collection loss or spillage. Semen samples were shipped via Federal Express overnight to the study’s andrology laboratory for semen analysis. Semen delivered to a central andrology laboratory by overnight mail in insulated mailing kits have been successful in maintaining specimens for other studies (Royster, Lobdell et al., 2000). Semen analysis after home collection has been reported to be reliable for all semen parameters with the exception of motility parameters (Morris, Jeffay et al., 2003;Stovall, Guzick et al., 1994). A percentage of sperm are alive after 24 hours and a next day motility assessment still can be made and may provide important information on sperm function and survivability (Stovall, Guzick et al., 1994).
We quantified 35 semen parameters including five reflecting general characteristics (volume, straw distance, sperm concentration, total sperm count, hypo-osmotic swollen), eight motility measures, 12 morphometry measures, 8 morphology measures, and two sperm chromatin stability assay measures, using established laboratory protocols inclusive of ongoing quality assurance and control procedures (American Society of Andrology, 1996).
The initial evaluation of the sample was conducted when the sample arrived at the laboratory consisting of recording the temperature, turbidity, color, liquefaction, and volume of the semen. A temperature logging monitor (Maxim Integrated, San Jose, CA, USA) placed on the collection jar determined the temperatures to which the semen had been exposed since collection. Motility assessments, viability estimates, sperm concentrations, the preparation of slides, and preservation of seminal plasma were conducted at this time. The glass capillary tube was evaluated on with a microscope to determine the distance the most progress sperm had traveled. Semen volume was measured to the nearest 0.1 ml. An aliquot of semen was heated to 37°C, placed in a 20 micron deep chamber, and sperm motility was assessed using the HTM-IVOS (Hamilton Thorne Biosciences, Beverly, MA) computer assisted semen analysis system (CASA). Sperm concentration was measured using the IVOS system and the IDENT™ stain (Zinnaman, Uhler et al., 1996). Sperm viability was conducted by hypo-osmotic swelling (HOS assay)(Jeyendran, Van der Ven et al., 1984). The HOS assay determines the structural and functional integrity of the cell membrane. Four microscope slides were prepared for sperm morphology and morphometry assessments. An aliquot of the whole semen was diluted in TNE buffer with glycerol and frozen for SCSA® analysis.
Sperm morphology was determined on a fixed, stained semen smear. Sperm morphology was classified by the two widely accepted classification systems; WHO 3rd Edition (traditional morphology) and WHO 5th Edition (strict morphology)(WHO 1992; WHO 2010). The main difference between these classification systems is how they classify a “borderline normal” sperm. They are reported as normal with the traditional scheme and abnormal with the strict scheme (Rothman, Bort et al., 2013). Morphometric analyses were conducted HTM-IVOS CASA (Hamilton Thorne Biosciences, Beverly, MA) and provided objective assessments of individual sperm head size and shape.
Progressive sperm motility was assessed by placing a flat capillary tube filled with hyaluronic acid placed into the fresh ejaculate and the progression of the vanguard sperm was measured when the specimen arrived at the laboratory the next day (Turner and Schrader, 2006). SCSA® was assayed according to the methods of Evenson, as modified by Breitenstein. 100μl of whole semen were diluted into 500μl TNE buffer and keep frozen at −70°C until analysis (Evenson, Jost et al., 1991; Brietenstein, Clark et al., 1994). The SCSA® procedure was conducted on a Coulter Epics Elite Flow Cytometer using the SCSA® program (SCSA diagnostics, Brookings, SD).
The second sample did not have the full range of measurements taken compared to the first sample, due to budgetary constraints. In the second sample, the analysis was limited to exclusively measurement of volume, concentration and motility.
Statistical Analysis
Five men were found to be azoospermic on both samples and were excluded from this analysis and were referred to clinical care. Descriptive analysis included the inspection of missing data and influential observations. The study cohort was assessed by select characteristics for male partners and quartiles of total cholesterol. Differences in characteristics between quartiles of total cholesterol were assessed using ANOVA and Fisher’s exact test, where appropriate. Linear mixed effects models were used to estimate the associations between lipid concentrations and semen quality parameters. Mixed modeling techniques were used to incorporate the inter-sample correlations for semen quality endpoints measured in both samples (volume, concentration, motility, and sperm head morphology). Models were adjusted for age (years), BMI (kg/m2), and race (non-Hispanic White, non-Hispanic Black, Hispanic, other) (Colaci, Afeiche et al., 2012;Keltz, Zapantis et al., 2010;Ramlau-Hansen, Thulstrup et al., 2007).
Models were also adjusted for fish consumption (as a proxy for dietary intake), and use of lipid lowering drugs (any report on the daily journal of taking a lipid lowering drug during the study follow-up), serum cotinine and alcohol consumption though adjustment for these factors did not appreciably change the results and were not included in the final models for parsimony.
A sensitivity analysis was conducted to evaluate the possible impact of unmeasured confounding by diet (e.g., dietary fat intake) or environmental factors in estimating the association between serum lipids and semen quality. Dietary fat intake could be considered as a potential unmeasured confounder, since intake may be correlated with lipid concentrations and has been associated with reduced semen quality (Attaman, Toth et al., 2012;Jensen, Heitmann et al., 2013). We simulated such a variable for a range of correlations between the unmeasured factor and semen quality (from half to double the observed effect), and between the unmeasured factor and lipids (ρ=0 to ρ=0.9), to represent mild to severe potential confounding factor. We compared the results of our final adjusted models to sensitivity models adjusting for this simulated dietary factor.
Semen quality parameters were also considered with Box-Cox transformation to achieve normality assumption in the linear mixed models. Following Handelsman,(Evenson, Larson et al., 2002) we found the optimal transformation parameter for each semen quality outcome, transformed the semen outcome, and reran the analyses to determine whether the obtained results were different from the primary analyses using untransformed semen outcomes. Specifically, for pre-specified lambda values ranging from 0 to 1, by 0.1, we applied the Box-Cox transformation formula. In this step, γ=0 is for the logarithm transformation and γ=1 is for no transformation. For each transformed semen quality outcome, we estimated the Shapiro-Wilks W statistic and the transformation corresponding to the largest W value is the optimal transformation.
RESULTS
The LIFE Study cohort comprised 501 male partners of couples attempting to become pregnant, among whom 347 (69%) achieved pregnancy. The mean average of male partners was (31.8±4.9) years; the majority of men were college educated (68%) and self-identified as non-Hispanic white race (72%). Age was significantly associated with total cholesterol levels (Table 1). Lipids were quantified for 491 (98.0%) males, with no differences in various characteristics by availability of blood (data not shown). The main reason for the absence of serum was insufficient volume following the analysis of environmental chemicals. A higher percentage of Hispanic than non-Hispanic men was in the upper quartile of total cholesterol as compared to the other quartiles. BMI was not associated with total cholesterol levels among study participants.
Table 1.
Sociodemographic description of male partners at baseline by quartile of total serum cholesterol concentrations, LIFE Study, 2005-2009.
| Characteristics: N (%) | Males Total cholesterol (mg/dL) |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | Q1: <167 118(24) |
Q2: 167-190 127(26) |
Q3: 191-216 123(25) |
Q4: >216 123(25) |
p-value | |
| Age, years; Mean (SD) | 31.8 (4.9) | 31.3(4.4) | 30.4(4.5) | 32.6(5.4) | 33.0(4.9) | <0.001 |
| BMI, kg/m2; Mean (SD) | 29.8 (5.6) | 29.1 (6.2) | 30.0(5.9) | 29.5(5.4) | 30.4(4.8) | 0.34 |
| Self-Identified Race/Ethnicity | ||||||
| Non-Hispanic White | 384 (78) | 100 (85) | 99 (78) | 101 (82) | 84 (68) | |
| Non-Hispanic Black | 23 (5) | 6 (5) | 6 (5) | 3 (2) | 8 (7) | 0.01 |
| Hispanic | 45 (9) | 2 (2) | 10 (8) | 14 (11) | 19 (15) | |
| Other | 39 (8) | 10 (8) | 12 (9) | 5 (4) | 12 (10) | |
| Education | ||||||
| < High School/Equivalent | 47 (10) | 7 (6) | 15 (12) | 11 (9) | 14 (11) | |
| Some College or Tech | 141 (29) | 32 (27) | 29 (23) | 44 (36) | 36 (29) | 0.22 |
| College Graduate or Higher | 303 (62) | 79 (67) | 83 (65) | 68 (55) | 73 (59) | |
| Baseline Serum Cotinine (ng/mL) | ||||||
| No exposure (0, 9.99) | 384 (78) | 90 (77) | 108 (85) | 98 (80) | 88 (72) | |
| Passive smoking (10, 99.99) | 22 (4) | 6 (5) | 3 (2) | 6 (5) | 7 (6) | 0.18 |
| Active smoking (100, 299.99) | 44 (9) | 11 (9) | 9 (7) | 6 (5) | 18 (15) | |
| Heavy smoking (300, 595.31) | 40 (8) | 10 (9) | 7 (6) | 13 (11) | 10 (8) | |
| Baseline Alcohol (per month) | ||||||
| < Once per month | 29 (7) | 9 (10) | 7 (6) | 5 (5) | 8 (8) | |
| Once per month | 39 (9) | 11 (12) | 9 (8) | 12 (11) | 7 (7) | |
| 2-3 days per month | 80 (19) | 15 (16) | 22 (20) | 22 (20) | 21 (20) | |
| Once a week | 104 (25) | 23 (24) | 28 (25) | 26 (24) | 27 (25) | 0.98 |
| 2-3 times per week | 127 (30) | 29 (31) | 32 (29) | 33 (30) | 33 (31) | |
| 4-6 times per week | 28 (7) | 4 (4) | 7 (6) | 9 (8) | 8 (8) | |
| Every day | 12 (3) | 3 (3) | 5 (5) | 2 (2) | 2 (2) | |
| Baseline Report of Participation in a Vigorous Exercise Program During the Last 12 Months |
207 (42) | 51(43) | 59 (46) | 53 (43) | 44 (36) | 0.37 |
| Any Use of Lipid Lowering Drugs During the Study Reported on the Daily Journals |
18 (4) | 8 (7) | 5 (4) | 3 (2) | 2 (2) | 0.16 |
Select lipid levels were associated with some but not all semen parameters. Specifically, a negative association was observed between total cholesterol and semen volume (β = −0.004, P<0.05) and percent hypo-osmotic swollen (β = −0.026, P<0.05) decrease after adjustment for age, BMI, study site, and race, while free cholesterol and was negatively associated with percent sperm head with acrosome (β = −0.043, P<0.05), sperm head area (β = −0.008, P<0.05), and sperm head perimeter (β = −0.005, P<0.05) (Table 2). Phospholipids also were negatively associated with sperm head area (β = −0.002, P<0.05) and percent with acrosome (β = −0.014, P<0.05).
Table 2.
Associations between male serum lipid concentrations and semen quality outcomes.
| Semen Quality Outcome (unit) | Cholesterol β(SE) | Free cholesterol β(SE) | Phospholipids β(SE) | Triglycerides β(SE) | Total lipid β(SE) | |
|---|---|---|---|---|---|---|
| Overall | Volume (ml) | −0.004 (0.002) | −0.010 (0.006) | −0.003 (0.002) | −0.001 (0.000) | −0.001 (0.000) |
| Sperm Concentration (*106/ml) | −0.021 (0.068) | −0.041 (0.203) | −0.004 (0.065) | 0.014 (0.017) | 0.006 (0.012) | |
| Total Sperm Count (*106/ml) | −0.292 (0.225) | −0.983 (0.673) | −0.164 (0.215) | −0.016 (0.057) | −0.028 (0.041) | |
| Distance Sperm Traveled in Straw (mm) | −0.002 (0.009) | 0.013 (0.027) | 0.002 (0.008) | 0.002 (0.002) | 0.001 (0.002) | |
| Hypo-osmotic swollen (%) | −0.026 (0.012) | −0.030 (0.035) | −0.011 (0.011) | 0.003 (0.003) | −0.000 (0.002) | |
| Motility | Amplitude of Lateral Head Displacement (μm) | −0.001 (0.001) | −0.002 (0.003) | −0.001 (0.001) | 0.000 (0.000) | 0.000 (0.000) |
| Avg. Path Velocity (μm/sec) | 0.002 (0.010) | 0.024 (0.029) | −0.005 (0.009) | 0.001 (0.002) | 0.001 (0.002) | |
| Beat Cross Frequency (Hz) | −0.006 (0.005) | −0.013 (0.015) | −0.006 (0.005) | 0.000 (0.001) | −0.000 (0.001) | |
| Curvilinear Velocity (μm/sec) | 0.005 (0.017) | 0.058 (0.051) | −0.005 (0.016) | 0.005 (0.004) | 0.003 (0.003) | |
| Linearity (%) | 0.000 (0.008) | 0.001 (0.025) | 0.001 (0.008) | 0.000 (0.002) | 0.000 (0.001) | |
| Percent Motility (%) | 0.007 (0.015) | 0.040 (0.046) | −0.006 (0.015) | 0.004 (0.004) | 0.002 (0.003) | |
| Straightness (%) | −0.000 (0.008) | 0.006 (0.025) | −0.000 (0.008) | 0.002 (0.002) | 0.001 (0.002) | |
| Straight-Line Velocity (μm/sec) | 0.003 (0.009) | 0.024 (0.027) | −0.003 (0.009) | 0.002 (0.002) | 0.001 (0.002) | |
| Morphometry | Sperm Head with Acrosome (%) | −0.017 (0.006) | −0.043 (0.018) | −0.014 (0.006) | −0.001 (0.001) | −0.002 (0.001) |
| Elongation Factor-Width/Length (%) | −0.002 (0.007) | 0.007 (0.020) | −0.003 (0.006) | 0.001 (0.002) | 0.001 (0.001) | |
| Sperm Head Area (μm2) | −0.003 (0.001) | −0.008 (0.003) | −0.002 (0.001) | −0.000 (0.000) | −0.000 (0.000) | |
| Sperm Head Length (μm) | −0.000 (0.000) | −0.002 (0.001) | −0.000 (0.000) | −0.000 (0.000) | −0.000 (0.000) | |
| Sperm Head Perimeter (μm) | −0.002 (0.001) | −0.005 (0.002) | −0.001 (0.001) | −0.000 (0.000) | −0.000 (0.000) | |
| Sperm Head Width (μm) | −0.000 (0.000) | −0.001 (0.001) | −0.000 (0.000) | 0.000 (0.000) | −0.000 (0.000) | |
| Round (%) | 0.000 (0.002) | −0.006 (0.007) | −0.002 (0.002) | −0.001 (0.001) | −0.001 (0.000) | |
| Pyriform (%) | 0.001 (0.008) | −0.006 (0.024) | 0.009 (0.008) | 0.000 (0.002) | 0.001 (0.001) | |
| Megalo head (%) | −0.002 (0.002) | −0.011 (0.007) | −0.003 (0.002) | −0.001 (0.001) | −0.001 (0.000) | |
| Micro head (%) | 0.003 (0.002) | 0.007 (0.005) | 0.002 (0.002) | −0.000 (0.000) | 0.000 (0.000) | |
| Coiled tail (%) | 0.015 (0.015) | 0.049 (0.043) | 0.011 (0.014) | 0.001 (0.004) | 0.001 (0.003) | |
| Other tail abnormalities (%) | −0.007 (0.005) | −0.028 (0.016) | −0.004 (0.005) | −0.002 (0.001) | −0.002 (0.001) | |
| Morphology | Amorphous (%) | 0.016 (0.014) | 0.019 (0.042) | 0.013 (0.014) | −0.004 (0.004) | −0.001 (0.003) |
| Bicephalic (%) | 0.002 (0.003) | −0.003 (0.008) | −0.001 (0.003) | −0.001 (0.001) | −0.001 (0.000) | |
| Cytoplasmic Droplet (%) | −0.009 (0.007) | −0.032 (0.020) | −0.007 (0.007) | −0.002 (0.002) | −0.001 (0.001) | |
| Immature Sperm (#immature) | 0.011 (0.025) | 0.015 (0.073) | 0.016 (0.023) | −0.001 (0.006) | 0.001 (0.005) | |
| Neck & Midpiece Abnormal (%) | −0.001 (0.013) | −0.004 (0.039) | 0.001 (0.013) | −0.003 (0.003) | −0.002 (0.002) | |
| Strict Criteria (%) | −0.005 (0.013) | 0.012 (0.039) | −0.003 (0.012) | 0.004 (0.003) | 0.002 (0.002) | |
| Taper (%) | −0.001 (0.004) | −0.004 (0.010) | 0.000 (0.003) | −0.001 (0.001) | −0.000 (0.001) | |
| WHO Normal (%) | −0.007 (0.016) | 0.007 (0.048) | −0.005 (0.015) | 0.005 (0.004) | 0.002 (0.003) | |
| Sperm chromatin stability |
DNA Fragmentation (%) | 0.001 (0.013) | −0.039 (0.039) | −0.005 (0.012) | −0.004 (0.003) | −0.002 (0.002) |
| High DNA Stainability (%) | 0.004 (0.007) | 0.011 (0.020) | 0.006 (0.006) | −0.001 (0.002) | −0.000 (0.001) |
Next day semen analysis
SE, standard error
Bolded: p < 0.05
Semen outcomes are not transformed. Mixed effects model (for volume, concentration, 24-hour motility, and sperm head morphology) and linear regression model (for the others) were used, and adjusted for age (years), BMI (kg/ m2), study site (Texas/Michigan) and race (non-Hispanic White, non-Hispanic Black, Hispanic, other).
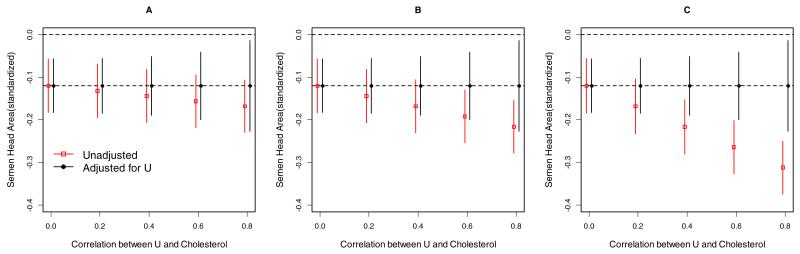
Our sensitivity analysis supported the robustness of our results in the presence of a possible mild to moderate unmeasured confounder (Figure 1). Still, we cannot entirely dismiss the possibility that an unmeasured dietary fat intake may account for the results. However, the unmeasured factor would need to exert twice the effect as the observed association with lipids, while being highly correlated with lipids (ρ=0.6 or larger) to completely explain away the observed association between cholesterol levels and semen quality. A degree of confounding this extreme seems implausible. Sensitivity analysis was used to evaluate the effects under the Box-Cox family of transformations and results were similar (Supplementary Table 1). Specifically, all except one of the primary significant findings were obtained in the sensitivity analysis. The sensitivity analysis also revealed five more significant findings, mostly in cholesterol with sperm head. We remain conservative with our reporting given that Box-Cox transformations are known to increase the type I error.
Figure 1.
Sensitivity analyses of lipid concentrations (X) and semen quality (standardized sperm head area) unadjusted and adjusted for a hypothesized unmeasured confounding factor U (e.g., standardized dietary factor that could influence both cholesterol levels and semen quality). Simulations are data-driven based on results from models of male standardized total cholesterol (i.e., mean 0, standard derivation 1) levels on semen quality. b/a is the ratio of the strength of the association between the unmeasured factor and semen quality compared to the association between total cholesterol levels and semen quality (A: b/a=0.5, B: b/a=1, C: b/a=2).
DISCUSSION
Overall, we observed that total and free cholesterol and phospholipid concentrations were negatively associated with several sperm head morphology parameters independently of BMI. Total cholesterol concentrations also were associated with reductions in semen volume and live/total count. These results highlight the role of serum lipids in male fecundity, and should be of concern given the rising prevalence of obesity and dyslipidemia.
There are many stages in the development of sperm where cholesterol and lipid homeostasis could potentially influence multiple semen quality parameters and ultimately the fertilization competency of sperm (Keber, Rozman et al., 2013;Maqdasy, Baptissart et al., 2013). The mammalian sperm plasma membrane primarily comprises phospholipids and cholesterol, which undergo several modifications upon removal from the testis. Cholesterol efflux from the sperm plasma membrane, referred to as capacitation, is responsible for changes in membrane fluidity necessary for the acrosome reaction and fertilization competence(Cross, 1998). In rodent models, dietary cholesterol and hypercholesterolemia impair testicular function,(Gupta and Dixit, 1988) and in rabbit models sperm head membrane lipids are altered by hypercholesterolemic diets (az-Fontdevila and Bustos-Obregon, 1993). Cholesterol and polyunsaturated acid enriched diets may also slow the kinetics of the acrosome reaction in rabbit spermatozoa and cause detrimental effects on spermatogenesis and overall sperm fertilizing capacity.
Our results indicate that higher levels of serum total cholesterol, free cholesterol and phospholipids are associated with a significantly lower percentage of sperm with intact acrosome, smaller sperm head area and perimeter. These results are consistent with sperm that are further along the acrosome reaction and capacitation path. These results seemingly conflict with findings from animal studies that report higher levels of cholesterol being associated with a slower acrosome reaction (Cross, 1998). In very small cross-sectional human studies, total cholesterol has been shown to be higher and phospholipid classes lower, in the seminal plasma of azoospermic men compared to normo- and oligospermic men (Sebastian, Selvaraj et al., 1987). Two small studies among infertile men showed declines in semen quality associated with lipid abnormalities (Ergun, Kose et al., 2007;Padron, Mas et al., 1989). Hyperlipoproteinamic patients were also observed to have low semen quality (Padron, Mas et al., 1989). Our data support these findings and represent the first and largest population based evidence for serum lipid concentrations and semen quality.
The relation between the lipid-associated changes in sperm morphology observed in our study and male fecundity are unknown, given the absence of research involving men not seeking clinical investigation. In a previous clinical study of couples undergoing IVF, smaller median sperm head area and greater uniformity of sperm head size was observed in men without a history of an unassisted pregnancy (Aziz, Fear et al., 1998). A similar statistically significant difference in sperm head area was observed in a previous examination of the predictive value of live sperm head morphometry for the achievement of pregnancy in vivo (Irvine, Macleod et al., 1994). We observed similar results in previous work using the LIFE study in that individual semen quality parameters, especially sperm head parameters, were positively and negatively significantly associated with time to pregnancy, though not after adjusting for couples’ ages and BMI (Buck Louis, Sundaram et al., 2013). Improvements in semen quality through lifestyle modifications to improve lipid concentrations may therefore improve overall couple fecundity.
There is less information regarding the direct associations of serum triglycerides with sperm parameters independent of hypercholesterolemia. At least one study however has shown that increased triglycerides may have deleterious effects on spermatiogenesis (Ergun, Köse et al., 2007). Increased serum VLDL, total triglycerides and testosterone levels were significantly correlated with decreased sperm motility in a group of infertile men. Similarly, negative correlations between total triglycerides and VLDL levels with multiple sperm, lydig cell and seminiferous tubule parameters were documented in the goat (Monfared 2013). The mechanism of these associations is not clear however one line of evidence indicates that hormone sensitive lipase (HSL) may play a key role. HSL is a multifunctional enzyme that liberates fatty acids from triglycerides and cholesteryl esters for energy production. HSL gene knockout mice appear normal but are sterile due to oligospermia (Osuga, Ishibashi et al., 2000). Any sperm that were found in the epididymis of these mice were non-motile. Furthermore, the epididymal epithelia cells were heavily vacuolated, presumable with lipid. Interestingly, steroidogenic tissues other than testes were not affected indicating that the disruption of the HSL gene caused direct effects on spermatogenesis rather than effects secondary to hormone insufficiency (Saltiel 2000). Since HSL catalyzes the hydrolysis of cholesteryl esters and triglycerides it is therefore reasonable that the resulting free cholesterol and/or fatty acids are important for spermatogenesis. As previously discussed, the profound remodeling of sperm cholesterol during maturation may be related to this process. Fatty acids are not only energy rich molecules, they are also powerful signal molecules. Free fatty acids are agonists for peroxisome proliferation activated receptors (PPARs) via heterodimerization with the retinoid × receptors (RXRs) (Kliewer, Sundseth et al., 1997). Interestingly, disruption of RXRs in mice leads to abnormal spermatogenesis (Kastner, Mark et al., 1996).
If corroborated, our findings have both clinical and public health relevancy across men’s lifespan. For example, monitoring serum lipids with appropriate lifestyle and/or pharmacologic intervention may improve health and wellness across the lifespan. This paradigm would include the promotion and maintenance of reproductive health irrespective of pregnancy intentions, given growing recognition of the interrelatedness between male fecundity and later onset adult diseases (Buck Louis, Yeung et al., 2013;Skakkebaek, Rajpert-De et al., 2001). Moreover if corroborated, our findings underscore the role of the clinicians in preconception care. With growing recognition of the importance of preconception guidance and care for women (http://www.cdc.gov/reproductivehealth/infertility/publichealth.htm), our findings underscore the importance of including male partners in such care or even targeting such guidance for men.
This study has several strengths, including a large number of male participants recruited irrespective of serum lipids or pregnancy outcome and for whom serum lipids were individually quantified. In addition, anthropometric assessments were conducted with all men allowing for valid and reliable BMI measurement. Our thorough sensitivity analysis demonstrated that our results were robust to unmeasured confounding due to diet or other environmental or lifestyle factors, lessening concerns regarding residual confounding. Although we exemplified dietary fat as a potential unmeasured factor, these results are generalizable to any other factor of the same magnitude. When interpreting the results, it is important to keep in mind that our findings are limited by reliance on a single non-fasting serum sample, no measurement of high/low density lipoprotein or standardized dietary assessment, and the cross-sectional nature of the lipids and semen quality parameter. However, the fasting status is not likely to be related to semen quality and, therefore, any introduced bias introduced would likely be non-differential and would only affect the precision of the estimates. Another important study limitation is the use of next day semen quality analysis, which is not ideal for time-sensitive endpoints such as motility and viability (Buck Louis, Sundaram et al., 2013). We were able to globally assess the presence of motile sperm at collection through the glass straw methods described above. However, we recognize that the lack of findings between cholesterol concentrations and motility may be a result of using the next day analysis. Though the variability in measurement is increased (reducing efficiency), there is no evidence to support that the use of the next day analysis introduces bias as the laboratory staff were blinded to the fecundity status of the male and their lipid levels. Moreover, there is no empirical evidence to support a systematic difference in the integrity of semen samples collected in the home by a couples’ time-to-pregnancy. We recognize that the next day analysis is not suitable for clinical purposes, but is utilized here for large population-based studies. In addition, no differences were observed between various semen endpoints (excluding motility) between samples collected at home the night before compared to samples analyzed within 1.5 hours (Olshan, Perreault et al., 2007; Luben, Olshan et al., 2007).Study participation was not dependent upon serum lipids minimizing the selective recruitment of men with higher/lower lipid concentrations including for a range of lipid subcomponents. Our sensitivity analyses are not consistent with the presence of unmeasured or residual confounding from diet or other unmeasured factors, unless the effect was incredibly strong. We recognize the need for future work that times serum lipid quantification relative to sensitive window for spermatogenesis as we assumed that mean lipid concentrations were approximately constant over a short period of time (such as spermatogenesis). It is also important to recognize the exploratory nature of this study and the potential for spurious findings given the large number of comparisons being made. However, these findings are biologically plausible and should be replicated in other study populations.
In conclusion, our findings demonstrate that serum lipids may affect semen quality parameters, specifically sperm head morphology, highlighting the importance of cholesterol and lipid homeostasis for male fecundity. The exact mechanisms remain elusive, but effects on sperm head characteristics suggest a possible male mediated effect on couple fecundity.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (contracts #N01-HD-3-3355, N01-HD-3-3356 and N01-HD-3-3358). We acknowledge the Reproductive Health Assessment Team, Biomonitoring and Health Assessment Branch, National Institute for Occupational Health and Safety (NIOSH) for the analysis of semen samples under a Memo of Understanding with the NICHD.
Reference List
- American Society of Andrology . Semen Analysis: Challenges of QC and New Technology. 1996. [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- az-Fontdevila M, Bustos-Obregon E. Cholesterol and polyunsaturated acid enriched diet: effect on kinetics of the acrosome reaction in rabbit spermatozoa. Mol Reprod Dev. 1993;35:176–180. doi: 10.1002/mrd.1080350211. [DOI] [PubMed] [Google Scholar]
- Aziz N, Fear S, Taylor C, Kingsland CR, Lewis-Jones DI. Human sperm head morphometric distribution and its influence on human fertility. Fertil Steril. 1998;70:883–891. doi: 10.1016/s0015-0282(98)00317-3. [DOI] [PubMed] [Google Scholar]
- Bataineh HN, Nusier MK. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med J. 2005;26:398–404. [PubMed] [Google Scholar]
- Bonde JP, Kold JT, Brixen LS, Abell A, Scheike T, Hjollund NH, et al. Year of birth and sperm count in 10 Danish occupational studies. Scand J Work Environ Health. 1998;24:407–413. doi: 10.5271/sjweh.362. [DOI] [PubMed] [Google Scholar]
- Breitenstein MJ, Clark JC, Schrader SM, Simon SD. The Use of a Half-Mirror for the Measurement of αt in the Sperm Chromatin Structure Assay. Journal of Andrology. 1994;14:P45. [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development--the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Kim S, et al. Semen quality and time-to-pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertility and Sterility. 2013 doi: 10.1016/j.fertnstert.2013.10.022. pii: S0015-0282, 03168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Yeung E, Sundaram R, Laughon SK, Zhang C. The exposome--exciting opportunities for discoveries in reproductive and perinatal epidemiology. Paediatr Perinat Epidemiol. 2013;27:229–236. doi: 10.1111/ppe.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MD. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009-2010. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville, MD: 2012. [Google Scholar]
- Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- Charlton M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009;(Suppl 2):S83–9. doi: 10.1002/lt.21914. [DOI] [PubMed] [Google Scholar]
- Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, et al. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–1199. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- Crawford AG, Cote C, Couto J, Daskiran M, Cunnarsson C, Haas K, et al. Prevalence of Obesity, Type II Diabetes Mellitus, Hyperlipidemia, and Hypertension in the United States: Findings from the GE Centricity Electronic Medical Record Database. Pop Health Management. 2010;13:151–61. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- Cross NL. Role of cholesterol in sperm capacitation. Biol Reprod. 1998;59:7–11. doi: 10.1095/biolreprod59.1.7. [DOI] [PubMed] [Google Scholar]
- Ergün A, Köse SK, Aydos K, Ata A, Avci A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch Androl. 2007;53:21–23. doi: 10.1080/01485010600888961. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Jost L, Baer R, Turner T, Schrader S. Individuality of DNA Denaturation Patterns in Human Sperm as Measured by the Sperm Chromatin Structure Assay. Reproductive Toxicology. 1991;5:115–125. doi: 10.1016/0890-6238(91)90039-i. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–14. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Dixit VP. Effect of dietary cholesterol on spermatogenesis. Z Ernahrungswiss. 1988;27:236–243. doi: 10.1007/BF02019512. [DOI] [PubMed] [Google Scholar]
- Gwynne JT, Strauss JF., III The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- Irvine DS, Macleod IC, Templeton AA, Masterton A, Taylor A. A prospective clinical study of the relationship between the computer-assisted assessment of human semen quality and the achievement of pregnancy in vivo. Hum Reprod. 1994;9:2324–2334. doi: 10.1093/oxfordjournals.humrep.a138446. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Carlsen E, Jorgensen N, Berthelsen JG, Keiding N, Christensen K, et al. Poor semen quality may contribute to recent decline in fertility rates. Hum Reprod. 2002;17:1437–1440. doi: 10.1093/humrep/17.6.1437. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- Jeyendran RS, Van der Ven HH, Perez-Palaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- Joffe M. Time trends in biological fertility in Britain. Lancet. 2000;355:1961–1965. doi: 10.1016/S0140-6736(00)02328-X. [DOI] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Jensen TK. Human fertility decline? Epidemiology. 2006;17:238–239. doi: 10.1097/01.ede.0000199518.37404.6c. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, et al. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- Keber R, Rozman D, Horvat S. Sterols in spermatogenesis and sperm maturation. J Lipid Res. 2013;54:20–33. doi: 10.1194/jlr.R032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27:539–544. doi: 10.1007/s10815-010-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proceedings of the National Academy of Sciences. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- Luben TJ, Olshan AF, Herring AH, Jeffay S, Strader L, Buus RM, et al. The Healthy Men Study: an evaluation of exposure to disinfection by-products in tap water and sperm quality. Environ Health Perspect. 2007;115:1169–76. doi: 10.1289/ehp.10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JM, Volle DH. Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol. 2013;368:30–46. doi: 10.1016/j.mce.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Monfared AL. Correlation of Serum Lipid Profile with Histological and Seminal Parameters of Testis in The Goat. Int J of Fertil and Steril. 2013;7:122–129. [PMC free article] [PubMed] [Google Scholar]
- Morris RA, Jeffay SC, Strader LF, Evenson DP, Olshan AF, Lansdell LW, et al. Evaluation of sperm chromatin structure assay (SCSA) in human sperm after simulated overnight shipment[abstract] J Androl. 2003;24(suppl):54. 2003. [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. 2012. NCHS Data Brief 1-8. [PubMed] [Google Scholar]
- Olshan AF, Perreault SD, Bradley L, Buus RM, Strader LF, Jeffay SC, et al. The healthy men study: design and recruitment considerations for environmental epidemiologic studies in male reproductive health. Fertil Steril. 2007;87:554–64. doi: 10.1016/j.fertnstert.2006.07.1517. [DOI] [PubMed] [Google Scholar]
- Osuga J-i, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron RS, Mas J, Zamora R, Riverol F, Licea M, Mallea L, et al. Lipids and testicular function. Int Urol Nephrol. 1989;21:515–519. doi: 10.1007/BF02549590. [DOI] [PubMed] [Google Scholar]
- Purohit A, Daradka HMM. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian Journal of Experimental Biology. 1999;37:396–398. [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmann SA, Bort AM, Quickley J, Pillow R. Sperm morphology classification: a rational method for schemes adopted by the World Health Organization. In: Carrell DT, Aston KI, editors. Spermatogenesis: Methods and Protocols. Humana Press; New York: 2013. pp. 27–38. [DOI] [PubMed] [Google Scholar]
- Royster MO, Lobdell DT, Mendola P, Perreault SD, Selevan SG, Rothmann SA, Robbins WA. Evaluation of a container for collection and shipment of semen with potential uses in population-based, clinical, and occupational settings. J Androl. 2000;21:478–484. [PubMed] [Google Scholar]
- Sallmen M, Weinberg CR, Baird DD, Lindbohm ML, Wilcox AJ. Has human fertility declined over time?: why we may never know. Epidemiology. 2005;16:494–499. doi: 10.1097/01.ede.0000165391.65690.e1. [DOI] [PubMed] [Google Scholar]
- Saltiel AR. Another hormone-sensitive triglyceride lipase in fat cells? Proc Natl Acad Sci. 2000;97:535–537. doi: 10.1073/pnas.97.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian SM, Selvaraj S, Aruldhas MM, Govindarajulu P. Pattern of neutral and phospholipids in the semen of normospermic, oligospermic and azoospermic men. J Reprod Fertil. 1987;79:373–378. doi: 10.1530/jrf.0.0790373. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De ME, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Stovall DW, Guzick DS, Berga SL, Krasnow JS, Zeleznik AJ. Sperm recovery and survival: two tests that predict in vitro fertilization outcome. Fertil Steril. 1994;62:1244–1249. doi: 10.1016/s0015-0282(16)57193-3. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP. Declining semen quality: can the past inform the present? Bioessays. 1999;21:614–621. doi: 10.1002/(SICI)1521-1878(199907)21:7<614::AID-BIES10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama M, Itoh S, Nagasaki T, Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977;79:93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Turner TW, Schrader SM. Sperm migration assay as a measure of recently ejaculated sperm motility in specimens shipped overnight. [abstract] J Androl. 2006;27(suppl):58. [Google Scholar]
- World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3rd ed Cambridge University Press; Cambridge: 1992. [Google Scholar]
- World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5th ed World Health Organization Press; Geneva: 2010. [Google Scholar]
- Yamamoto Y, Shimamoto K, Sofikitis N, Miyagawa I. Effects of hypercholesterolaemia on Leydig and Sertoli cell secretory function and the overall sperm fertilizing capacity in the rabbit. Hum Reprod. 1999;14:1516–21. doi: 10.1093/humrep/14.6.1516. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Uhler ML, Vertuno E, Fisher SG, Clegg ED. Evaluation of computer-assisted semen analysis (CASA) with IDENT stain to determine sperm concentration. Journal of Andrology. 1996;17:288–292. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.