Abstract
Introduction
The use of single-tablet ART regimens and its implications on adherence among HIV-infected women have not been well-described.
Methods
Participants were enrolled in the Women’s Interagency HIV Study (WIHS), a longitudinal study of HIV infection in U.S. women. We examined semiannual trends in single-tablet regimen use and ART adherence, defined as self-reported 95% adherence in the past 6 months, during 2006–2013. In a nested cohort study, we assessed the comparative effectiveness of a single-tablet versus a multiple-tablet regimen with respect to adherence, virologic suppression, quality of life, and AIDS-defining events, using propensity score matching to account for demographic, behavioral, and clinical confounders. We also examined these outcomes in a subset of women switching from a multiple- to single-tablet regimen, using a case-crossover design.
Results
15,523 person-visits, representing 1,727 women (53% black, 29% Hispanic, 25% IDU, median age 47), were included. Use of single-tablet regimens among ART users increased from 7% in 2006 to 27% in 2013; adherence increased from 78% to 85% during the same period (both p<0.001). Single-tablet regimen use was significantly associated with increased adherence (adjusted RR 1.05, 95% CI 1.03–1.08) and virologic suppression (RR 1.06, 95% CI 1.01–1.11), while associations with improved quality of life and fewer AIDS-defining events did not achieve statistical significance. Similar findings were observed among the subset of switchers.
Conclusion
Single-tablet regimen use was associated with increased adherence and virologic suppression. Despite this, 15% of women prescribed ART were still not optimally adherent; additional interventions are needed to maximize therapeutic benefits.
Keywords: adherence, antiretroviral therapy, HIV, time factors, United States, viral load, women
INTRODUCTION
Among HIV-infected people who are prescribed potent antiretroviral therapy (ART), treatment adherence is important to maximize its health benefits with respect to virologic suppression and prevention of disease progression.(1, 2) However, it is well-known that adherence can be hampered by many factors including dosing requirements, side effects, and behavioral and psychosocial factors that serve as barriers to optimal use, such as substance use and depression.(3, 4) Characteristics associated with race/ethnicity have also been associated with adherence, with African-Americans less likely than other groups to report optimal adherence to ART.(5–7)
A lower daily pill burden has been associated with better adherence and treatment outcomes.(8, 9) One notable innovation among potent ART regimens was the introduction of a once-daily fixed-dose co-formulation in 2006 combining tenofovir (TDF), emtricitabine (FTC), and efavirenz (EFV), which reduced the potential pill burden and dosing frequencies required of earlier ART regimens.(10) Two additional once-daily combination pills retaining the TDF/FTC backbone but replacing EFV with one or more agents have since become available in the United States: a co-formulation containing rilpivirine (RPV) in 2011;(11) and a co-formulation containing elvitegravir (EVG) and the boosting agent cobicistat (COBI) in 2012.(12) These new co-formulations offer single-tablet regimen alternatives for women planning to become pregnant by replacing EFV, which may have the potential to cause fetal harm.(13, 14)
A few studies have shown single-tablet regimens to either maintain or increase treatment adherence. A multicenter clinical trial of 166 treatment-experienced virologically-suppressed individuals in the U.S. found that switching to a single-tablet ART regimen helped patients maintain adherence and increased some aspects of quality of life.(15, 16) In an observational cohort study of 118 homeless or unstably housed individuals in San Francisco, taking a single-tablet regimen was associated with greater adherence and viral suppression compared with a multiple-tablet regimen.(17) The generalizability of these findings to women has not been fully established, as these studies were comprised mostly of men. Numerous studies report lower adherence in women,(5, 6, 18) possibly related to higher toxicity profiles, a higher prevalence of depression, or competing demands such as childcare responsibilities.(19–21) The extent to which adherence in women may be affected by single-tablet regimen use in the context of these factors is unknown.
Given the limited data on use of these therapies and their influence on adherence in U.S. women, we examined semiannual trends in single-tablet regimen use and adherence among ART-treated HIV-infected women in the Women’s Interagency HIV Study (WIHS) between 2006 and 2013. Using a nested cohort study design, we compared the effectiveness of single-tablet versus multiple-tablet regimen use with respect to adherence and related health outcomes, including virologic suppression, disease progression, and quality of life, using propensity score matching to account for potential confounding by indication in a broad sample of ART-experienced WIHS participants. In a subset of participants who switched from a pre-existing regimen to a single-tablet regimen, we conducted a case-crossover study to test for a post-switch increase in adherence and virologic suppression, as an alternate way to account for confounding since each participant’s treatment outcomes after switching are compared with her outcomes before switching.
METHODS
Source population
The WIHS is a longitudinal study of over 4,000 HIV-infected and -uninfected women who have been followed at 6-month intervals at six U.S. sites, with detailed examinations, specimen collection, and structured interviews assessing health behaviors, medical history, and medication use.(22, 23) Women were recruited in 3 waves (1994–1995, 2001–2002, 2010–2012) from HIV primary care clinics, hospital-based programs, community outreach sites, women’s support groups, and other locations. In contrast to clinic-based cohorts that collect data through routine care, the WIHS is interval-based, meaning that visits occur independently of clinical care and therefore capture behaviors (e.g., ART non-adherence) that may be less likely to be reported to care providers. The demographic composition of study participants in the WIHS is representative of the U.S. female HIV-infected population.(24)
Inclusion criteria
Inclusion criteria included HIV infection and ART use. We included any person-visit in the WIHS between April 1, 2006 and March 31, 2013 during which an HIV-infected participant self-reported ART use in the previous six months and had a valid HIV-1 viral load measurement. In the time trend analysis only, we excluded ART users who enrolled in the WIHS in 2011 or later (N=701 person-visits) to avoid a potential cohort effect by this younger group. Because most WIHS participants during the study period are ART-experienced, and such individuals differ in adherence levels compared with those who are ART-naïve,(25) we limited analyses examining the association between single-tablet regimen use and adherence-related outcomes to ART-experienced women.
We conducted sensitivity analyses among a pre-defined subgroup of women who were less likely to conceive and therefore more likely to be indicated for use of the EFV/TDF/FTC co-formulation due to the following characteristics: age 45+; report of having undergone menopause; a history of sterilization (e.g., hysterectomy, tubal ligation, or oophorectomy); or use of hormonal birth control in the past 6 months. We also performed a sensitivity analysis excluding RPV/TDF/FTC and EVG/COBI/TDF/FTC users since they only comprised 6% of person-visits on a single-tablet regimen.
Exposure of interest
Our exposure of interest was single-tablet regimen use, defined as current use of one of the three available single-tablet ART formulations (EFV/TDF/FTC, RPV/TDF/FTC, EVG/COBI/TDF/FTC), and no other antiretroviral drugs, at each 6-month study visit.
Outcomes of interest
ART adherence is assessed in the WIHS by asking the participant the percentage of time during the past 6 months that ART was taken as prescribed,(26) categorized as: 100% of the time, 95–99%, 75–94%, and <75%. We dichotomized the response to 95% or greater adherence, based on prior work that has found this level of adherence to optimize virologic outcomes.(27) We also examined an alternate definition of 100% adherence versus <100% adherence.(28) This decision was supported by WIHS data showing 77–78% virologic suppression among women reporting either 95–99% or 100% adherence, but only 60% suppression among women reporting 75–94% adherence. Virologic suppression was defined as having an HIV-1 viral load <80 copies/mL. We assessed quality of life (QOL) based on a summary score derived from a shortened version of the Medical Outcomes Study-HIV.(29, 30) This score, which comprises six subdomains including physical function, pain, energy/fatigue, emotional well-being, social functioning, and role functioning, ranges from 0, representing worst QOL, to 100, representing best QOL. We defined improvement in QOL as a dichotomous variable capturing any increase in the QOL score from the previous visit. Finally, incident AIDS-defining clinical events were assessed via self-report at each visit or through matches with cancer or tuberculosis registries, using the 1993 CDC clinical AIDS definition.(31) We also examined a composite outcome of clinical AIDS or death. Death was ascertained based on active follow-up with participants or next of kin, or through death registry matches.
Other variables
We considered the following variables as potential confounders: age at visit, race/ethnicity, calendar year, recruitment period (2010–2012 versus earlier), income, education, employment, insurance status, enrollment in the AIDS Drug Assistance Program (ADAP), study site, CD4+ count, viral load, number of children, birth of child since last visit, history of sterilization, menopause status, current and past recreational drug use and alcohol use, housing status, and severe depressive symptoms (score ≥ 23), as assessed by the Center for Epidemiologic Studies Depression Scale [CES-D].(32)
Statistical methods
We examined time trends in once-daily single-tablet regimen use and in ART adherence, for each 6-month period between April 2006 and March 2013. Within each period, the numerator was the number of women in each category (e.g., on a single-tablet regimen), and the denominator was the number of women on ART. We tested for time trends using Poisson regression with generalized estimating equations (GEE).
Nested cohort study
To assess the effectiveness of single-tablet regimen use on adherence-related treatment outcomes, we compared outcomes between person-visits on a single tablet regimen and those not on a single-tablet regimen after propensity score matching to address potential confounding by indication.(33) We estimated the propensity score as the predicted probability of being on a single-tablet regimen, given the aforementioned confounders, by logistic regression. Using the propensity score, we matched person-visits of women on a single-tablet regimen with similar person-visits not on a single-tablet regimen, to eliminate the association between the confounding factors and use of a single-tablet regimen. Nearest neighbor matching was used for all confounders, except for history of sterilization and recruitment period, for which exact matching was used. We matched each single-tablet regimen person-visit to 3 non-single-tablet regimen person-visits for increased efficiency. Adequate balance on confounders was assessed based on an estimate of the standardized bias, defined as the difference in the means of each covariate before and after matching, divided by the standard deviation.(34) Standardized bias estimates ranged from <0.001 to 0.067, suggesting that the groups were well-balanced on all measured confounders. We used log-binomial regression (or Poisson regression when models did not converge) to estimate risk ratios for ART adherence, virologic suppression, improvement in QOL, and an AIDS-defining event at the visit following the index person-visit (i.e., six months after). We performed sensitivity analyses that stratified on baseline presence of viremia, to assess whether baseline viral load played a role in subsequent virologic suppression. Because QOL data in the WIHS are collected at every other visit, we used QOL data from the subsequent visit for person-visits with missing data. All analyses used GEE to account for correlated data within individuals.(35)
Case-crossover study
In a subset of women who switched from a pre-existing regimen to a single-tablet regimen, we tested for a post-switch increase in adherence and virologic suppression among those who remained on the single-tablet regimen for two consecutive visits, controlling for time-varying confounders, using a case-crossover study design.(36) Only time-varying confounders were needed because we compared outcomes in different person-visits corresponding to the same participant. We did not determine risk ratios for QOL or AIDS-defining events due to insufficient data. Among those not fully adherent to their ART regimen (i.e., <100% adherence), we compared the reasons for missing ART medications while on the preexisting regimen versus the single-tablet regimen, using a standardized questionnaire.(26)
We used SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2 (R Foundation for Statistical Computing, Geneva), including the MatchIt package for propensity score matching,(37) for analysis.
RESULTS
There were 15,523 person-visits between April 2006 and March 2013, representing 1,727 ART-treated women, included in this analysis. Briefly, 53% were black, 29% Hispanic, and 15% white. The median age at visit was 47 (interquartile range [IQR]: 41–52). 71% had a history of any recreational drug use (25% injection drugs), and 17% were currently using recreational drugs. 71% had an income of $24,000 or less, and 19% had a CES-D score of 23+, indicative of severe depressive symptoms. The median CD4+ count at the time of visit was 528 cells/uL (IQR 346–733), and the median viral load was undetectable (75th percentile = 92 copies/mL). 44% had a history of sterilization. Among participant visits reporting use of a non-single-tablet regimen, 70% were on a protease inhibitor (PI)-based regimen, while 26% were on a non-nucleotide reverse transcriptase inhibitor (NNRTI)-based regimen. 94.6% of participant visits reporting use of a single-tablet regimen were on EFV/TDF/FTC, with 5% on RPV/TDF/FTC and the remainder on EVG/COBI/TDF/FTC. Among the 511 single-tablet regimen users during the study period, 13% were ART-naïve when first starting the regimen.
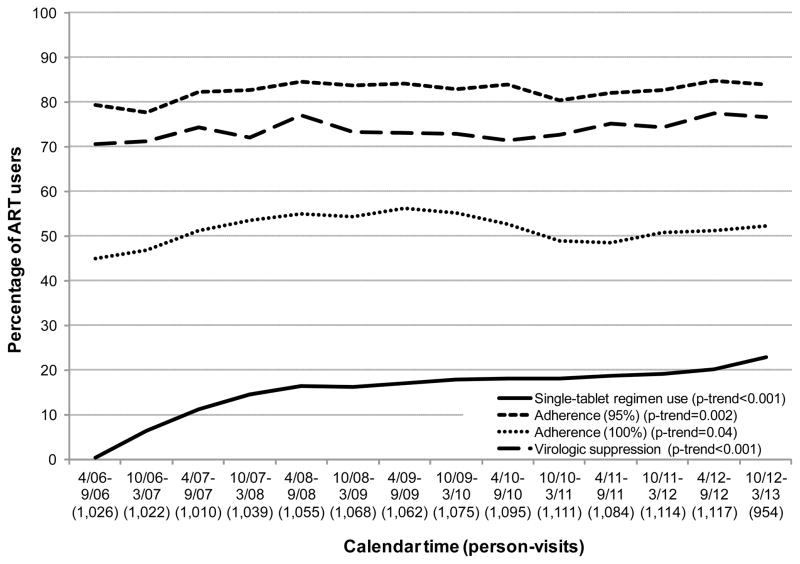
Figure 1 shows trends between 2006 and 2013 in single-tablet regimen use, ART adherence, and virologic suppression among established participants using ART in the WIHS. Use of single-tablet regimens significantly increased from 7% in 2006 to 27% in 2013 (ptrend<0.001). During the same period, adherence increased from 78% to 85% (ptrend<0.001), while virologic suppression increased from 71% to 77% (ptrend<0.001). After taking into account the increased use of single-tablet regimens, the calendar-time increases in adherence and virologic suppression were attenuated by 53% and 21%, respectively, suggesting that single-tablet regimens contributed considerably to the increase in adherence over time. These relationships persisted when focusing on women more likely to have no contraindication for EFV/TDF/3TC (75% of the study population), i.e., women no longer of child-bearing age, sterile women, or women on hormonal contraception.
Figure 1.
Trends in single-tablet regimen use, adherence, and virologic suppression among ART users, Women’s Interagency HIV Study, 2006–2013.
Among treatment-experienced women, there were 1,846 person-visits between 2006 and 2013 on a single-tablet regimen available for the nested cohort study, propensity-score matched on a 1:3 basis with 5,348 person-visits on a multiple-tablet regimen. Table 1 shows selected characteristics of women at these visits, before and after matching. Being on a single-tablet regimen was associated with a 5% increase in adherence, defined as taking one’s medications at least 95% of the time during the previous six months (adjusted risk ratio [RR] 1.05, 95% confidence interval [CI] 1.03–1.08) (Table 2). Defining adherence as taking one’s medications 100% of the time resulted in a larger association (RR 1.18, 95% CI 1.10–1.26).
Table 1.
Demographic, clinical, and behavioral characteristics of ART users at index visit, by single-tablet regimen use, Women’s Interagency HIV Study, 2006–2013
| Characteristics at index visit | Single-tablet regimen, Nperson-visits=1,846 | Multiple-tablet regimen before matching, Nperson-visits=11,387 | Multiple-tablet regimen after matching, Nperson-visits=5,348 | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Demographic | ||||||
| Age (years) (median, IQR) | 47 | 42–52 | 46 | 40–52 | 47 | 41–53 |
| Age | ||||||
| 18–29 years | 42 | 2.3 | 219 | 1.9 | 73 | 1.4 |
| 30–39 years | 280 | 15.2 | 2359 | 20.7 | 1016 | 19.0 |
| 40–49 years | 871 | 47.2 | 4720 | 41.5 | 2166 | 40.5 |
| 50–59 years | 577 | 31.3 | 3366 | 29.6 | 1736 | 32.5 |
| 60+ years | 76 | 4.1 | 723 | 6.3 | 357 | 6.7 |
| Race/ethnicity | ||||||
| Black (non-Hispanic) | 1038 | 56.2 | 5848 | 51.4 | 2926 | 54.7 |
| Hispanic | 526 | 28.5 | 3378 | 29.7 | 1545 | 28.9 |
| White or other | 282 | 15.3 | 2161 | 19.0 | 877 | 16.4 |
| Income | ||||||
| $12,000 or less | 826 | 44.7 | 5309 | 46.6 | 2443 | 45.7 |
| $12,001–$24,000 | 445 | 24.1 | 2776 | 24.4 | 1310 | 24.5 |
| $24,001–$36,000 | 256 | 13.9 | 1366 | 12.0 | 702 | 13.1 |
| $36,001–$75,000 | 230 | 12.5 | 1230 | 10.8 | 584 | 10.9 |
| $75,000 | 89 | 4.8 | 706 | 6.2 | 309 | 5.8 |
| Education (at WIHS study entry) | ||||||
| Did not complete high school | 744 | 40.3 | 4168 | 36.6 | 2178 | 40.7 |
| Completed high school | 540 | 29.3 | 3349 | 29.4 | 1602 | 30.0 |
| Some college | 483 | 26.2 | 2889 | 25.4 | 1193 | 22.3 |
| Completed college | 79 | 4.3 | 981 | 8.6 | 375 | 7.0 |
| Employed | 728 | 39.4 | 4128 | 36.3 | 2105 | 39.4 |
| Had insurance | 1754 | 95.0 | 11030 | 96.9 | 5102 | 95.4 |
| Enrolled in the AIDS Drug Assistance Program (ADAP) | 1419 | 76.9 | 8676 | 76.2 | 4127 | 77.2 |
| Study site | ||||||
| Bronx | 354 | 19.2 | 2113 | 18.6 | 1046 | 19.6 |
| Brooklyn | 363 | 19.7 | 2218 | 19.5 | 1061 | 19.8 |
| Washington, D.C. | 286 | 15.5 | 1520 | 13.3 | 803 | 15.0 |
| Los Angeles | 299 | 16.2 | 2526 | 22.2 | 887 | 16.6 |
| San Francisco | 217 | 11.8 | 1507 | 13.2 | 667 | 12.5 |
| Chicago | 327 | 17.7 | 1503 | 13.2 | 884 | 16.5 |
|
| ||||||
| Clinical | ||||||
| CD4+ count (cells/uL) (median, IQR) | 569 | 390–771 | 511 | 321–721 | 559 | 363–776 |
| HIV-1 viral load | ||||||
| Undetectable | 1535 | 83.2 | 8020 | 70.4 | 3885 | 72.6 |
| 81–99,999 copies/mL | 292 | 15.8 | 3080 | 27.0 | 1406 | 26.3 |
| 100000+ copies/mL | 19 | 1.0 | 287 | 2.5 | 57 | 1.1 |
| No. children (median, IQR) | 2 | 1–3 | 2 | 1–3 | 2 | 1–4 |
| Gave birth since previous visit | 4 | 0.2 | 69 | 0.6 | 10 | 0.2 |
| History of sterilization | ||||||
| Tubal ligation | 702 | 38.0 | 3978 | 34.9 | 2126 | 39.8 |
| Hysterectomy | 279 | 15.1 | 1759 | 15.4 | 862 | 16.1 |
| Oophorectomy | 40 | 2.2 | 178 | 1.6 | 136 | 2.5 |
| Any of above | 849 | 46.0 | 5014 | 44.0 | 2588 | 48.4 |
| Menopause | 572 | 31.0 | 3547 | 31.1 | 1731 | 32.4 |
|
| ||||||
| Behavioral/psychosocial | ||||||
| History of recreational drug use | 1353 | 73.3 | 8083 | 71.0 | 3903 | 73.0 |
| Current recreational drug use | 371 | 20.1 | 1768 | 15.5 | 1002 | 18.7 |
| History of alcohol use | 1543 | 83.6 | 9136 | 80.2 | 4420 | 82.6 |
| Current alcohol use | ||||||
| Abstainer | 1058 | 57.3 | 7381 | 64.8 | 3318 | 62.0 |
| Light (<3 drinks/week) | 645 | 34.9 | 2999 | 26.3 | 1510 | 28.2 |
| Moderate or heavier (3+/week) | 113 | 6.1 | 851 | 7.5 | 434 | 8.1 |
| Lives in own house/apartment | 30 | 1.6 | 156 | 1.4 | 86 | 1.6 |
| Severe depressive symptoms, based on CES-D score of 23+ | 1621 | 87.8 | 10192 | 89.5 | 4740 | 88.6 |
CES-D = Center for Epidemiologic Studies Depression Scale, IQR = interquartile range, WIHS = Women’s Interagency HIV Study.
Matching on a 1:3 basis using the estimated propensity of being on a single-tablet regimen, adjusted for all factors in table and recruitment period.
Table 2.
Associations of single-tablet regimen use with adherence, virologic suppression, quality of life, and AIDS-defining events or death, Women’s Interagency HIV Study, 2006–2013
| Outcome | Multiple-tablet regimen person-visits with outcome, (N, %) | Single-tablet regimen person-visits with outcome, (N, %) | Adjusted risk ratio for single- tablet regimen use (95% CI) | P-value |
|---|---|---|---|---|
| 95% adherence | 4,258 (79.6) | 1,617 (87.6) | 1.05 (1.03–1.08) | <0.001 |
| 100% adherence | 2,601 (48.6) | 1,144 (62.0) | 1.18 (1.10–1.26) | <0.001 |
| Virologic suppression (<80 copies/mL) | 3,890 (72.7) | 1,527 (82.7) | 1.06 (1.01–1.11) | 0.03 |
| Improvement in quality of life* | 2,109 (39.4) | 760 (41.2) | 1.03 (0.96–1.11) | 0.37 |
| Clinical AIDS-defining event | ||||
| After 6 months | 138 (2.6) | 42 (2.3) | 0.96 (0.62–1.49) | 0.86 |
| After 1 year | 223 (4.7) | 56 (3.5) | 0.87 (0.56–1.36) | 0.54 |
| After 2 years | 306 (8.5) | 65 (5.4) | 0.61 (0.37–0.996) | 0.048 |
| Clinical AIDS-defining event or death | ||||
| After 6 months | 168 (3.1) | 51 (2.8) | 0.95 (0.66–1.37) | 0.78 |
| After 1 year | 274 (5.8) | 73 (4.5) | 0.92 (0.65–1.31) | 0.64 |
| After 2 years | 331 (9.1) | 78 (6.5) | 0.69 (0.48–0.997) | 0.048 |
AIDS = acquired immunodeficiency syndrome, CI = confidence interval.
Total number of person-visits is 5,348 in multiple-tablet regimen group, 1,846 in single-tablet regimen group.
Risk ratios estimated using log-binomial regression with generalized estimating equations, after propensity score matching.
Based on modified Medical Outcomes Study quality of life index.
A single-tablet regimen was also significantly associated with increased virologic suppression (RR 1.06, 95% CI 1.01–1.11). This association was maintained when stratifying by viremia: among those with no detectable viremia when the regimen was assessed, the RR for maintaining suppression was 1.04 (95% CI 1.01–1.07); among those with viremia, the RR for becoming suppressed was 1.19 (95% CI 1.02–1.39). Single-tablet regimen use was associated with better QOL and fewer AIDS-defining events in the next six months, but these results were not statistically significant (RR 1.03, 95% CI 0.96–1.11 and RR 0.96, 95% CI 0.62–1.49, respectively). Extending follow-up of AIDS-defining events to two years resulted in a more pronounced effect (RR 0.61, 95% CI 0.37–0.996). These inferences remained when excluding RPV/TDF/FTC and EVG/COBI/TDF/FTC from analysis.
For the case-crossover study, there were 163 women who switched regimens over time and maintained single-tablet regimen use for at least two visits. 35% of these women were previously on the same drug components prior to switching to the single-tablet regimen (e.g., TDF/FTC + EFV) and 28% had been on a similar regimen based on the same drug class (i.e., 2 NRTI + one NNRTI) prior to switch. 58% had been on a regimen based on a different drug class, primarily PI-based. 34% had been on a twice- or three times daily regimen prior to switching to a single-tablet regimen. The single-tablet regimen was associated with increased adherence (85% to 90%, RR 1.08, 95% CI 1.002–1.14) and virologic suppression (77% to 85%, RR 1.08, 95% CI 0.97–1.20), compared with levels on the prior regimen (Table 3). Important time-varying characteristics associated with better outcomes included a higher baseline CD4+ count, less alcohol use, and no recreational drug use. 70% of switchers maintained their baseline viral load after switching, while 18% had a lower viral load and 12% had a higher viral load. Among the reasons why women were not fully adherent to their medication on their prior regimen (i.e., took <100% of medication in the past six months), the reasons most often stated were “had a change in daily routine” (12%), “simply forgot” (11%), “fell asleep or slept through dose time” (10%), and “did not feel like taking any pills” (8%) (Table 4). The percentages decreased for almost all reasons after switching to the single-tablet regimen, and the decrease was greatest for the reasons, “had a change in daily routine” (12% to 6%, p=0.04) and “did not feel like taking any pills” (8% to 2%, p=0.01).
Table 3.
Time-varying factors associated with adherence and virologic suppression, among switchers (N=163), Women’s Interagency HIV Study, 2006–2013
| 95% adherence | 100% adherence | Virologic suppression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Unadjusted RR (95% CI) | P | Adjusted RR (95% CI) | P | Unadjusted RR (95% CI) | P | Adjusted RR (95% CI) | P | Unadjusted RR (95% CI) | P | Adjusted RR (95% CI) | P |
| Single-tablet regimen use | 1.07 (1.00–1.14) | 0.06 | 1.08 (1.002–1.16) | 0.04 | 1.09 (0.95–1.24) | 0.23 | 1.05 (0.92–1.20) | 0.50 | 1.07 (0.98–1.17) | 0.13 | 1.08 (0.97–1.20) | 0.17 |
| CD4+ cell count, 2 visits prior (ref. = <200 cells/uL) | ||||||||||||
| 200–349 cells/uL | 1.16 (0.93–1.44) | 0.19 | 1.12 (0.90–1.38) | 0.32 | 1.31 (0.76–2.25) | 0.33 | 1.30 (0.82–2.05) | 0.27 | 1.70 (1.05–2.73) | 0.03 | 1.43 (0.97–2.14) | 0.07 |
| 350–499 cells/uL | 1.23 (0.99–1.52) | 0.06 | 1.17 (0.95–1.44) | 0.15 | 1.52 (0.91–2.54) | 0.11 | 1.45 (0.93–2.28) | 0.10 | 1.86 (1.19–2.92) | 0.01 | 1.45 (1.00–2.11) | 0.048 |
| 500+ cells/uL | 1.24 (1.00–1.53) | 0.046 | 1.17 (0.94–1.44) | 0.16 | 1.42 (0.86–2.35) | 0.17 | 1.33 (0.85–2.07) | 0.22 | 1.87 (1.20–2.91) | 0.01 | 1.44 (1.00–2.07) | 0.05 |
| Detectable HIV-1 viral load, 2 visits prior | 0.91 (0.74–1.12) | 0.38 | 1.01 (0.91–1.12) | 0.86 | 0.85 (0.69–1.05) | 0.13 | 1.00 (0.81–1.25) | 0.98 | 0.95 (0.85–1.07) | 0.39 | 0.63 (0.51–0.79) | <0.001 |
| Current recreational drug use | 0.78 (0.64–0.94) | 0.01 | 0.83 (0.69–0.99) | 0.04 | 0.44 (0.27–0.71) | <0.001 | 0.56 (0.35–0.89) | 0.02 | 0.91 (0.75–1.09) | 0.30 | 0.99 (0.84–1.15) | 0.86 |
| Current alcohol use (ref. = abstainer) | ||||||||||||
| <3 drinks/week | 0.96 (0.87–1.06) | 0.44 | 0.99 (0.90–1.09) | 0.90 | 0.67 (0.53–0.85) | <0.001 | 0.74 (0.59–0.92) | 0.01 | 1.01 (0.90–1.14) | 0.86 | 0.99 (0.89–1.10) | 0.82 |
| 3+ drinks/week | 0.78 (0.59–1.02) | 0.07 | 0.85 (0.65–1.13) | 0.26 | 0.44 (0.26–0.73) | 0.001 | 0.61 (0.37–0.99) | 0.045 | 0.91 (0.70–1.20) | 0.51 | 1.01 (0.80–1.27) | 0.96 |
| Stable housing | 1.14 (0.98–1.32) | 0.09 | 1.10 (0.95–1.27) | 0.23 | 1.13 (0.85–1.49) | 0.41 | 1.15 (0.84–1.58) | 0.39 | 1.33 (1.04–1.70) | 0.02 | 1.19 (0.98–1.45) | 0.09 |
CI = confidence interval, Ref. = reference group, RR = risk ratio. Risk ratios estimated using Poisson regression with generalized estimating equations and adjusted for all other factors in table, calendar time, and recruitment period.
Table 4.
Reported reasons for non-adherence to their antiretroviral therapy regimen, among switchers (N=163)
| Before (i.e., on multiple-tablet regimen) | After (i.e., on single-tablet regimen) | ||||||
|---|---|---|---|---|---|---|---|
| Reason | N | % of switchers with <100% adherence (N=69) | % of all switchers (N=163) | N | % of switchers with <100% adherence (N=61) | % of all switchers (N=163) | P-value |
| Had a change in daily routine | 25 | 36 | 12 | 12 | 20 | 6 | 0.04 |
| Simply forgot | 22 | 32 | 11 | 11 | 18 | 5 | 0.07 |
| Fell asleep or slept through dose time | 21 | 30 | 10 | 15 | 25 | 7 | 0.38 |
| Did not feel like taking any pills | 17 | 25 | 8 | 4 | 7 | 2 | 0.01 |
| Felt too sick to take medications | 11 | 16 | 5 | 4 | 7 | 2 | 0.11 |
| Wanted to avoid side effects | 9 | 13 | 4 | 4 | 7 | 2 | 0.26 |
| Did not want others to notice you taking medications | 7 | 10 | 3 | 6 | 10 | 3 | 0.99 |
| Felt too depressed to take medications | 7 | 10 | 3 | 3 | 5 | 1 | 0.34 |
| Ran out of pills | 7 | 10 | 3 | 3 | 5 | 1 | 0.34 |
| Had too many pills to take | 6 | 9 | 3 | 4 | 7 | 2 | 0.75 |
| Felt like the drug was toxic or harmful | 6 | 9 | 3 | 3 | 5 | 1 | 0.50 |
| Were on drugs or drank too much | 5 | 7 | 2 | 5 | 8 | 2 | 0.99 |
| Had difficulty following special instructions | 5 | 7 | 2 | 2 | 3 | 1 | 0.45 |
| Any of these | 47 | 68 | 23 | 31 | 51 | 15 | 0.05 |
Participants answered “often” or “sometimes” for each reason, versus “rarely” or “never”
DISCUSSION
In this treatment-experienced population of HIV-infected women in the United States, we found that single-tablet regimen use was associated with significant improvements in adherence and virologic suppression. We also found suggestive evidence that it may also improve overall QOL and reduce the incidence of clinical events such as AIDS-defining illness and death. These associations were consistent based on two complementary approaches: a nested cohort study that compared periods of single-tablet ART use with periods of multiple-tablet ART use among similar individuals, and a case-crossover study that limited assessment to women who recently switched regimens. Our approach allowed us to make generalizations about the effectiveness of single-tablet regimen use in real-world conditions that are not restricted to those found in clinical trials.
Our results are broadly consistent with those reported in the literature,(15, 17, 38) extending these findings to women who often have characteristics predisposing them to lower adherence to HIV treatments. The levels of adherence among women that we observed are consistent with other recent studies in the U.S.,(6, 7) and add to an accruing body of evidence supporting benefits of regimen simplification. (8, 9, 39) Our demonstration of improved virologic suppression as a consequence of single-tablet regimen use provides a partial explanation for published secular improvements in suppression among HIV-infected individuals over time.(40) However, the magnitude of the increases attributable to single-tablet regimens (5–18%) during the study period suggests that these regimens provide only incremental improvements on treatment outcomes in our population. Other factors, such as improved retention in care, may also contribute to these increases and are the focus of interventional studies.(41, 42)
Use of single-tablet formulations reached only about 20% of this group through 2013, lower than previously reported in some other settings.(8) This level may reflect the clinical history of this treatment-experienced cohort, with some women having been enrolled in the study for up to 19 years. Switching to a single-tablet regimen may not have been considered a priority to their providers if they were already stable on their current regimen, if they already developed resistance to one of the components of the available single-tablet regimens, or if they were planning to become pregnant. In contrast, the few women who were ART-naïve prior to initiating therapy during the study period started on a single-tablet regimen 48% of the time.
Continued monitoring of the effects of emerging adherence strategies is warranted to strengthen the evidence base, especially as the health care environment continues to evolve in the U.S. and internationally. For example, it has been postulated that as generic versions of individual components of single-tablet regimens become available, some individuals may switch back to multiple-tablet formulations due to their anticipated lower cost, particularly in resource-poor settings.(43) The potential effects of such a change on adherence outcomes in our population will be important to follow over time. Future work should also follow the outcomes of previously ART-naïve women initiating single-tablet regimens, including younger women currently being recruited to join the WIHS as new participants.
Our study has limitations. One limitation is that ART adherence is based on self-report, rather than medication event monitoring systems or unannounced pill counts.(17) However, self-report has been shown to have comparable validity with other more expensive monitoring systems,(44, 45) and is recommended for routine adherence monitoring in patients despite the potential for reporting bias.(46) Other limitations relate to our measurement of adherence. Assessing adherence at 6-month intervals only captures behaviors only in a broad sense. Use of 95% adherence as the outcome of interest, which is based on older studies of unboosted protease inhibitors,(27) may be too conservative for more recent regimens that may not require levels of adherence as high,(47) and therefore may mask additional benefits of single-tablet regimens. Despite these drawbacks, the uniformity and regularity of adherence assessment over the 8-year study period improves the robustness of our findings. We grouped all multiple-tablet regimens together to compare these collectively with single-tablet regimens, but this makes it difficult to distinguish between benefits derived from the dosing schedule versus the regimen components.(46) Finally, it is possible that the switching effects that we found in the case-crossover study are a transient consequence of counseling, and future work should also examine the sustainability of these improvements.
Despite these limitations, our study has several strengths. We report trends in single-tablet regimen use and extend known inferences on their effectiveness to HIV-infected women in the U.S., a growing population with less available research addressing their unique circumstances. Our data come from a well-established prospective study population that is demographically representative of the national female HIV-infected population. The WIHS’s detailed longitudinal data on health behaviors, medical history, and medication use were instrumental in being able to create balanced groups to minimize the possibility of confounding by indication (although residual confounding remains possible). Finally, the WIHS follows many women who either continue to participate in the study past child-bearing age or have undergone sterilization procedures, and therefore it is particularly suitable to examine single-tablet regimen use among such women who can safely be prescribed EFV despite its contraindications in terms of teratogenicity.
Adherence has been described as “a set of interacting behaviors informed by individual, social, and environmental forces”.(48) Our study found that about 85% of ART-treated women in the WIHS in 2013 were adherent at the 95% level, but only 50% were adherent at the 100% level, even with the availability of single-tablet regimens. Thus, the “overlapping, combination approaches” to HIV prevention advocated by the U.S. National HIV/AIDS Strategy also apply to adherence.(49) Some examples of additional evidence-based approaches that may be relevant to our study population include those that involve self-management tools and individual- and group-level education and counseling.(46) While the simplification of treatment regimens has contributed to improved adherence and virologic suppression in women, it is just one component of a multi-faceted strategy to be able to truly maximize the therapeutic benefits of ART.
Acknowledgments
We would like to thank Xiaonan Xue, PhD for statistical advice, and the WIHS participants for their time and commitment.
Principal contributions: D.B.H. conceived and designed the study, conducted the main analysis, and drafted the article. N.A.H. and R.C.K. contributed to the study design. All authors contributed substantially to the interpretation of the data, revised the article critically for important intellectual content, and gave approval of the final version.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange).
The Women’s Interagency HIV Study (WIHS) Collaborative Study Group includes the following:
New York City/Bronx Consortium: Montefiore Medical Center (Kathryn Anastos, MD (Principal Investigator); Anthony Cajigas, MD; Esther Robison, PhD; Rodney Wright, MD); University of California Davis (Harold Burger, MD, PhD; Barbara Weiser, MD); Albert Einstein College of Medicine (Robert Kaplan, PhD; Marla Keller, MD); Weill Medical College of Cornell University (Marshall Glesby, MD); Rutgers (Don Hoover, PhD); Community Advisor (Nilsa Ramos-Santiago).
Brooklyn, NY: State University of New York Health Science Center at Brooklyn (Howard Minkoff, MD (Principal Investigator); Deborah Gustafson, PhD (Co-Principal Investigator); Michael Augenbraun, MD; Howard Crystal, MD; Jack DeHovitz, MD, MPH; Helen Durkin, PhD; Susan Holman, RN, MS; Jason Lazar, MD; Maja Nowakowski, PhD; Rebecca Schwartz, PhD; David Seifer, MD; Anjali Sharma, MD, MS; Tracey Wilson, PhD).
Washington, DC, Metropolitan Consortium: Georgetown University Medical Center (Mary Young, MD (Principal Investigator); Lakshmi Goparaju, PhD); George Washington University Medical Center (Sylvia Silver, DA); Whitman-Walker Clinic (Kunthavi Sathasivam, MD); Montgomery County Health Department (Carol Jordan, RN, MPH); Inova Health System of Northern Virginia (David Wheeler, MD; Barbara Lawrence, BS); Community Advisors (Kimberley Kelsey, Kathy Moore).
The Connie Wofsy Study Consortium of Northern California: University of California, San Francisco (Ruth Greenblatt, MD (Principal Investigator); Peter Bacchetti, PhD; Deborah Cohan, MD, MPH; Nancy Hessol, MSPH; Phyllis Tien, MD); Alameda County Medical Center (Howard Edelstein, MD); Alta Bates Medical Center (Claire Borkert, MD); Community Advisor (Nilda Rodriguez).
Los Angeles County/Southern California Consortium: Keck School of Medicine, University of Southern California and Los Angeles County & USC Medical Center (Alexandra M. Levine, MD (Principal Investigator); Yvonne Barranday, BA; Marek Nowicki, PhD; Leigh Pearce, PhD; Jean Richardson, DrPH); the Santa Barbara County Department of Health Services (Elizabeth Downing, MD); University of Hawaii (Cecilia Shikuma, MD); Community Advisor (Elisa Sanchez).
Chicago Consortium: Cook County Hospital (Mardge H. Cohen, MD (Principal Investigator); Audrey French, MD; Kathleen M. Weber, BSN); University of Illinois at Chicago (Ronald Hershow, MD); Rush Presbyterian-St. Luke’s Medical Center (Beverly Sha, MD); Northwestern Memorial Hospital (Susan Cohn, MD); Community Advisor (Marta Santiago).
Data Coordinating Center: Johns Hopkins Bloomberg School of Public Health (Stephen Gange, PhD (Principal Investigator); Elizabeth Golub, PhD, MPH (Co-Principal Investigator); Alison Abraham, PhD; Christine Alden, BA; Keri Althoff, PhD, MPH; Lorie Benning, MS; Christopher Cox, PhD; Gypsyamber D’Souza, PhD; Lisa Jacobson, ScD; Bryan Lau, PhD; Sharada Modur, PhD; Alvaro Muñoz, PhD; Christopher Pierce, MHS; Aaron Platt, BS; Michael Schneider, MS; Eric Seaberg, PhD, MPH; Gayle Springer, MLA; Sol Su, ScD; Eryka Wentz, MA; Won Yoo, BS; Jinbing Zhang, MS).
NIH: National Institute of Allergy and Infectious Diseases (Gerald Sharp, DrPH; Carolyn Williams, PhD); Eunice Kennedy Shriver National Institute of Child Health and Human Development (Kevin Ryan, PhD; Heather Watts, MD); National Institute of Drug Abuse (Katherine Davenny, MPH; Richard Jenkins, PhD); National Cancer Institute (Geraldina Dominguez, PhD).
Footnotes
Preliminary findings were presented at the 8th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, 2–4 June 2013.
Conflicts of interest and source of funding: J.M.C. reports an unrelated past grant from Gilead. K.A. reports an unrelated past consultancy with Bristol-Myers Squibb. For the remaining authors none were declared. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 2.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas GM. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 2011;88(21–22):948–52. doi: 10.1016/j.lfs.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazo M, Gange SJ, Wilson TE, Anastos K, Ostrow DG, Witt MD, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis. 2007;45(10):1377–85. doi: 10.1086/522762. [DOI] [PubMed] [Google Scholar]
- 6.Beer L, Heffelfinger J, Frazier E, Mattson C, Roter B, Barash E, et al. Use of and adherence to antiretroviral therapy in a large U.S. sample of HIV-infected adults in care, 2007–2008. Open AIDS J. 2012;6:213–23. doi: 10.2174/1874613601206010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoni JM, Huh D, Wilson IB, Shen J, Goggin K, Reynolds NR, et al. Racial/ethnic disparities in ART adherence in the United States: findings from the MACH14 study. JAIDS. 2012;60(5):466–72. doi: 10.1097/QAI.0b013e31825db0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. doi: 10.1371/journal.pone.0031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48(4):484–8. doi: 10.1086/596482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horberg MA, Klein DB. An update on the use of Atripla in the treatment of HIV in the United States. HIV AIDS (Auckl) 2010;2:135–40. doi: 10.2147/hiv.s6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Clercq E. Where rilpivirine meets with tenofovir, the start of a new anti-HIV drug combination era. Biochem Pharmacol. 2012;84(3):241–8. doi: 10.1016/j.bcp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Marchand C. The elvitegravir Quad pill: the first once-daily dual-target anti-HIV tablet. Expert Opin Investig Drugs. 2012;21(7):901–4. doi: 10.1517/13543784.2012.685653. [DOI] [PubMed] [Google Scholar]
- 13.FDA. [Accessed 12 Oct 2012];Important information about Sustiva (efavirenz) and pregnancy. Available at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124885.htm.
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 12 Mar 2013];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 15.Dejesus E, Young B, Morales-Ramirez JO, Sloan L, Ward DJ, Flaherty JF, et al. Simplification of antiretroviral therapy to a single-tablet regimen consisting of efavirenz, emtricitabine, and tenofovir disoproxil fumarate versus unmodified antiretroviral therapy in virologically suppressed HIV-1-infected patients. J Acquir Immune Defic Syndr. 2009;51(2):163–74. doi: 10.1097/QAI.0b013e3181a572cf. [DOI] [PubMed] [Google Scholar]
- 16.Hodder SL, Mounzer K, Dejesus E, Ebrahimi R, Grimm K, Esker S, et al. Patient-reported outcomes in virologically suppressed, HIV-1-Infected subjects after switching to a simplified, single-tablet regimen of efavirenz, emtricitabine, and tenofovir DF. AIDS Patient Care STDS. 2010;24(2):87–96. doi: 10.1089/apc.2009.0259. [DOI] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24(18):2835–40. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puskas CM, Forrest JI, Parashar S, Salters KA, Cescon AM, Kaida A, et al. Women and vulnerability to HAART non-adherence: a literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep. 2011;8(4):277–87. doi: 10.1007/s11904-011-0098-0. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg MJ, Gore ME, French AL, Gandhi M, Glesby MJ, Kovacs A, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39(5):717–24. doi: 10.1086/423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips KD, Moneyham L, Murdaugh C, Boyd MR, Tavakoli A, Jackson K, et al. Sleep disturbance and depression as barriers to adherence. Clin Nurs Res. 2005;14(3):273–93. doi: 10.1177/1054773805275122. [DOI] [PubMed] [Google Scholar]
- 21.Merenstein D, Schneider MF, Cox C, Schwartz R, Weber K, Robison E, et al. Association of child care burden and household composition with adherence to highly active antiretroviral therapy in the Women’s Interagency HIV Study. AIDS Patient Care STDS. 2009;23(4):289–96. doi: 10.1089/apc.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9(2):117–25. [PubMed] [Google Scholar]
- 23.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC. [Accessed 15 Oct 2012];HIV among women. Available at: http://www.cdc.gov/hiv/topics/women/index.htm.
- 25.Horberg M, Silverberg M, Hurley L, Delorenze G, Quesenberry C. Influence of prior antiretroviral experience on adherence and responses to new highly active antiretroviral therapy regimens. AIDS Patient Care STDS. 2008;22(4):301–12. doi: 10.1089/apc.2007.0101. [DOI] [PubMed] [Google Scholar]
- 26.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 27.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 28.Saberi P, Johnson MO, McCulloch CE, Vittinghoff E, Neilands TB. Medication adherence: tailoring the analysis to the data. AIDS Behav. 2011;15(7):1447–53. doi: 10.1007/s10461-011-9951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Weber K, Robison E, Hu Z, Jacobson LP, Gange SJ. Assessing the effect of HAART on change in quality of life among HIV-infected women. AIDS Res Ther. 2006;3:6. doi: 10.1186/1742-6405-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozzette SA, Hays RD, Berry SH, Kanouse DE, Wu AW. Derivation and properties of a brief health status assessment instrument for use in HIV disease. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(3):253–65. doi: 10.1097/00042560-199503010-00006. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 33.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–9. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 36.Wang PS, Schneeweiss S, Glynn RJ, Mogun H, Avorn J. Use of the case-crossover design to study prolonged drug exposures and insidious outcomes. Ann Epidemiol. 2004;14(4):296–303. doi: 10.1016/j.annepidem.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- 38.Airoldi M, Zaccarelli M, Bisi L, Bini T, Antinori A, Mussini C, et al. One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence. 2010;4:115–25. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggiolo F, Ripamonti D, Arici C, Gregis G, Quinzan G, Camacho GA, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3(5):371–8. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 40.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157(5):325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llibre JM, Clotet B. Once-daily single-tablet regimens: a long and winding road to excellence in antiretroviral treatment. AIDS Rev. 2012;14(3):168–78. [PubMed] [Google Scholar]
- 44.Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV Clin Trials. 2011;12(5):244–54. doi: 10.1310/hct1205-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS. 2008;22(9):735–43. doi: 10.1089/apc.2007.0229. [DOI] [PubMed] [Google Scholar]
- 46.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–9. doi: 10.1345/aph.1P587. [DOI] [PubMed] [Google Scholar]
- 48.Steiner JF. Rethinking adherence. Ann Intern Med. 2012;157(8):580–5. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 49.White House Office of National AIDS Policy. [Accessed 12 Mar 2013];National HIV/AIDS Strategy for the United States. Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.