Abstract
Background
In the PROMOTE-pediatrics trial, HIV-infected Ugandan children randomized to receive lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) experienced a lower incidence of malaria compared to children receiving non-nucleoside-reverse-transcriptase-inhibitor (NNRTI)-based-ART. Here we present the results of the non-inferiority (NI) analysis of virologic efficacy and comparison of immunologic outcomes.
Methods
ART-naïve or -experienced (HIV RNA < 400 copies/ml) children ages 2 months to 6 years received either LPV/r or NNRTI-based-ART. The proportion of children with virologic suppression (HIV RNA <400copies/ml) at 48 weeks was compared using a pre-specified NI margin of −11% in per-protocol analysis. Time to virologic failure by 96 weeks, change in CD4 counts and percentages, and incidence of adverse event rates were also compared.
Results
Of 185 children enrolled, 91 initiated LPV/r and 92 initiated NNRTI-based ART. At baseline, the median age was 3.1 years (range: 0.4 to 5.9) and 131 (71%) were ART-naïve. The proportion of children with virologic suppression at 48 weeks was 80% (67/84) in the LPV/r-arm vs. 76% (59/78) in the NNRTI-arm, a difference of 4% (95%CI: −9% to +17%). Time to virologic failure, CD4 changes, and the incidence of DAIDS grade III/IV adverse events were similar between arms.
Conclusions
LPV/r-based ART was not associated with worse virologic efficacy, immunologic efficacy, or adverse event rates compared to NNRTI-based ART. Considering these results and the reduction in malaria incidence associated with LPV/r previously reported for this trial, wider use of LPV/r to treat HIV-infected African children in similar malaria endemic settings could be considered.
Keywords: Children, African, lopinavir, virologic outcomes
Introduction
In the PROMOTE-pediatrics trial, HIV-infected Ugandan children randomized to receive lopinavir/ritonavir (LPV/r) based antiretroviral therapy (ART) experienced a lower incidence of malaria compared to children receiving non-nucleoside reverse transcriptase inhibitor (NNTRI) based ART1. While LPV/r has direct anti-plasmodial activity in vitro2, the decreased malaria incidence in the LPV/r-arm of PROMOTE was driven primarily by a reduction in the recurrence of malaria after treatment with artemether–lumefantrine, stemming from a beneficial pharmacologic interaction that prolonged the half-life of lumefantrine [1]. Children receiving LPV/r also had a lower prevalence of gametocytemia, suggesting that wider use could lead to decreased malaria transmission3.
The results of PROMOTE-pediatrics suggest that expanded use of LPV/r for the treatment of HIV-infected children living in areas of high malaria endemicity settings could be a rational policy. However, the HIV-related outcomes of LPV/r– compared to NNRTI-based ART must also be considered. In one randomized trial of infants less than 36 months of age, LPV/r – based ART led to superior virologic outcomes compared to NNRTI-based ART, but results from trials in older children were equivocal and some data have suggested that LPV/r may be associated with impaired CD4 recovery4,5.
Here we report results of the protocol specified a non-inferiority comparison of the proportion of children with HIV RNA levels < 400 copies/ml after 48 weeks of ART. To assess the durability of virologic efficacy, we compared time to confirmed virologic failure over 96 weeks. We additionally compared changes in CD4+ T-cell measures and adverse event incidence during the follow-up period.
Methods
Details about the PROMOTE-pediatrics trial including eligibility criteria and the study protocol have been published (Clinical Trial Registration Number:NCT00978068)1. In brief, this was an open-label randomized clinical trial designed to determine if the use of LPV/r-based ART would reduce malaria incidence compared to the use of NNRTI-based ART. Subjects were HIV-infected children at least 2 months but less than 6 years old living in Tororo, Uganda who were either ART-naïve and ART-eligible per Ugandan guidelines or ART-experienced, receiving NNRTI-based first line ART with an HIV RNA Level <400 copies/ml in the preceding 6 months. Children less than 2 years old who had been exposed to maternal nevirapine (NVP) and/or received NVP as perinatal transmission prophylaxis were excluded because use of an NNRTI as treatment would be clinically contraindicated.
At enrollment, children were randomized 1:1 to receive LPV/r plus two nucleoside reverse transcriptase inhibitors (NRTIs) or an NNRTI plus two NRTIs. In the NNRTI arm, NVP was used for all children < 3 years old and efavirenz(EFV) for most children >3 years old . NRTIs were zidovudine(ZDV) or abacavir(ABC) plus lamivudine(3TC); stavudine was also utilized initially, but then replaced by AZT or ABC after 2009, in accordance with changes in Ugandan and WHO guidelines6. NVP was dosed at 160–200 mg/m2 (max 200mg) once daily for the first 14 days and then twice daily7,8. EFV was dosed as 15 mg/kg (max 600 mg) once daily8. LPV/r was dosed by weight bands per 2008 United States Department of Health and Human Services guidelines7.
Children were followed at the study clinic with monthly routine visits and for all acute illnesses at the study clinic. CD4 counts and percentages (FACS Calibur, BD Biosciences, San Jose, CA, USA) and HIV RNA levels (COBAS® Amplicor HIV-1 Monitor Test v1.5 and Ampliprep Taqman Assay, Roche Molecular Diagnostics, Pleasanton, CA, USA; Abbott m2000 RealTime PCR, Abbott Molecular Diagnostics, Germany) were determined every 12 weeks for the first year and every 24 weeks thereafter. Adherence was assessed using 3-day recall at each routine visit and calculated as the percentage of prescribed doses reportedly taken. Children who had persistent HIV RNA levels of > 400 c/ml had in-depth adherence assessments, with changes to second line ART made on a case-by-case basis, per Ugandan guidelines.
The primary outcome for this analysis, the proportion of children with virologic suppression (HIV RNA level < 400 c/ml) after 48 weeks, was compared by test of proportions. Because the primary aim of the PROMOTE-pediatrics trial was to compare efficacy in malaria prevention, the study sample size was based on estimates of malaria incidence. To compare virologic efficacy between arms, we chose to utilize a non-inferiority analysis and pre-specified a non-inferiority margin of −11% in the difference between arms in the proportion with HIV RNA level < 400 c/ml with a 95% confidence interval. Analyses were per-protocol to minimize the risk of falsely concluding no difference between arms (Type II error). However, we also analyzed the primary outcome using modified intention-to-treat techniques, in which children were categorized according to originally assigned study arm, and those who died, were lost to follow up, or had missing data at 48 weeks were considered to have not suppressed. The proportions with virologic suppression were also compared at 96 weeks per-protocol. To assess the durability of virologic efficacy up to 96 weeks, we generated a Kaplan-Meir survival model of time to virologic failure, stratified by ART-status at enrollment, with virologic failure defined at the time of the first of two successive HIV RNA > 400 c/ml (after a minimum of 24 weeks of treatment for ART-naïve children), and compared risk of failure by arm using a log-rank test. Means of CD4 measures and adherence were compared using Student's T-test with 2-tailed p-values. The incidence of Division of AIDS (DAIDS, National Institute of Allergy and Infectious Diseases)9 Grade III or IV adverse events was compared using negative binomial regression using all available follow-up time and per protocol approaches. Children were enrolled from September 2009 through October 2011; data accumulated up to October 1, 2012 were included in these analyses. Stata Version 11 was used for all analyses (StataCorp, College Station, TX, USA).
PROMOTE-pediatrics was approved by the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council for Science and Technology, and the University of California, San Francisco, Committee for Human Research.
Results
After screening 421 children, 185 children were enrolled (Figure 1) with 91 initiating LPV/r-based ART and 92 initiating or continuing NNRTI-based ART (33 EFV, 59 NVP). At enrollment, the median age of the cohort was 3.1 years (range: 0.4 to 5.9) and 131 (71%) were ART-naïve. Baseline characteristics of the children who initiated therapy were similar between arms (Table 1). The mean (range) of total follow-up time per subject was 109 (0-156) weeks. At monthly routine assessments, mean reported adherence was 99.5% in both arms (p=0.99).
Figure 1. Study Flow Chart.
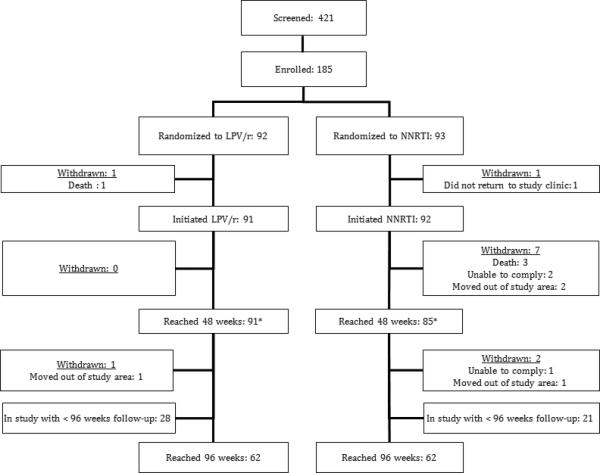
LPV/r, lopinavir/ritonavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor.
Table 1.
Baseline characteristics of subjects
| LPV/r (n=91*) | NNRTI (n=92*) | P-value† | |
|---|---|---|---|
| Age, years | 3.1 (2.4 to 4.3) | 3.1 (2.4 to 4.7) | 0.63 |
| Female | 44 (48%) | 45 (48%) | 0.53 |
| WHO Stage | 0.82 | ||
| I | 64 (70%) | 70 (76%) | |
| II | 19 (21%) | 16 (17%) | |
| III | 2 (2%) | 1 (1%) | |
| IV | 6 (7%) | 5 (5%) | |
| ART-status | 0.55 | ||
| ART-naïve | 64 (70%) | 65 (71%) | |
| ART-experienced | 27 (29%) | 27 (29%) | |
| CD4 number, cells/μl | |||
| ART-naïve | 582 (597 to 969) | 593 (389 to 788) | 0.89 |
| ART-experienced | 1275 (861 to 1885) | 1099 (802 to 1906) | 0.59 |
| CD4 Percentage | |||
| ART-naïve | 17 (12 to 23) | 16 (11 to 23) | 0.77 |
| ART-experienced | 31 (27 to 38) | 30 (24 to 37) | 0.60 |
| HIV RNA Level, log(copies/ml) | |||
| ART-naïve | 5.3 (4.8 to 5.9) | 5.5 (4.8 to 5.9) | 0.75 |
| ART-experienced | All BLD | All BLD | - |
Excludes the 2 children who withdrew or died before initiating study drugs.
Student's T-Test or Chi-square.
Values are medians (interquartile range) or number (percentage); LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor; BLD, below level of detection, 400 copies/ml.
A total of 91 (99%) of children in the LPV/r arm and 85 (89%) of children in the NNRTI arm remained in follow-up at 48 weeks, the time point of the primary analysis. One child discontinued study medication due to an adverse reaction (Stevens-Johnson Syndrome after 3 weeks of therapy with NVP). Eight children withdrew from the NNRTI arm and one from the LPV/r arm (Figure 1), all due to inability to comply with study procedures, and none related to medication tolerance. HIV RNA levels at 48 weeks were missing from 13 children (7 LPV/r and 6 NNRTI) due to missed study visits or laboratory problems.
The proportion of children with virologic suppression at 48 weeks was 80% (67/84) in the LPV/r arm compared to 76% (59/78) in the NNRTI arm, corresponding to a difference of 4.1% with a 95% confidence interval of – 8.7% to 17%, thereby excluding the pre-specified non-inferiority margin of −11%. In modified intention-to-treat analysis, the proportion of children with virologic suppression was 74% (67/91) in the LPV/r arm compared to 65% (60/92) in the NNRTI arm (difference of 8.4%; 95%CI: −4.9% to +22%).
The proportion of children with virologic suppression at 96 weeks was 89% (46/52) in the LPV/r arm versus 84% (41/49) in the NNRTI arm (difference: 4.8%, 95%CI: −9% to 18%) in per-protocol analysis. Of the 124 children enrolled with at least 96 weeks of follow-up (Figure 1), 6 had changed from NNRTI to LPV/r and 18 children were missing HIV RNA levels due to lab error or missed visits (LPV/r: 10, NNRTI: 8).
A total of 37 children developed confirmed virologic failure by 96 weeks (Figure 2 and SDC Figure 1). Among the ART-naive, the 96-week virologic failure rate was 26% (95%CI: 17%-39%) in the LPV/r arm and 28% (18%-41%) in the NNRTI-arm (Log-rank test p=0.8) in survival modeling. Among the ART-experienced, the 96-week virologic failure rate was 8% (95%CI:2%-27%) in the LPV/r arm and 12% (4%-32%) in the NNRTI-arm (Log-rank test p=0.6).
Figure 2. Time to Virologic Failure, ART-naïve.
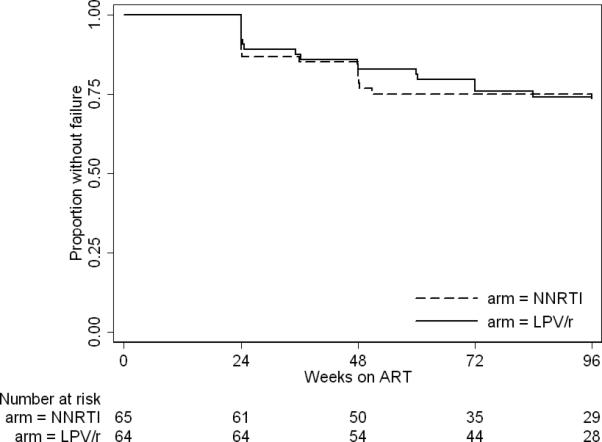
Time to virologic failure, the first of 2 successive HIV RNA levels > 400 copies/ml in Kaplan-Meier modeling. LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor
Compared to the NNRTI arm, a greater portion of the children who developed virologic failure in the LPV/r arm subsequently achieved virologic suppression after adherence counseling. Of the 18 children with virologic failure in the LPV/r arm, 14 (78%) subsequently achieved virologic suppression after adherence counseling. In contrast, of the 19 with virologic failure in the NNRTI arm, only 4 (21%) subsequently achieved virologic suppression (p=0.0006). A total of 6 children switched to second-line ART (all from NNRTI to LPV/r); 5 achieved virologic suppression and 1 did not yet have follow-up viral load testing.
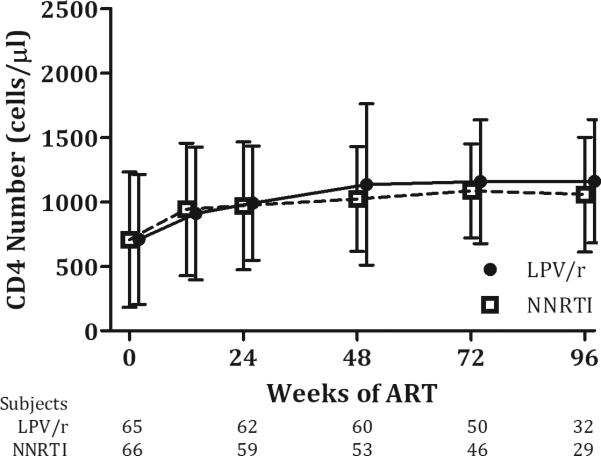
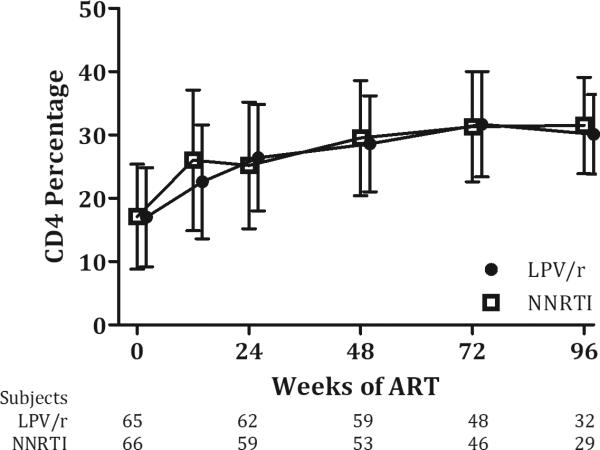
ART-naïve children experienced comparable increases in CD4 counts, with mean change from baseline to 48 weeks of 405 cells/μl (SD 324) for the LPV/r arm and 426 cells/μl (498) for the NNRTI arm (p=0.7); similar recovery in CD4 percentage was also seen, with mean (SD) change of 11.8(7.4) in the LPV/r arm and 13.3(7.1) in the NNRTI arm (p=0.27). Among the ART-experienced, there were mean (SD) losses in CD4 count at 48 weeks for both the LPV/r [−296(610)] and NNRTI arms [−5.8 (733)](p=0.13), but CD4 percentages showed mean improvements: LPV/r [0.5 (8.8)], NNRTI [3.5(9.5)] (p=0.23). There were no significant differences in mean CD4 number or percentage between arms at any time point (all p-values > 0.05) (Figures 3a, 3b, and SDC Figures 2a and 2b). There was a trend toward lower mean CD4 counts among ART-experienced children randomized to LPV/r versus NNRTI at 48-96 weeks, but CD4 percentages remained comparable at all time-points.
Figure 3. Change in CD4 measures over time, ART-naive.
a) CD4 Number b) CD4 Percentage
CD4 count and percentage by duration of time receiving ART during the study. Values are means with standard deviation error bars. Data were included from subjects on per-protocol basis with some patients missing scheduled visits at each time point, as shown below.
DAIDS grade 3 or 4 adverse events were rare, and there were no significant differences in their incidence between the two ART arms (SDC Table 1). Neutropenia (<750 cells/μl) and thrombocytopenia (<50,000/ μl) were the most commonly reported adverse events. There were 47 neutropenia events (24.0 per 100 person years) in the LPV/r arm and 51 (28.3 per 100 person years) in the NNRTI arm. The rate of grade 3 or 4 elevated alanine aminotransferase (ALT) was slightly higher in children in the NNRTI arm compared to the LPV/r arm(3.32 vs. 1.53 events per person year, respectively, P=0.26). There was one permanent ART change in a patient who developed Stevens Johnson Syndrome while receiving NVP. One patient changed from NVP to EFV due to hepatitis. Two children (one from each arm) temporarily changed to EFV to avoid interactions with therapy for tuberculosis. There were 4 deaths, none attributed to study medications. Two children with severe malnutrition (one each randomized to NVP and LPV/r) died within 6 weeks of enrollment of unclear causes; they had been admitted to the hospital and provided with nutritional supplementation. One child died of measles complicated by pneumonia (NVP) despite measles vaccination. One child experienced a sudden death of unclear etiology (EFV).
Discussion
Our finding of comparable virologic outcomes in children receiving LPV/r and NNRTI-based ART supports to the idea that LPV/r-based ART could be used to treat HIV-infected African children while also preventing malaria. We previously showed that children in the LPV/r-arm of this study experienced a lower incidence of malaria1, primarily due a reduction in the incidence of recurrent malaria after treatment with artemether–lumefantrine. It is thus important to note that the benefits of LPV/r in malaria prevention would only apply to areas where artemether–lumefantrine is in use. But artemether–lumefantrine is recommended by the WHO as first line treatment for malaria and is in use throughout much of Africa10. With the results present here, we show that the anti-malarial benefits of LPV/r for HIV-infected children in this setting did not come at a cost of virologic or CD4+ T-cell recovery.
Comparable virologic outcomes for protease inhibitor (PI) versus NNRTI-based ART have been reported from larger randomized trials in adults and children. In the Optimal Combination Therapy after Nevirapine Exposure (A5208) trial, NVP and LPV/r-based ART lead to the same rate of virologic failure or death (14% vs. 14%, hazard ratio, 0.97; 95% CI, 0.6 to 1.6) among 500 ART-naive HIV-infected African women without prior exposure to single-dose nevirapine11. In the PENPACT 1 trial, 263 HIV-infected children randomized to either NNRTI- or PI -based-ART had similar proportions with HIV RNA < 400 copies/ml throughout follow-up 12. However, in the IMPAACT p1060 trial, the HIV-infected infants (without perinatal exposure to NVP) randomized to receive NVP had an elevated risk of virologic failure (confirmed HIV RNA > 400 c/ml) or death by 24 weeks (hazard ratio: 2.51; 95% CI, 1.41 to 4.47) and a trend towards lower rates of suppression (HIV RNA < 400 c/ml) at 48 weeks (75% vs 85%, respectively, p=0.06) compared to infants receiving LPV/r 5. Our study did not confirm the superiority of LPV/r reported in the p1060 trial, but we did note trends in favor of the LPV/r. Differences between our results and those of p1060 are likely explained, at least in part, by the age differences between our and their cohorts (median 3.1 vs. 1.8 years, respectively). If we restrict our analyses to include only children < 3 years of age (n=75), the rates of virologic suppression are more in favor of LPV/r as reported in p1060 (data not shown). Also of note, the older children in the NNRTI-arm of our study received EFV, which has been associated with superior outcomes compared to NVP in African children13.
The good outcomes of children switching from NNRTI to LPV/r-based ART in our study add to limited data on the safety of switching ART in children with virologic control 14-18. HIV-infected children occasionally need to change ART due to adverse events, anticipated drug interactions, or formulation availability. Prior studies have demonstrated safety in changing from PI to EFV15 or NVP4,18- based ART, but to our knowledge, no randomized trials have previously reported on the safety of switching from NNRTI- to PI-based ART among children with virologic suppression
We observed a notable difference between treatment arms in the outcomes of children recognized to have virologic failure. Most children in the LPV/r arm ultimately achieved suppression with adherence counseling and without a change in ART, but most children in the NNRTI arm continued to fail despite counseling. This could be the result of the lower barrier to resistance seen with NNRTI's compared to PIs such as LPV/r 19 or reflect ongoing issues of poorer tolerance and adherence to NNRTI's. This finding highlights the importance of early recognition of adherence problems and the role that frequent HIV RNA level testing could play in identifying those at risk for resistance.
Virologic failure rates by 96 weeks were >25% in both treatment arms. The reasons for such high failure rates are uncertain, but adherence issues were identified in the majority of cases. Caregivers received adherence counseling prior to treatment initiation and at every monthly visit. Adherence was reported at 99.5% over all clinic visits, but we believe that caregiver reports were inflated. When a case of virologic failure was identified, additional counseling was performed and in difficult cases, counselors visited the homes of families to help them problem solve. In some cases, adherence was improved with simple changes in practice such as substituting tablets for liquids. But in other cases, socioeconomic factors presented greater challenge and no clear solution, such as the case of the single mothers who assigned responsibility for medication administration to young siblings. Our experience suggests that treatment programs in similar settings should devote as much effort as resources allow to adherence support in order to optimize ART outcomes.
Consideration of the optimal first line ART for HIV-infected African children must take into account not only the response to initial therapy, but also the consequences of failure on resistance mutation accumulation and the response to second line therapy. Our ability to assess the long-term implications of study regimens would have been improved with information about the accumulation of resistance mutations and additional data about the outcomes of children on second line therapy. Some studies have shown good responses to PI-based therapy following NNRTI-based ART in Africa, but data are limited in children 20-22. One small retrospective study showed poorer virologic responses when NNRTIs were used as second line following PIs, compared to when PIs followed NNRTIs23. However, in PENPACT 1, which took place in Europe and North and South America, children who received PIs (versus NNRTI) as first line had fewer NRTI mutations at the time of virologic failure and good suppression rates in response to NNRTI-based second line treatment 4.
We also compared changes in CD4 measures by treatment arm. In HIV-infected adults, several studies have suggested that PIs 24-26, and LPV/r in particular27, may be associated with improved CD4 count recovery. Conversely, some data suggest impaired CD4 responses in children receiving LPV/r compared to NNRTI-based ART. Among the NVP-exposed infants in the p1060 trial, the LPV/r arm demonstrated a trend toward lower CD4 percentages recovery at 48 weeks28 compared to the NNRTI arm. In the NEVEREST trial, ART-experienced infants receiving LPV/r were randomized to either continue LPV/r or switch to NVP; a greater portion of those who stayed on LPV/r experienced a 10% decline in CD4 percentage at 52 weeks (15% vs 3%)4. The marginal differences in CD4 measures that have been reported are of unclear clinical significance, but suggest the possibility of an interaction of LPV/r with lymphocyte recovery. In this trial, we did not find significant differences in CD4 count or percentage between the arms of our study. ART-experienced LPV/r –treated children had lower median CD4 counts, with a net decline from baseline to 48 weeks but CD4 percentages remained notably stable.
Both NNRTIs and LPV/r were well tolerated in our study, with only one grade III/IV adverse event leading to permanent discontinuation. As in other trials of NNRTIs and PIs in children, the most common adverse event was neutropenia, but the clinical consequences of these events appear to have been minor5,28. All cases resolved without complications in our study. Indeed high rates of reported neutropenia may also reflect genetic and/or geographic differences that lead to relatively low neutrophil levels in Ugandan, compared to North American children 29. There is some concern that LPV/r may impair growth; greater change in weight-for-age Z-score was seen in children receiving NNRTIs compared to those receiving PIs in the p1060 trial. In our trial severe malnutrition was noted in only one patient, who died before initiating medications, but detailed analyses of impacts of study drugs on growth are ongoing.
Considering our results, what is the role for LPV/r in the treatment of HIV-infected African children? The cost of LPV/r has declined dramatically over the past decade and there is wider access to pediatric formulations30. Clinical trials have yielded varying results about whether LPV/r is superior to NNRTIs. At the moment, it may be that LPV/r use should be targeted to the populations of children in which it has shown particular benefit. The World Health Organization now recommends LPV/r over NNRTIs as the first-line therapy for all HIV-infected children under 3 years of age31. The results of PROMOTE-pediatrics s suggest that the use of LPV/r- over NNRTI-based ART could lower the incidence of malaria for HIV-infected children up to age 6 years living in malaria endemic regions where artemether–lumefantrine is the malaria treatment of choice1. And for a malaria-endemic country, the relatively higher cost of LPV/r compared to NNRTs may be offset by the avoidance of malaria-associated medications, hospitalizations, and mortality. As the use of LPV/r expands in Africa, it will be important that data about clinical outcomes and the declining cost of LPV/r continue to be evaluated as African countries determine their treatment guidelines.
Supplementary Material
SDC Figure 1. Time to Virologic Failure, ART-experienced
Time to virologic failure, the first of 2 successive HIV RNA levels > 400 copies/ml in Kaplan-Meier modeling. LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor
SDC Figure 2. Change in CD4 measures over time, ART-experienced
a) CD4 Number b) CD4 Percentage
CD4 count and percentage by duration of time receiving ART during the study. Values are means with standard deviation error bars. Data were included from subjects on per-protocol basis with some patients missing scheduled visits at each time point.
Acknowledgements
The authors would like to acknowledge the children and families and study team of PROMOTE-pediatrics for the time and effort that made the trial possible. Funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD059454 and TDR:K23HD60459) at the National Institutes of Health. Lopinavir/ritonavir was donated by AbbVie. We thank Albert Plenty for his assistance with statistical analysis. TR and JA contributed significantly to the design of the study, acquisition and interpretation of data, and drafted/edited the manuscript. AK, GI and FW contributed significantly to the acquisition of data and edited of the manuscript. GD, PR, DH and MK contributed significantly to the design of the study, acquisition and interpretation of data, and edited the manuscript. All authors approved the final version.
Footnotes
Disclaimers: Portions of these data were presented at the 18th (2010, Boston, MA) and 20th (2013, Atlanta, GA) Conferences on Retrovirus and Opportunistic Infections.
Clinical Trial Registration: Prevention of Malaria and HIV disease in Tororo (PROMOTE) pediatrics trial registration: NCT00978068
Conflicts of interest:
No conflicts of interest are declared.
REFERENCES
- 1.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012 Nov 29;367(22):2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 2005 Jul;49(7):2983–2985. doi: 10.1128/AAC.49.7.2983-2985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikilezi G, Achan J, Kakuru A, et al. Prevalence of Asymptomatic Parasitemia and Gametocytemia among HIV-Infected Ugandan Children Randomized to Receive Different Antiretroviral Therapies. Am J Trop Med Hyg. 2013 Apr;88(4):744–746. doi: 10.4269/ajtmh.12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010 Sep 8;304(10):1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012 Jun 21;366(25):2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach - 2010 revision. Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 7.Working Group on Antiretroviral Therapy and Medical Management of HIVInfected Children [April 18, 2013];Guidelines for the use of antiretroiviral agents in pediatric HIV infection. 2008 Feb;28 http://aidsinfo.nih.gov/guidelines/archive/pediatric-guidelines/. [Google Scholar]
- 8.Antiretroviral therapy of HIV infection in infants and children in resourcelimited settings: towards universal access. World Health Organization; 2006. p. 158. ( http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf) [Google Scholar]
- 9.Division of AIDS - National Institute of Allergy and Infectious Diseases [April 18, 2013];Division of AIDS Table for the Grading of he severity of adult and pediatric adverse events, version 1.0. 2004 Dec; clarification August 2009. . ( http://rsc.techres. com/Document/safetyandpharmacovigilance/Table_for_Grading_Severit y_of_Adult_Pediatric_Adverse_Events.pdf).
- 10.Guidelines for the treatment of malaria. (second edition) 2010 [Google Scholar]
- 11.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010 Oct 14;363(16):1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babiker A, Castro nee Green H, Compagnucci A, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIVinfected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011 Apr;11(4):273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of Long-Term Viral Failure Among Ugandan Children and Adults Treated With Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 14.Viergever RF, ten Berg MJ, van Solinge WW, Hoepelman AI, Gisolf EH. Changes in hematological parameters after switching treatment of HIVinfected patients from zidovudine to abacavir or tenofovir DF. HIV Clin Trials. 2009 Mar-Apr;10(2):125–128. doi: 10.1310/hct1002-125. [DOI] [PubMed] [Google Scholar]
- 15.McComsey G, Bhumbra N, Ma JF, Rathore M, Alvarez A. Impact of protease inhibitor substitution with efavirenz in HIV-infected children: results of the First Pediatric Switch Study. Pediatrics. 2003 Mar;111(3):e275–281. doi: 10.1542/peds.111.3.e275. [DOI] [PubMed] [Google Scholar]
- 16.Mallolas J, Podzamczer D, Milinkovic A, et al. Efficacy and safety of switching from boosted lopinavir to boosted atazanavir in patients with virological suppression receiving a LPV/r-containing HAART: the ATAZIP study. J Acquir Immune Defic Syndr. 2009 May 1;51(1):29–36. doi: 10.1097/QAI.0b013e31819a226f. [DOI] [PubMed] [Google Scholar]
- 17.Valantin MA, Bittar R, de Truchis P, et al. Switching the nucleoside reverse transcriptase inhibitor backbone to tenofovir disoproxil fumarate + emtricitabine promptly improves triglycerides and low-density lipoprotein cholesterol in dyslipidaemic patients. J Antimicrob Chemother. 2010 Mar;65(3):556–561. doi: 10.1093/jac/dkp462. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Tome MI, Amador JT, Pena MJ, Gomez ML, Conejo PR, Fontelos PM. Outcome of protease inhibitor substitution with nevirapine in HIV-1 infected children. BMC Infect Dis. 2008;8:144. doi: 10.1186/1471-2334-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs. 2012 Jun 18;72(9):e1–25. doi: 10.2165/11633630-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kekitiinwa A, Asiimwe AR, Kasirye P, et al. Prospective long-term outcomes of a cohort of Ugandan children with laboratory monitoring during antiretroviral therapy. Pediatr Infect Dis J. 2012 Aug;31(8):e117–125. doi: 10.1097/INF.0b013e31825cb9d6. [DOI] [PubMed] [Google Scholar]
- 21.Murphy RA, Sunpath H, Castilla C, et al. Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr. 2012 Oct 1;61(2):158–163. doi: 10.1097/QAI.0b013e3182615ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoffelen AF, Wensing AM, Tempelman HA, Geelen SP, Hoepelman AI, Barth RE. Sustained virological response on second-line antiretroviral therapy following virological failure in HIV-infected patients in rural South Africa. PLoS One. 2013;8(3):e58526. doi: 10.1371/journal.pone.0058526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanoni BC, Sunpath H, Feeney ME. Pediatric response to second-line antiretroviral therapy in South Africa. PLoS One. 2012;7(11):e49591. doi: 10.1371/journal.pone.0049591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006 Oct 24;20(16):2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 25.Dronda F, Moreno S, Moreno A, Casado JL, Perez-Elias MJ, Antela A. Longterm outcomes among antiretroviral-naive human immunodeficiency virusinfected patients with small increases in CD4+ cell counts after successful virologic suppression. Clin Infect Dis. 2002 Oct 15;35(8):1005–1009. doi: 10.1086/342695. [DOI] [PubMed] [Google Scholar]
- 26.Barreiro P, Soriano V, Casas E, Gonzalez-Lahoz J. Different degree of immune recovery using antiretroviral regimens with protease inhibitors or nonnucleosides. AIDS. 2002 Jan 25;16(2):245–249. doi: 10.1097/00002030-200201250-00014. [DOI] [PubMed] [Google Scholar]
- 27.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008 May 15;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010 Oct 14;363(16):1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubega IR, Fowler MG, Musoke PM, et al. Considerations in using US-based laboratory toxicity tables to evaluate laboratory toxicities among healthy malawian and Ugandan infants. J Acquir Immune Defic Syndr. 2010 Sep;55(1):58–64. doi: 10.1097/QAI.0b013e3181db059d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Spet 19, 2013];Untangling the web of antiretrovirtal price reductions. (16th edition). 2013 Jul; http://www.msfaccess.org.
- 31.World Health Organization . Consolidated guidelines on the use of antiretrovrial drugs for treating and preventing HIV infection. Geneva, Switzerland: 2013. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC Figure 1. Time to Virologic Failure, ART-experienced
Time to virologic failure, the first of 2 successive HIV RNA levels > 400 copies/ml in Kaplan-Meier modeling. LPV/r, ritonavir-boosted lopinavir; NNRTI, non-nucleoside-reverse-transcriptase-inhibitor
SDC Figure 2. Change in CD4 measures over time, ART-experienced
a) CD4 Number b) CD4 Percentage
CD4 count and percentage by duration of time receiving ART during the study. Values are means with standard deviation error bars. Data were included from subjects on per-protocol basis with some patients missing scheduled visits at each time point.