Abstract
High serum/plasma cholesterol levels have been suggested as a risk factor for Alzheimer disease (AD). Some reports, mostly retrospective epidemiological studies, have observed a decreased prevalence of AD in patients taking the cholesterol lowering drugs, statins. The strongest evidence causally linking cholesterol to AD is provided by experimental studies showing that adding/reducing cholesterol alters amyloid precursor protein (APP) and amyloid beta-protein (Aβ) levels. However, there are problems with the cholesterol-AD hypothesis. Cholesterol levels in serum/plasma and brain of AD patients do not support cholesterol as a causative factor in AD. Prospective studies on statins and AD have largely failed to show efficacy. Even the experimental data are open to interpretation given that it is well-established that modification of cholesterol levels has effects on multiple proteins, not only APP and Aβ. The purpose of this review, therefore, is to examine the above-mentioned issues and discuss the pros and cons of the cholesterol-AD hypothesis, and the involvement of other lipids in the mevalonate pathway, such as isoprenoids and oxysterols, in AD.
Keywords: Alzheimer disease, amyloid beta-protein, apolipoprotein E, cholesterol, isoprenoids, oxysterols, statins
Twenty three years ago the late Larry Sparks and his colleagues made the intriguing observation that brains of patients with advanced coronary heart disease had senile plaques similar to those seen in Alzheimer patients (Sparks et al. 1990). In a subsequent paper, they reported that feeding rabbits a diet high in cholesterol induced plaques in brain tissue of treated animals (Sparks et al. 1994). Those initial observations stimulated a large body of research ranging from retrospective and prospective epidemiological studies on the cholesterol lowering drugs statins and Alzheimer disease (AD) to animal, cellular and molecular studies on cholesterol and amyloid beta-protein (Aβ). Elevated serum/plasma cholesterol levels have been proposed to be a risk factor for developing AD (Pappolla et al. 2003) but there are issues with that hypothesis (Wood et al. 2005;Daviglus et al. 2010). A modification of that hypothesis is the notion that high serum cholesterol levels during middle age are associated with an increased risk of AD (Solomon et al. 2009a). Effectiveness of statins in treating or preventing AD is controversial particularly with respect to prospective studies (McGuinness et al. 2013). The strongest support for a role of cholesterol in AD comes from studies in animal models of AD and in vitro studies using primary and immortalized cells. A majority of the animal and cell culture studies have reported that increasing cholesterol levels increases Aβ abundance whereas the opposite effects are noted when cholesterol levels are reduced and that work has been extensively reviewed (Wood et al. 2003;Posse de Chaves 2012;Maulik et al. 2013;Burns and Rebeck 2010;Ong et al. 2013).
Data from human studies of cholesterol levels whether in serum/plasma or brain are not consistent in supporting a role of cholesterol in AD, and this conundrum will be examined in this review. This lack of consistently in the human literature differs in comparison with results of most animal and cell culture studies. What appears to be a paradox between the human and animal/cell culture studies will be critically examined from the perspective of the multiple roles of cholesterol in addition to the cholesterol-mediated actions in AD. Cholesterol is not the only important lipid produced in the mevalonate pathway as noted in Figure 1, and this review will examine whether a case can be made for linking other mevalonate-derived lipids (farnesyl pyrophosphate, geranylgeranyl pyrophosphate, 24S-hydroxycholesterol) to AD. Emphasis of this review will be on the role of cholesterol as factor in the development of AD. However, we will examine the alternative hypothesis that AD and specifically Aβ perturb cholesterol homeostasis.
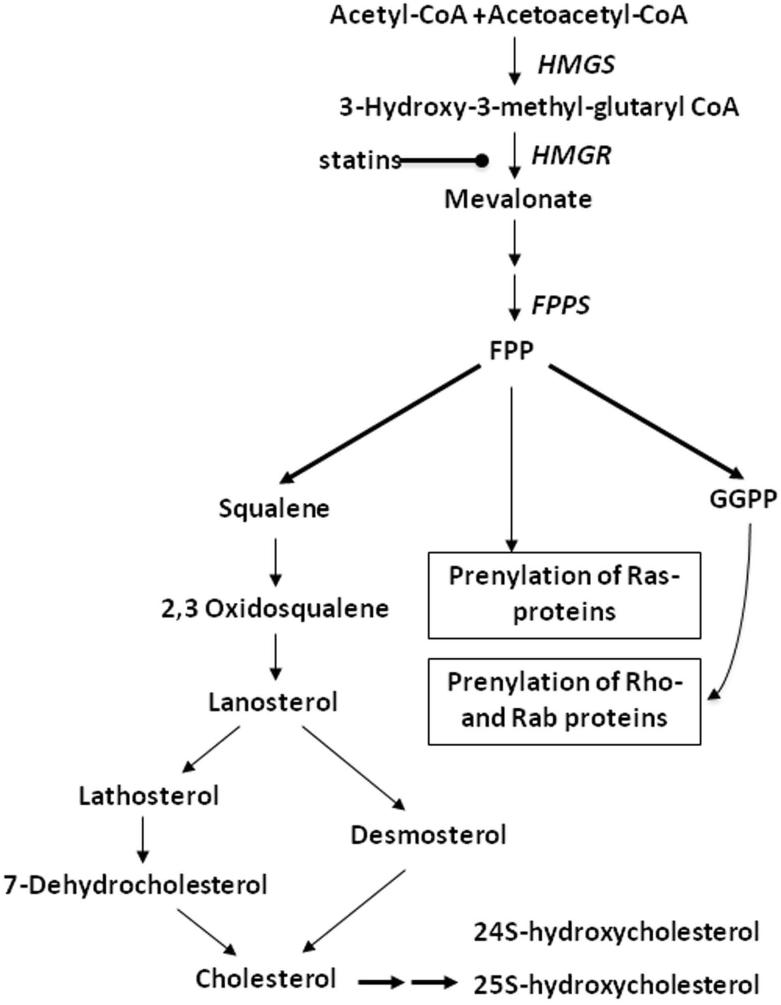
Figure 1. Mevalonate/Isoprenoid/Cholesterol synthesis pathway.
Acetyl-CoA and acetoacetyl-CoA catalyzed by 3-hydroxy-3-methylglutaryl CoA synthase (HMGS) are converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), which is then converted to mevalonate through the action of HMG-CoA reductase (HMGR) and the cofactor NADPH. This reaction is the rate-limiting step for cholesterol synthesis, and statins are a substrate for HMG-CoA reductase. Through a series of reactions, mevalonate is converted to farnesyl pyrophosphate (FPP) by farnesyl pyrophosphate synthase (FPPS). FPP is a key branch point for synthesis of geranylgeranyl pyrophosphate (GGPP), cholesterol, ubiquinone, and dolichol. Both FPP and GGPP are required for prenylation of GTP-binding proteins such as Rho, Rac, and Ras, enabling those proteins to be inserted in membranes. FPP is also the precursor of squalene. Conversion of squalene to cholesterol requires over 19 reactions. Cholesterol is subsequently converted to oxysterols such as 24S-hydroxycholesterol and 25S-hydroxycholesterol.
Serum/Plasma Cholesterol Levels and AD
Elevated serum cholesterol levels have been proposed to increase the risk of developing AD (Pappolla et al. 2003). An early study on cholesterol and AD reported that apoE genotype and AD were dependent on total cholesterol levels, age and gender (Jarvik et al. 1995). The authors concluded that total serum cholesterol may accelerate development of AD. However, that conclusion is not supported by their data, which showed that mean serum cholesterol levels did not significantly differ between AD patients and controls. In another study, total serum cholesterol levels were not significantly different between AD and control subjects, but it was observed that low-density lipoprotein cholesterol (LDL-C) was significantly higher and high-density lipoprotein cholesterol (HDL-C) was significantly lower in AD patients than control subjects (Kuo et al. 1998). In contrast, LDL-C levels were reported to not be significantly different in AD patients compared with control subjects (Romas et al. 1999), but total cholesterol levels were significantly lower in AD patients than control subjects. Although the mean total cholesterol levels were significantly different, the physiological effects of such small changes (3.9% difference) are unclear. A recent study found that plasma cholesterol levels were approximately 10% higher in AD patients as compared with control subjects and that this difference was significant (Popp et al. 2013). An earlier study by the same group reported that plasma cholesterol levels of AD and control individuals were not significantly different (Popp et al. 2012).
The argument has been made that elevated serum cholesterol levels at midlife may be predictive of developing AD, and that longitudinal studies may be more revealing as compared with cross-sectional studies (Kivipelto et al. 2001). In a longitudinal study at the first exam, cholesterol levels were significantly higher in AD patients compared with control subjects but were not significantly different at the second exam ranging from 11-26 years after the first exam. Odds ratio analysis using different models indicated that midlife cholesterol levels were a significant risk for developing AD. However, there are problems with that interpretation. The mean cholesterol levels at the first exam were 278 mg/dl for the AD group and 259 mg/dl for the control group. At the second exam, cholesterol levels were 232 and 224 mg/dl for the AD and control groups. Normal cholesterol levels are considered to be under 200 mg/dl. Both AD and control groups were hypercholesterolemic at midlife but only 48 individuals were eventually diagnosed with AD compared with 1,352 subjects not receiving an AD diagnosis. Missing from the study was a groups of individuals with normal cholesterol levels both at midlife and late-life with or without a subsequent diagnosis of AD. Another study reported that high cholesterol levels at midlife were associated with the risk of developing late-life dementia (Whitmer et al. 2005). In that study there were 2844 individuals with cholesterol levels above 240 mg/dl and out of that group 266 or 9% were identified with dementia using medical records. Based on a Cox proportional hazards model, the authors concluded that high midlife cholesterol levels were associated with an increased risk of developing AD. Similar conclusions were reached in a later study using Cox proportional hazards models of cholesterol levels and development of AD (Solomon et al. 2009a). There were 9,844 subjects whose cholesterol levels were determined when in their early 40’s. Less than 5% were later diagnosed in their late 60's with AD using electronic records. Approximately 2% were diagnosed with vascular dementia. Mean cholesterol levels were similar for all three groups at midlife: no dementia, 224 mg/dl; AD, 228mg/dl; vascular dementia, 226 mg/dl. A prudent interpretation of those results is that elevated cholesterol levels at midlife is a risk factor for developing AD for a very small number of individuals but other causative factors are at play.
Data supporting elevated cholesterol levels as a risk factor for AD are not robust. Further weakening the cholesterol-AD hypothesis are studies showing that serum cholesterol levels do not differ between AD and control individuals. A meta-analysis of 10 studies published between 1986 and 1999 found that cholesterol levels were actually significantly lower in AD patients than in control subjects (Knittweis and McMullen 2000). The well-recognized Framingham study found that total serum cholesterol levels were not associated with the risk for developing AD (Tan et al. 2003). Serum cholesterol levels were determined at baseline and across 15 biennial cycles as well as neurological and neuropsychological examinations for dementia. Serum cholesterol levels in normal individuals 65 years of age or older were not associated with the risk of subsequently developing dementia or AD (Li et al. 2005). The Honolulu-Asia Aging study found that serum cholesterol levels at midlife were not associated with later development of dementia or AD as assessed by a neurological and neuropsychological exams and neuroimaging (Stewart et al. 2007). The observation was made in that study that cholesterol levels in individuals with dementia and AD had declined over at least 15 years. It was suggested that the decline in cholesterol levels might actually contribute to the development of dementia (Stewart et al. 2007).
Elevated total serum cholesterol levels whether at midlife or later do not have a major role in the development of AD. This conclusion is not surprising based on the following data: 1) the estimated number of US adults with cholesterol levels of 200 mg/dl and higher is 98.9 million and of that number 31.9 million have cholesterol levels of 240 mg/dl and higher (Go et al. 2013); and 2) 4.7 million individuals are estimated to have AD (Hebert et al. 2013). If high serum cholesterol levels were a risk factor for AD then the incidence and prevalence of AD would be higher. Another issue to consider is the biological rationale for examining serum cholesterol levels in AD patients. Serum cholesterol and brain cholesterol levels are not in equilibrium (Kabara 1973). Normally, changes in serum cholesterol do not affect brain cholesterol homeostasis, and no relevant cholesterol flux from the periphery into brain seems to take place (Dietschy and Turley 2004). This line of evidence is supported by data from our laboratories on apoE-knockout mice and cholesterol-fed rats. ApoE-knockout mice have peripheral atherosclerotic lesions and show highly elevated serum and liver cholesterol values, but brain cholesterol levels of these animals do not differ compared with age-matched wild-type mice (Kirsch et al. 2003a). In the same study, treatment of young Wistar rats for at least 6 months with 2% cholesterol had no effect on brain cholesterol levels of synaptic plasma membranes (SPM) compared with control animals. Plasma and liver cholesterol levels were significantly increased in the animals on the 2% cholesterol diet.
Brain Cholesterol Levels and AD
Elevated serum and plasma cholesterol levels do not appear to be a risk factor for AD. Serum and plasma cholesterol levels are not in equilibrium with brain cholesterol as discussed above. There is evidence that the blood-brain barrier may be dysfunctional in AD (Erickson and Banks 2013) which could alter brain cholesterol homeostasis. If high serum/plasma cholesterol levels were a factor in the development of AD then it follows that cholesterol should be in greater abundance in brain tissue of AD patients as compared with normal individuals. Cholesterol levels have been determined in different brain regions and the cerebrospinal fluid (CSF) of AD patients compared with control subjects. There have been reports of reduced cholesterol levels, increased cholesterol levels, and no changes in cholesterol levels in AD patients versus control subjects (Wood et al. 2005). Cholesterol levels were lower in the temporal gyrus of autopsied brains of AD patients than control subjects (Mason et al. 1992). Cholesterol levels in the frontal cortex gray matter of AD patients was modestly but significantly higher (2.65 ± 0.14 mg/g wet tissue weight) with the apoE4 genotype compared with apoE4 control subjects (2.04 ± 0.18) (Sparks 1997). Cholesterol levels were similar in the cerebral cortex of AD and control individuals (Heverin et al. 2004) but a small but significant increase (approx 15 μg vs 12 μg/mg brain tissue) in cholesterol levels was noted in the basal ganglia of AD patients compared with control subjects. Cholesterol levels did not differ in hippocampal tissue of AD patients compared with control subjects (Eckert et al. 2000). We reported that cholesterol levels and HMG-CoA reductase gene expression were similar in post-mortem human brain frontal cortex of AD and control individuals (Eckert et al. 2009). However, in two recent studies it was reported that CSF cholesterol levels were significantly lower (approximately 12%) in AD patients than controls (Popp et al. 2012;Popp et al. 2013).
Simply put, brain cholesterol levels of AD patients are highly variable, and the data do not support the hypothesis that total brain cholesterol abundance is a causative factor in AD. Contributing to the variability amongst the studies obviously are issues pertaining to sample selection, preparation and assays methods. Another consideration is that total cholesterol levels are at best a gross estimate of the role of cholesterol in any biological process. Cholesterol exists for example in different domains within membranes (Wood et al. 2007;Schroeder et al. 2010;Sonnino and Prinetti 2013;Wood et al. 2011) . Data from animal and cell culture studies have shown that large changes can occur in membrane cholesterol domains that take place in the absence or minimal changes in total cholesterol levels. We have shown for example that the distribution of cholesterol between the two membrane leaflets of SPM was altered in animal models of aging, alcoholism (Igbavboa et al. 1996;Wood et al. 1989), statin administration (Kirsch et al. 2003b;Burns et al. 2006;Eckert et al. 2013), and apoE genotype (Hayashi et al. 2002;Igbavboa et al. 1997). Another type of cholesterol domain attracting great interest albeit with some controversy is lipid rafts (Sonnino and Prinetti 2013;Head et al. 2013). Lipid rafts are thought to be scaffolds for interactions of proteins and lipids. These cholesterol enriched membrane domains have been implicated in certain neurodegenerative diseases such Smith-Lemli-Opitz syndrome, Huntington's, Niemann-Pick Type C and AD (Korade and Kenworthy 2008). A study in lipid rafts from human AD brain samples reported changes in fatty acid composition between AD and control samples but that cholesterol content was similar in the two groups (Martin et al. 2010). A different approach to lipid analysis is time-of-flight secondary ion mass spectrometry (ToF-SIMS) which is now gaining acceptance as a tool for imaging lipids in biological samples (Passarelli and Winograd 2013). ToF-SIMS was used in a recent study to determine cholesterol distribution in cortical layers of white and grey matter in AD patients and controls, and it was found that the cholesterol signal in the lower half of the cerebral cortex was higher in AD patients than controls (Lazar et al. 2013). The novel findings reported by Lazar et al., provide the first indication that cholesterol distribution within a specific brain region may differ in AD as compared with control samples. It would have been helpful in that study if total levels of cholesterol in the cerebral cortex were determined using other methodology. Such data would have provided insight as to whether the observed changes were due to differences in the total amount of cholesterol as compared with a change in how cholesterol was distributed. Faulty cholesterol distribution plays a key role in lysosomal storage diseases such as Niemann-Pick Type C (NPC) and it has been suggested that lysosomal dysfunction may be present in AD (Nixon 2004). Hydroxypropyl-β-cyclodextrin which binds cholesterol and has been used in NPC animal models and patients (Matsuo et al. 2013) improved behavior in a mouse model of AD (Yao et al. 2012). Aβ levels also were reduced and Aβ clearance increased. Certainly investigating lipid domains whether at subcellular levels or within brain regions gives a micro versus macro view of the potential role of cholesterol in AD. What is not forthcoming from such an approach is that it does not provide insight as to whether cholesterol is a causative factor in AD or is this crucial lipid a casualty of the disease (Wood et al. 2003;Posse de Chaves 2012). That notion is discussed later in this review.
Farnesyl pyrophosphate and geranylgeranyl pyrophosphate in AD
Support is lacking for elevated serum or brain cholesterol levels as causative factors in AD. Cholesterol is one of many molecules synthesized within the mevalonate pathway (Fig. 1). The two upstream isoprenoids, farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) have attracted much attention in diseases and conditions as diverse as cancer(Wiemer et al. 2009), osteoporosis(Mo et al. 2012), cardiovascular disease(Liao and Laufs 2004), premature and normal aging(Reddy and Comai 2012;Hooff et al. 2012) and neurodegenerative diseases including AD(Weber et al. 2005;Cole and Vassar 2006;Hooff et al. 2010;Li et al. 2012). FPP is a key branch point in the mevalonate pathway, and it is a precursor of GGPP and cholesterol. Both FPP and GGPP are required for the post-translational processing of small GTPases. Progress on understanding how regulation of FPP and GGPP levels affects the function of small GTPases in normal brain and neurodegenerative diseases such as AD has been impeded because of analytical difficulties in FPP and GGPP isolation and detection. We have recently overcome these technical obstacles and reported for the first time FPP and GGPP levels in human brain tissue of normal individuals and AD patients (Eckert et al. 2009). FPP and GGPP levels of AD patients were significantly higher 36% and 56% respectively, when compared with age-matched controls. Total cholesterol levels were similar in brain tissue of AD and control samples even though FPP is a precursor of cholesterol; FPP is also the precursor of GGPP. Consistent with elevation of FPP and GGPP levels, we showed that gene expression of FPP synthase and GGPP synthase (protein products catalyze FPP and GGPP production) were also elevated in AD brain tissue. HMG-CoA reductase expression levels were unchanged. This study suggests a specific targeting of FPP and GGPP regulation in AD. However, these results need to be confirmed using a larger sample size. It remains to be determined if increases in FPP and GGPP in AD brain are specific to AD or are a manifestation of neurodegeneration which might occur in other diseases.
The consequences of elevated FPP and GGPP levels on protein prenylation require investigation. GTPases must be prenylated either by FPP or GGPP in order to be inserted into membranes and to become active (McTaggart 2006). The majority of small Rho-GTPases are prenylated by GGPP involving geranylgeranyltransferase-I (GGTase-I), which catalyzes the covalent attachment of GGPP via thioether linkage to the CAAX-motif of those proteins. FPP normally prenylates for example Ras proteins (HRAS, KRAS, NRAS) through actions of farnesyl transferase (FTase) but when FTase is inhibited FPP can become a substrate for GGTase-I and prenylation of KRAS and NRAS but not HRAS Ras can occur (Roskoski 2003;Downward 2003)
The Rho family of GTPases such as Rac1, RhoA, and Cdc42 are major regulators of synaptic plasticity, acting on dendrite morphogenesis and stability, growth cone motility and collapse (Linseman and Loucks 2008;Sekino et al. 2007;Ramakers 2002) and affecting neuronal architecture and synaptic connectivity (Pilpel and Segal 2004). There are data indicating that certain small Rho family GTPases such as Rac1, RhoA, and Cdc42 contribute to AD pathogenesis (Désiré et al. 2005;Mendoza-Naranjo et al. 2007;Ma et al. 2008;Wang et al. 2009;Oh et al. 2010;Huesa et al. 2010). In addition, the level of Ras in the brain is increased in the early stage of AD (Gärtner et al. 1999;Gärtner et al. 1995). Rho and Ras proteins are prenylated by GGPP and FPP, respectively and AD may have a global effect on protein prenylation. Reducing protein prenylation by decreasing levels of FPP and GGPP and inhibiting prenyltransferases have been strategies used to counteract neuronal dysfunction in animal and cell models of AD (Cole and Vassar 2006;Li et al. 2012). However, it has been reported that administration of simvastatin which reduces FPP, GGPP and cholesterol stimulated long-term potentiation (LTP) in the CA1 region of the hippocampus in slices from wild-type C57BL/6 mice which was due to depletion of FPP and not GGPP (Mans et al. 2012). Geranylgeraniol (GGOH), the dephosphorylated derivative of GGPP rescued impairment of hippocampal long-term potentiation induced by statins and suppression of cholesterol 24-hydroxylase (Kotti et al. 2006;Kotti et al. 2008). Protein prenylation is required for synaptic plasticity (Tolias et al. 2011), and the long-term consequences of reducing prenylation in brain are not known. Effects of altering isoprenoid levels on brain function is not without controversy and requires much more investigation.
24S-hydroxycholesterol in AD
The cholesterol metabolite 24S-hydroxycholesterol (24S-OHC) is an oxysterol (Fig. 1) proposed to be a marker of brain cholesterol metabolism (Lütjohann and von Bergmann 2003). Cholesterol 24-hydroxylase catalyzes the synthesis of 24S-OHC and 24-hydroxylase has been identified in specific regions of neurons (endoplasmic reticulum, cell body, dendrites) and brain areas (e.g., hippocampal CA1 pyramidal cells, hippocampal and cerebellar interneurons) (Lund et al. 2003;Ramirez et al. 2008). There are reports that 24S-OHC levels are altered in CSF and plasma of AD patients as compared with control subjects (Papassotiropoulos et al. 2002;Popp et al. 2012;Popp et al. 2013). Significantly elevated 24S-OHC was observed in CSF of AD patients (approximately 2.1 ng/mL) in contrast to control subjects (approximately 1.3 ng/mL) (Papassotiropoulos et al. 2002). Plasma 24S-OHC levels were similar for the AD and control samples. Opposite results were found in another study looking at CSF and plasma 24S-OHC in AD and control samples (Popp et al. 2012). CSF levels were 2.99 ng/mL and 2.68 ng/mL for AD patients and controls respectively, which were not significantly different. Plasma 24S-OHC levels were significantly higher (78.82 ng/mL) for AD patients than control samples (62.28 ng/mL). Similar findings were reported recently showing that AD patients had higher levels of plasma 24S-OHC as compared with control subjects but that CSF 24S-OHC levels were comparable (Popp et al. 2013). However, significantly lower 24S-OHC plasma levels were found in AD patients as compared to patients with mild cognitive impairment and individuals with subjective cognitive complaints (Solomon et al. 2009b).
The data on 24S-OHC and AD are equivocal. Another concern is that reductions in serum and plasma 24S-OHC levels have been reported recently in patients with multiple sclerosis and Huntington disease (van de Kraats et al. 2013;Leoni et al. 2013). Changes in 24S-OHC levels may simply reflect neuronal dysfunction common to certain neurodegenerative diseases.
Animal and Cell Culture AD Models
What would appear to be the strongest evidence supporting cholesterol as a factor in AD comes from animal and cell culture studies. There have been several recent reviews covering in vivo and in vitro studies that examined perturbation of the mevalonate pathway and effects on amyloid precursor protein (APP) and Aβ (Eckert et al. 2010;Williamson and Sutherland 2011;Li et al. 2012;Posse de Chaves 2012;Gamba et al. 2012;Maulik et al. 2013). Generally, reducing cholesterol levels decrease Aβ abundance and are neuroprotective whereas increasing cholesterol levels have opposite effects. Applying those findings to what occurs in AD patients is challenging due to the multifaceted roles of cholesterol in cells. Reducing or increasing cholesterol can have numerous structural and functional effects in addition to effects on AD associated proteins. One study reported that reducing brain cholesterol synthesis by crossing cholesterol 24-hydroxylase knockout mice with an AD mouse model (B6.Cg-Tg(APPswe, PSEN1E9)85Dbo/J) had a slight effect on Aβ levels but increased lifespan of the AD mice (Halford and Russel 2009)Basic studies on cholesterol, membrane structure and function have been reviewed in detail previously (Schroeder et al. 2001;Wood et al. 2002;Vance et al. 2005;Wood et al. 2007;Lee 2011). Removing cholesterol increases fluidity of biological membranes. Cholesterol alters lipid packing, curvature and interdigitation of the membrane leaflets. Modifying cholesterol levels in SPM and synaptosomes altered sodium-dependent Υ-aminobutyric acid (GABA) (North and Fleischer 1983). Reducing cholesterol in membranes produced a loss in GABA uptake, and the uptake was restored by the addition of cholesterol. Activity of Ca2+ + Mg2+-ATPase but not Na+,K+-ATPase was reduced by oxidation of cholesterol (Wood et al. 1995). Increasing membrane cholesterol in erythrocytes reduced Na+,K+-ATPase activity whereas opposite effects were observed when cholesterol was increased (Yeagle 1983). The conventional wisdom regarding an explanation for cholesterol-induced changes in protein function has been alterations in membranes properties such as fluidity, lipid packing, curvature and changes in interdigitation or overlapping of the membrane leaflets. However, it is becoming recognized that certain proteins may have specific cholesterol binding sites (Lee 2011). A cholesterol binding domain was identified in the nicotinic acetylcholine receptor (AChR) (Corbin et al. 1998). Recently, it was reported that cholesterol depletion accelerates the internalization of the AChR (Borroni and Barrantes 2011). A specific cholesterol binding site was shown for the human β2-adrenergic receptor (Hanson et al. 2008).
The multifaceted effects of cholesterol pose a problem for explaining how either reducing or increasing cholesterol modifies APP and Aβ. A specific mechanism has not been forthcoming. Several years ago (Avdulov et al. 1997) we found that the binding affinity of cholesterol to Aβ40 polymers (KD of 3.24 × 10−9 M) was strikingly higher as compared with other lipids (phosphatidylcholine KD of 7.07 × 10−7 M; stearic acid KD of 9.42 × 10−8 M). Cholesterol also was reported to bind to Aβ at the α-secretase cleavage site (Yao and Papadopoulos 2002). Just recently, a cholesterol-binding domain was identified within an APP transmembrane domain (Barrett et al. 2012),and a cholesterol binding site was reported for the Aβ 22-35 amino acid fragment (Di Scala et al. 2013). Both Aβ and APP contain lipophilic domains, which would enhance cholesterol binding. Aβ 25-35 favors the membrane hydrophobic interior (Mason et al. 1996) which would facilitate interaction. These novel findings provide a potential mechanism for APP, Aβ cholesterol interactions. One speculative prediction is that cholesterol binding to APP could provide an optimal physico-chemical environment for the activity of secretases and stimulate Aβ production. What conditions facilitate and inhibit binding need to be examined. The distribution for example of cholesterol in the two membrane leaflets could be altered which may impact cholesterol binding to APP/Aβ. Such changes in distribution can occur in the absence of changes in the total amount of brain cholesterol. Cholesterol in the SPM exofacial leaflet of mice expressing human apoE4 was two-fold greater as compared with the exofacial leaflet of apoE 3 mice but total SPM cholesterol did not differ between the two groups (Hayashi et al. 2002). The larger amount of cholesterol in the SPM exofacial leaflet of the apoE4 mice could enhance binding of Aβ to the membrane resulting in perturbation of the membrane with consequences on lipid rafts, ion transport, endocytosis and exocytosis which would be inimical to cells. We have reported that the SPM exofacial leaflet of 24-25 mo old mice contained twice as much cholesterol as compared with the exofacial leaflet of 3-4 mo old mice (Igbavboa et al. 1996). Lipid raft protein and lipid composition differ in SPM of apoE4 mice as compared with apoE3 mice and the apoE4 lipid raft composition was similar to aged wild-type mice (Igbavboa et al. 2005). Support for membrane cholesterol facilitating actions of Aβ was shown in an in vitro study of hippocampal neurons (Nicholson and Ferreira 2009). Mature neurons cultured for 21 days showed greater Aβ-induced toxicity and had higher membrane cholesterol content than neurons cultured for shorter time periods. Just recently it was shown in model membranes with cholesterol asymmetry of the two leaflets similar to that of aged and apoE4 SPM that the C-terminus of Aβ42 resided in the exofacial leaflet and such repositioning may promote Aβ oligomerization or oxidative reactivity (Liguori et al. 2013). Taken together, the results of the cholesterol studies on hippocampal neurons (Nicholson and Ferreira 2009), leaflet model membranes (Liguori et al. 2013) and SPM leaflets (Igbavboa et al. 1996;Hayashi et al. 2002) suggest that neuronal membranes of aged individuals, and those with the apoE4 allele would be more susceptible to Aβ perturbation than either younger individuals or those carrying the apoE2 or apoE3 alleles as illustrated in the membrane leaflet model in Figure 2.
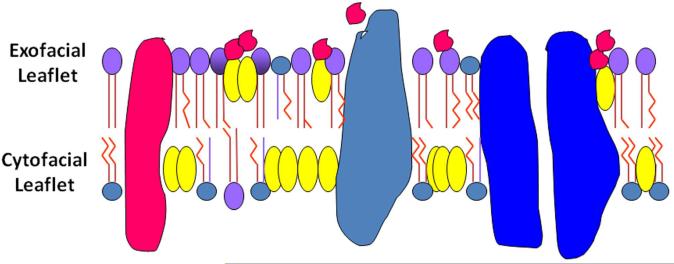
Figure 2. Model of Aβ acting on the synaptic plasma membrane exofacial leaflet.
Cholesterol is asymmetrically distributed in synaptic plasma membranes (SPM) (Wood et al. 2011). It is predicted that conditions where the SPM exofacial leaflet has been shown to have an abnormal enrichment of cholesterol (apoE4 and aged membranes) (Hayashi et al. 2002;Igbavboa et al. 1996) those membranes would be more susceptible to Aβ (red structures) perturbation than either younger individuals or those carrying the apoE2 or apoE3 alleles. Modeling of cholesterol distribution in the two leaflets similar to aged and apoE4 SPM found that the C-terminus of Aβ42 resided in the exofacial leaflet and such repositioning may promote Aβ oligomerization or oxidative reactivity (Liguori et al. 2013). Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), sphingomyelin (SM);  Globular structures represent proteins.
Globular structures represent proteins.
This section focused on cholesterol as a causative factor in the development/progression of AD particularly with respect to APP and Aβ. However, there is a smaller body of work indicating that Aβ impacts cholesterol homeostasis (Wood et al. 2002;Wood et al. 2003;Posse de Chaves 2012). Aβ40 and Aβ28 inhibited cholesterol esterification in plasma (Koudinov et al. 1996). Free and esterified cholesterol synthesis was reduced by Aβ40 in HepG2 cells (Koudinova et al. 1996). Cholesterol esterification was inhibited by Aβ40 in rat primary neurons (Liu et al. 1998). Aβ42 inhibited protein prenylation and disrupted protease cleavage of sterol regulatory element-binding protein-2 (SREBP-2) (Mohamed et al. 2012). Cleaved SREBP-2 localizes to the nucleus where it stimulates transcription by binding to nonpalindromic sterol response elements (SRE) in the promoters of several genes including: HMG-COA reductase, FPP synthase, and squalene synthase (Horton et al. 2002). SREBP-2 is the primary transcription factor of the SREBP family for regulation of cholesterol but it was reported that SREBP-1, SREBP-2 and HMGCR mRNA levels were reduced as was cholesterol in rat cortical neurons expressing APP (Pierrot et al. 2013). The authors concluded that APP may act to regulate cholesterol turnover. Cholesterol trafficking into and out of cells was altered by Aβ (Liu et al. 1998;Michikawa et al. 2001). Aβ stimulated cholesterol containing MTT formazan-transporting vesicles from B12 cells and increased cholesterol esterification in rat primary neurons (Liu et al. 1998). Oligomeric Aβ40 but not monomers or fibrils induced release of cholesterol, phospholipids and GM1 from rat primary neurons and astrocytes (Michikawa et al. 2001). The Golgi complex plays an important role in cholesterol trafficking (Mendez 1995;Heino et al. 2000). We had the idea that Aβ might target cholesterol homeostasis in the Golgi complex (Igbavboa et al. 2003). Aβ42 caused a redistribution (movement from the cis to the trans region) of cholesterol within the Golgi complex and also induced translocation of plasma membrane cholesterol to the Golgi complex in astrocytes. This Aβ-induced trafficking of cholesterol from the plasma membrane to the Golgi complex was due to retrograde movement of the cholesterol transport protein, caveolin-1 (Igbavboa et al. 2009).
Cholesterol transport proteins have attracted notice in the AD field. There has been for example growing interest in the role of the ABC family of proteins and their involvement in Aβ trafficking (Hirsch-Reinshagen and Wellington 2007;Wolf et al. 2012). Certainly, apoE has received the most attention of the cholesterol transport proteins in AD. The majority of those studies have focused on the apoE4 allele and risk of AD and functional studies of the apoE isoforms and Aβ dynamics; topics that have been covered previously in several excellent reviews (Kim et al. 2009;Holtzman et al. 2012;Liu et al. 2013). For the late onset sporadic AD (> 95% cases), the strongest genetic risk factor is one’s genotype for apolipoprotein E (APOE). There are three common APOE alleles in humans: APOE-ε2, APOE-ε3, and APOE-ε4, with an allele frequency of 7, 78, and 15%, respectively (Strittmatter and Roses 1996). While the APOE-ε2 allele confers some protection against AD (Corder et al. 1994), the APOE-ε4 allele is associated with an increased risk of AD (Corder et al. 1993;Poirier et al. 1993). One APOE-ε4 allele increases the risk of AD by three times (heterozygotes) and two APOE-ε4 alleles increase the risk of AD by 15 times (homozygotes) (Farrer et al. 1997). However, the question of how the APOE-ε4 allele promotes AD has not been answered. The protein product of the APOE gene, apoE, is a 299-amino acid glycoprotein in humans. The three APOE alleles lead to the production of three isoforms of apoE with different amino acid residues at position 112 and 158: apoE2 (Cys112, Cys158), apoE3 (Cys112, Arg158), and apoE4 (Arg112, Arg158), resulting in differences in structure and function of apoE isoforms (Zhong and Weisgraber 2009). ApoE is expressed by several cell types but it is primarily expressed in the liver and in the brain (Mahley 1988). In the circulation, apoE is associated with different classes of lipoprotein particles. In the brain, apoE is predominantly produced by astrocytes and associated with high-density lipoprotein (HDL)-like particles. ApoE interacts with multiple lipoprotein receptors and plays an important role in cholesterol transport and lipid metabolism. ApoE4 is less efficient than the other apoE isoforms in recycling of membrane lipids and neuronal repair (Poirier 1994). Several other studies also indicate apoE isoforms function differently in brain cholesterol metabolism (Michikawa et al. 2000;Gong et al. 2002;Rapp et al. 2006), potentially due to the structural differences among apoE isoforms that determine their lipid- and receptor-binding properties. ApoE has been implicated as a chaperone that modulates Aβ aggregation and deposition or clearance (Verghese et al. 2011). Expression of human Apo E2, E3 and E4 in mice leads to isoform-specific differences in amyloid load, with E4 > E3 > E2 (Holtzman et al. 2000). Further, the clearance of soluble Aβ in the brain interstitial fluid (ISF) has been shown to depend on the isoform of human apoE expressed (E4 < E3 ≤ E2) (Castellano et al. 2011). However, a recent study demonstrates that there is little direct interaction between soluble Aβ and apoE in the extracellular fluids and proposes that apoE isoforms affect Aβ metabolism by interfering with the interactions between Aβ and its receptors/transporters (Verghese et al. 2013). Thus, the puzzle how apoE4 increases the risk of AD has not been solved.
ApoE not only affects Aβ but Aβ acts on apoE regulation. Aβ increases cellular apoE protein abundance (Hu et al. 1998;Igbavboa et al. 2003;Igbavboa et al. 2006;LaDu et al. 2000;LaDu et al. 2001). The mechanism for that increase was not established until recently. Aβ42 stimulated apoE mRNA and protein expression in astrocytes (Rossello et al. 2012). In the same study it was reported that upregulation of apoE by Aβ involved the β2 -adrenergic receptor (β2AR) and the transcription factor AP-2β. Aβ directly binds to the β2AR (Wang et al. 2010). Cells expressing the AP-2 transcription factor showed Aβ42-induced activation of a co-expressed luciferase reporter gene construct under the control of an apoE promoter fragment containing AP-2 binding sites in contrast to cells not expressing AP-2 (Rossello et al. 2012).
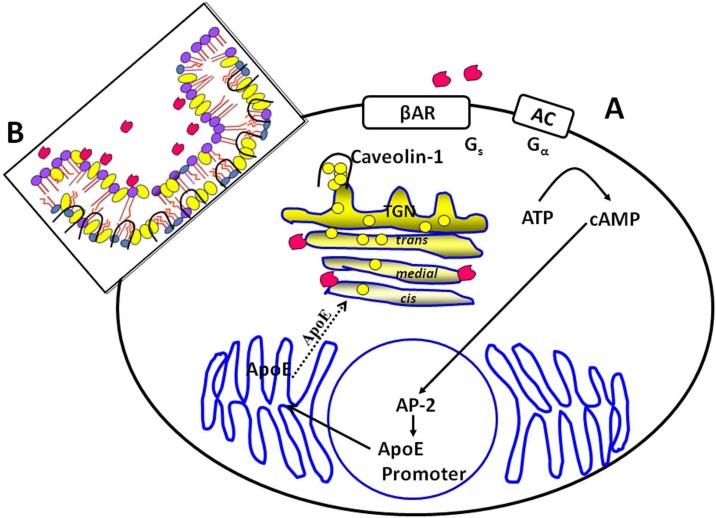
The functional significance of the Aβ-stimulated increase in apoE and retrograde transport of cholesterol by caveolin-1 is not known. A model is proposed which is summarized in Figure 3. Aβ increases apoE synthesis via the β2AR. Newly synthesized apoE in astrocytes migrates through the Golgi complex (Dekroon and Armati 2001). A functional effect of this increase in apoE abundance is that more apoE containing cholesterol is removed from the cis region and transported to the trans region of the Golgi as illustrated in Figure 3, panel A. We know that Aβ reduces cholesterol in the cis region and increases it in the trans region (Igbavboa et al. 2003). Consequences of this increase in cholesterol in the trans region could have a negative impact on Golgi mediated protein and lipid trafficking. Cholesterol loading of the Golgi impedes vesicular transport from the trans Golgi network (Ying et al. 2003). A portion of the increased cholesterol abundance in the trans region is accounted for by Aβ stimulating caveolin-1 transport of plasma membrane cholesterol (Fig. 3, panel B) (Igbavboa et al. 2009). Aβ disruption of caveolae may alter normal function of this membrane structure which is thought to be involved in signaling, lipid storage, endocytosis and remodeling of the extracellular environment (Parton and del Pozo 2013). We are proposing that Aβ stimulation of apoE synthesis is harmful to cell function. However, both apoE and β-adrenergic receptors have been reported to facilitate neuroprotection afforded by astrocytes (Junker et al. 2002;Laureys et al. 2010;Rebeck et al. 2002). ApoE mimetics are neuroprotective in models of AD and neuronal injury (Christensen et al. 2011;Li et al. 2010;Wang et al. 2007). β-adrenergic receptor agonists increase apoE protein expression (Cedazo-Mínguez et al. 2001;Igbavboa et al. 2006). One possibility is that the Aβ stimulation of apoE synthesis in astrocytes may have pathological consequences on Golgi structure and function but have protective effects when secreted by astrocytes and taken up by neuronal receptors of the low density lipoprotein receptor family.
Figure 3. Model of Aβ perturbation of cholesterol homeostasis in astrocytes.
Panel A shows amyloid β-protein (Aβ) (red structures) induces stimulation of apoE synthesis via the β2 -adrenergic receptor (βAR), cAMP and the transcription factor AP-2 (Igbavboa et al. 2003;Igbavboa et al. 2006;Rossello et al. 2012). Newly synthesized apoE is proposed to move to the Golgi and translocate cholesterol from the cis to the trans region. Panel B is a model of Aβ acting on caveolae resulting in translocation of caveolin-1 complexed with cholesterol to the trans-Golgi region depicted in panel A (Igbavboa et al. 2009;Igbavboa et al. 2003). Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), sphingomyelin (SM); 
Summary
When Michael Brown and Joseph Goldstein were awarded the Nobel Prize in Physiology or Medicine for their work on regulation of cholesterol they said in their Nobel lecture that "cholesterol has exerted an almost hypnotic fascination for scientists from the most diverse areas of science and medicine" (Brown and Goldstein 1985). That statement resonates with those of us studying cholesterol in the central nervous system and those who have focused on cholesterol in AD. Cholesterol dysregulation has been proposed as a factor in several divergent diseases such as suicide and depression (Zhang 2011), inborn errors of cholesterol metabolism (Herman and Kratz 2012), Huntington disease (Karasinska and Hayden 2011), and Parkinson disease (de Lau et al. 2006). The pioneering studies of Larry Sparks and his colleagues on cholesterol and AD initiated a field of research that continues today. An intriguing characteristic of the cholesterol-AD interaction is that several different properties/functions of cholesterol are associated with AD and in particular Aβ, which raises several major questions: 1) is cholesterol a causative factor in AD; 2) the incongruity of findings between human and animal/cell culture studies; 3) are other lipids in the mevalonate pathway linked to AD; and 4) does AD impact cholesterol homeostasis? The purpose of this review was to examine those questions.
Table 1 summarizes the pros and cons for the hypothesis that bulk changes in cholesterol levels are either causative factors or biomarkers of AD. That hypothesis is not supported by the data. There is large variability in the human data on cholesterol levels whether in serum, plasma and brain which argues against cholesterol as a causative factor in AD. An alternative hypothesis might be that brain cholesterol domains versus total or bulk cholesterol levels may be contributing factors in AD. To date, there is little support for that hypothesis in human brain tissue of AD patients, and studies on cholesterol domains in human brain are needed. Cholesterol is a member of the large mevalonate family, and two members of that family, FPP and GGPP, through their involvement in protein prenylation have been proposed to play roles in AD. Data on FPP and GGPP levels in human AD brain samples were reported in one study, which found that both lipids were significantly elevated as compared with control subjects. More studies are needed to confirm those results. Another product of the mevalonate pathway is the oxysterol 24S-OHC which some have proposed as a marker of CNS metabolism in AD patients. A lack of consistency however in studies on plasma and CSF levels of 24S-OHC in AD patients calls in to question the importance of that lipid in AD. Equally important is that changes in 24S-OHC have been reported in samples of patients with other neurodegenerative diseases suggesting that changes in 24S-OHC may be a non-specific marker of neuronal dysfunction and/or cell death. That interpretation may also apply to FPP and GGPP.
Table 1.
Summary of Pros and Cons of the Hypothesis that Cholesterol is a Causative Factor in Alzheimer Disease
| ENDPOINT | PROS | CONS |
|---|---|---|
| Serum/plasma cholesterol levels |
Some studies report ↑in AD pts compared with controls. |
Some studies report no differences or ↓ In studies reporting ↑, small differences between groups. |
| Brain/CSF cholesterol levels |
Some studies report ↑in AD pts compared with controls. |
Some studies report no differences or ↓in AD pts. |
| Brain FPP and GGPP levels |
FPP and GGPP levels ↑ in AD pts compared with controls. |
First study on FPP and GGPP levels in AD patients and needs to be replicated. Address question if changes are common to other neurodegenerative diseases. |
| Serum/CSF 24S-OHC levels |
Some studies report ↑ in AD pts compared with controls. |
Some studies report no differences, or ↓in AD pts. In studies reporting ↑,small differences between groups. Changes observed in other diseases. |
| Statins reduce AD | Retrospective epidemiological studies |
Majority of prospective studies do not support statin efficacy in AD. |
| Animal/cell culture studies |
Reducing cholesterol decreases Aβ levels, opposite effects when cholesterol is increased. |
Changing cholesterol levels affects multiple proteins besides APP/Aβ. |
Though the human data pose problems for the cholesterol-AD hypothesis, the animal and cell culture studies provide the strongest support. However, interpretation of those studies must be viewed within the context of the numerous effects of cholesterol on several different proteins besides APP and Aβ. Specific mechanisms have not been identified pertaining to how manipulation of cholesterol levels modifies APP and Aβ. Lipid rafts have been proposed as a potential mechanism but a problem with that interpretation is that multiple proteins have been identified in membrane lipid raft fractions and methods to perturb lipid rafts have non-specific effects. Aβ directly interacts with cholesterol and what remains to be established is whether such an interaction is a factor in the development of AD or a consequence of the disease. With respect to consequences, much of the support for Aβ effects on cholesterol structure and function is from in vitro studies in different cell types including primary neurons and astrocytes. It remains to be determined and a challenge to establish if brain cholesterol structure and functions such as membrane asymmetry, cholesterol trafficking, and sterol binding to proteins are altered in AD patients. The role of cholesterol may not be a causative factor in AD but instead a casualty of the disease, which may be a syndrome common to certain neurodegenerative diseases.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health, AG-23524, AG-18357, AG-31846, Hanna-Bragard-Apfel Foundation and the Alzheimer Forschung Initiative. The authors have no conflict of interest to declare.
Reference List
- Avdulov NA, Chochina SV, Igbavboa U, Vassiliev AV, Wood WG. Lipid binding to amyloid β-peptide: Preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J. Neurochem. 1997;69:1746–1752. doi: 10.1046/j.1471-4159.1997.69041746.x. [DOI] [PubMed] [Google Scholar]
- Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, Beel AJ, Sanders CR. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni V, Barrantes FJ. Cholesterol modulates the rate and mechanism of acetylcholine receptor internalization. J. Biol. Chem. 2011;286:17122–17132. doi: 10.1074/jbc.M110.211870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Nobel Lectures. 1985:284–324. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Burns MP, Igbavboa U, Wood WG, Duff K. Cholesterol distribution, not total levels, correlate with altered amyloid precursor protein processing in statin-treated mice. NeuroMol. Med. 2006;8:319–328. doi: 10.1385/nmm:8:3:319. [DOI] [PubMed] [Google Scholar]
- Burns MP, Rebeck GW. Intracellular cholesterol homeostasis and amyloid precursor protein processing. Biochim Biophys Acta. 2010;1801:853–859. doi: 10.1016/j.bbalip.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Science Trans Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Mínguez A, Hamker U, Meske V, Veh RW, Hellweg R, Jacobi C, Albert F, Cowburn RF, Ohm TG. Regulation of apolipoprotein E secretion in rat primary hippocampal astrocyte cultures. Neuroscience. 2001;105:651–661. doi: 10.1016/s0306-4522(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Christensen DJ, Ohkubo N, Oddo J, Van Kanegan MJ, Neil JE, Li FQ, Colton CA, Vitek MP. Apolipoprotein E and peptide mimetics mimetics modulate inflammation by binding the SET protein and activating protein phosphatase 2A. J Immunol. 2011;186:2535–2542. doi: 10.4049/jimmunol.1002847. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. Isoprenoids and Alzheimer's disease: a complex relationship. Neurobiology of Disease. 2006;22:209–222. doi: 10.1016/j.nbd.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Corbin J, Wang HH, Blanton MP. Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor with [125I]azido-cholesterol. Biochim Biophys Acta. 1998;1414:65–74. doi: 10.1016/s0005-2736(98)00153-9. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Haines JL, Pericak Vance M. A. Protective effects of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. National Institues of Health State-of-the-Science Conference Statement: Preventing Alzheimer's Disease and Cognitive Decline. NIH Consens State Sci Statements. 2010;27:1–30. [PubMed] [Google Scholar]
- de Lau ML, Koudstaal PJ, Hofman A, Breteler MMB. Serum cholesterol levels and the risk of Parkinson's disease. Am. J. Epidemiol. 2006;164:998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- Dekroon RM, Armati PJ. Synthesis and processing of apolipoprotein E in human brain cultures. Glia. 2001;33:298–305. doi: 10.1002/1098-1136(20010315)33:4<298::aid-glia1028>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Désiré L, Bourdin J, Loiseau N, Peillon H, Picard V, De Oliveria C, Bachelot F, Leblond B, Taverne T, Beausoleil E, Lacombe S, Drouin D, Schweighoffer F. Rac1 inhibition targets amyloid precursor protein processing by gamma-secretase and decreases abeta production in vitro and in vivo. J. Biol. Chem. 2005;280:37516–37525. doi: 10.1074/jbc.M507913200. [DOI] [PubMed] [Google Scholar]
- Di Scala C, Yahi N, Lelièvre C, Garmy N, Chahinian H, Fantini J. Biochemical identification of a linear cholesterol-binding domain within Alzheimer's β amyloid peptide. ACS Chem Neurosci. 2013;20:509–517. doi: 10.1021/cn300203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nature Cancer Reviews. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Eckert GP, Cairns NJ, Maras A, Gattaz WF, Müller WE. Cholesterol modulates the membrane disordering effects of β-amyloid peptides in the hippocampus: Specific changes in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2000;11:181–186. doi: 10.1159/000017234. [DOI] [PubMed] [Google Scholar]
- Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Müller WE, Wood WG. Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiology of Disease. 2009;35:251–257. doi: 10.1016/j.nbd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Reik C, Muller WE. Simvastatin alters membrane cholesterol distribution and beta-amyloid levels in brains of APP751SL mice. Die Pharmazie. 2013;68:590–594. [PubMed] [Google Scholar]
- Eckert GP, Wood WG, Müller WE. Lipid membranes and Beta-Amyloid: A Harmful Connection. Current Protein & Peptide Science. 2010;11:319–325. doi: 10.2174/138920310791330668. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Banks WA. Blood-brain barrier dysfunction as cause and consequence of Alzheimer's disease. J. Cereb. Blood Flow Metab. 2013:1–14. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman BT, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Gamba P, Testa G, Sottero B, Gargiulo S, Poli G, Leonarduzzi G. The link between altered cholesterol metabolism and Alzheimer's disease. Ann NY Acad Sci. 2012;1259:54–64. doi: 10.1111/j.1749-6632.2012.06513.x. [DOI] [PubMed] [Google Scholar]
- Gärtner U, Holzer M, Arendt T. Elevated expression of p21ras is an early event in Alzheimer's disease and precedes neurofibrillary degeneration. Neuroscience. 1999;91:1–5. doi: 10.1016/s0306-4522(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Gärtner U, Holzer M, Heumann R, Arendt T. Induction of p21ras in Alzheimer pathology. Neuroreport. 1995;10:1441–1444. doi: 10.1097/00001756-199507100-00020. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Wood D, Turner MB. Heart Disease and Stroke Statistics-2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human apoE3 and apoE4 knock-in mice. J. Biol. Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- Halford RW, Russel DW. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer's disease, but does extend lifespan. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3502–3506. doi: 10.1073/pnas.0813349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola V-P, Chien EYT, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human β2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Igbavboa U, Hamanaka H, Kobayashi M, Fujita SC, Wood WG, Yanagisawa K. Cholesterol is increased in the exofacial leaflet of synaptic plasma membranes of human apolipoprotein E4 knock-in mice. Neuroreport. 2002;13:383–386. doi: 10.1097/00001756-200203250-00004. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskelton: Impact on signaling and function: Membrane /lipid rafts, mediators of cytoskeletal arrangement and cell signaling. 2013 doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman GE, Kratz L. Disorders of sterol synthesis: beyond Smith-Lemli-Opitz syndrome. Am J Med Genet C Semin Med Genet. 2012;160:301–321. doi: 10.1002/ajmg.c.31340. [DOI] [PubMed] [Google Scholar]
- Heverin M, Bogdanovic N, Lutjohann D, Bayer TA, Pikuleva I, Bretillon L, Diczfalusy U, Winblad B, Björkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J. Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Wellington CL. Cholesterol metabolism, apolipoprotein E, adenosine triphosphate-binding cassette transporters, and Alzheimer's disease. Curr. Opin. Lipidol. 2007;18:325–332. doi: 10.1097/MOL.0b013e32813aeabf. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;3:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooff GP, Wood WG, Hyun KJ, Igbavboa U, Ong WY, Müller WE, Eckert GP. Brain isoprenoids farnesyl pyrophosphate and geranylgeranyl pyrophosphate are increased in aged mice. Mol Neurobiol. 2012;46:179–185. doi: 10.1007/s12035-012-8285-6. [DOI] [PubMed] [Google Scholar]
- Hooff GP, Wood WG, Müller WE, Eckert GP. Isopernoids, small GTPases and Alzheimer's disease. Biochim Biophys Acta. 2010;1801:896–905. doi: 10.1016/j.bbalip.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, LaDu MJ, Van Eldik LJ. Apolipoprotein E attenuates beta-amyloid-induced astrocyte activation. J. Neurochem. 1998;71:1626–1634. doi: 10.1046/j.1471-4159.1998.71041626.x. [DOI] [PubMed] [Google Scholar]
- Huesa G, Baltrons MA, Gómez-Ramos P, Morán A, García A, Hidalgo J, Francés S, Santpere G, Galea E. Altered distribution of RhoA in Alzheimer's disease and abetaPP overexpressing mice. J Alzheimers Dis. 2010;19:37–56. doi: 10.3233/JAD-2010-1203. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Avdulov NA, Chochina SV, Wood WG. Transbilayer distribution of cholesterol is modified in brain synaptic plasma membranes of knockout mice deficient in the low density lipoprotein receptor, apolipoprotein E, or both proteins. J. Neurochem. 1997;69:1661–1667. doi: 10.1046/j.1471-4159.1997.69041661.x. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Avdulov NA, Schroeder F, Wood WG. Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J. Neurochem. 1996;66:1717–1725. doi: 10.1046/j.1471-4159.1996.66041717.x. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Eckert GP, Malo TM, Studniski A, Johnson LNA, Yamamoto N, Kobayashi M, Fujita SC, Appel TR, Müller WE, Wood WG, Yanagisawa K. Murine synaptosomal lipid raft protein and lipid composition are altered by expression of human apoE3 and 4 and by increasing age. J. Neurol. Sci. 2005;229-230:225–232. doi: 10.1016/j.jns.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Johnson-Anuna LN, Rossello X, Butterick TA, Sun GY, Wood WG. Amyloid beta-protein 1-42 increases cAMP and apolipoprotein E levels which are inhibited by β1 and β2-adrenergic receptor antagonists in mouse primary astrocytes. Neuroscience. 2006;142:655–660. doi: 10.1016/j.neuroscience.2006.06.056. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Pidcock JM, Johnson LNA, Malo TM, Studniski A, Sun GY, Wood WG. Cholesterol distribution in the Golgi complex of DITNC1 astrocytes is differentially altered by fresh and aged amyloid beta-peptide1-42. J. Biol. Chem. 2003;278:17150–17157. doi: 10.1074/jbc.M301150200. [DOI] [PubMed] [Google Scholar]
- Igbavboa U, Sun GY, Weisman GA, Wood WG. Amyloid β-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer's disease: A case-control study. Neurology. 1995;45:1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- Junker V, Becker A, Hühne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of β-adrenoceptors activates astrocytes and provides neuroprotection. European Journal of Pharmacology. 2002;446:25–36. doi: 10.1016/s0014-2999(02)01814-9. [DOI] [PubMed] [Google Scholar]
- Kabara JJ. A critical review of brain cholesterol metabolism. Prog. Brain Res. 1973;40:363–382. doi: 10.1016/S0079-6123(08)60700-1. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, Hayden MR. Cholesterol metabolism in Huntington disease. Nat Rev Neurol. 2011;7:561–572. doi: 10.1038/nrneurol.2011.132. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch C, Eckert GP, Koudinov AR, Müller WE. Brain cholesterol, statins and Alzheimer's Disease. Pharmacopsychiatry. 2003a;36:S113–S119. doi: 10.1055/s-2003-43058. [DOI] [PubMed] [Google Scholar]
- Kirsch C, Eckert GP, Müller WE. Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem. Pharmacol. 2003b;65:843–856. doi: 10.1016/s0006-2952(02)01654-4. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissien A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittweis JW, McMullen WA. The effect of apoE on dementia is not through atherosclerosis: The Rotterdam Study: To the Editor. Neurology. 2000;54:2356–2358. doi: 10.1212/wnl.54.12.2356-a. [DOI] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti TJ, Head DD, McKenna CE, Russell DW. Biphasic requirement for geranylgeraniol in hippocampal long-term potentiation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11394–11399. doi: 10.1073/pnas.0805556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti TJ, Ramirez DM, Pfeiffer BE, Huber KM, Russell DW. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3869–3874. doi: 10.1073/pnas.0600316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudinov AR, Koudinova NV, Berezov TT. Alzheimer's peptides Aβ1-40 and Aβ1-28 inhibit the plasma cholesterol esterification rate. Biochem Mol Biol Int. 1996;38:747–752. [PubMed] [Google Scholar]
- Koudinova NV, Berezov TT, Koudinov AR. Multiple inhibitory effects of Alzheimer's peptide Aβ1-40 on lipid biosynthesis in cultured human HepG2 cells. FEBS Lett. 1996;395:204–206. doi: 10.1016/0014-5793(96)01042-3. [DOI] [PubMed] [Google Scholar]
- Kuo Y-M, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, Roher AE. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain Aβ 1-42 levels. Biochem. Biophys. Res. Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E receptors mediate the effects of β-amyloid on astrocyte cultures. J. Biol. Chem. 2000;275:33974–33980. doi: 10.1074/jbc.M000602200. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate Aβ-induced glial neuroinflammatory responses. Neurochem. Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, Kooijman R, Smolders I, Michhhhotte Y, De Keyser J. Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog Neurobiol. 2010;91:189–199. doi: 10.1016/j.pneurobio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Lazar AN, Bich C, Panchal M, Desbenoit N, Petit VW, Touboul D, Dauphinot L, Marquer C, Laprévote O, Brunelle A, Duyckaerts C. Acta Neuropathol. 2013;125:133–144. doi: 10.1007/s00401-012-1041-1. [DOI] [PubMed] [Google Scholar]
- Lee AG. Biological membranes: the importance of molecular detail. Trends in Biochemical Sciences. 2011;36:493–500. doi: 10.1016/j.tibs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Leoni V, Long JD, Mills JA, Di Donato S, Paulsen JS, the members of the PREDICT-HD study group Plasma 24S-hydroxycholesterol correlation with markers of Huntington disease progression. Neurobiology of Disease. 2013;55:37–43. doi: 10.1016/j.nbd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FQ, Fowler KA, Neil JE, Colton CA, Vitek MP. An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J Pharmacol Exp Ther. 2010;334:106–115. doi: 10.1124/jpet.110.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Shofer JB, Kukull WA, Peskind ER, Tsuang DW, Breitner JC, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. 2005;65:1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang W, Cheng S, Cao D. Isoprenoids and related pharmacological interventions: Potential application in Alzheimer's disease. Mol Neurobiol. 2012;46:64–77. doi: 10.1007/s12035-012-8253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2004;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori N, Nerenberg PS, Head Gordon T. Embedding Aβ42 in heterogeneous membranes depends on cholesterol asymmetries. Biophys J. 2013;105:899–910. doi: 10.1016/j.bpj.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Frontier Bioscience. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Schubert D. Amyloid β peptide alters intracellular vesicle trafficking and cholesterol homeostasis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13266–13271. doi: 10.1073/pnas.95.22.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- Lütjohann D, von Bergmann K. 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry. 2003;36:S102–S106. doi: 10.1055/s-2003-43053. [DOI] [PubMed] [Google Scholar]
- Ma Q-L, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM. p21-activated Kinase-aberrant Activation and Translocation in Alzheimer Disease Pathogenesis. Journal of Biological Chemistry. 2008;283:14132–14143. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mans RA, McMahon LL, Li L. Simvastatin-mediated enhancement of lonf-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience. 2012;202:1–9. doi: 10.1016/j.neuroscience.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V, Fabelo N, Santpere G, Puig B, Marin R, Ferrer I, Díaz M. Lipid alterations in lipid rafts from Alzheimer's disease human brain cortex. J Alzheimers Dis. 2010;19:489–502. doi: 10.3233/JAD-2010-1242. [DOI] [PubMed] [Google Scholar]
- Mason RP, Estermyer JD, Kelly JF, Mason PE. Alzheimer's disease amyloid beta peptide 25-35 is localized in the membrane hydrocarbon core: X-ray diffraction analysis. Biochem. Biophys. Res. Commun. 1996;222:78–82. doi: 10.1006/bbrc.1996.0699. [DOI] [PubMed] [Google Scholar]
- Mason RP, Shoemaker WJ, Shajenko L, Chambers TE, Herbette LG. Evidence for changes in the Alzheimer's disease brain cortical membrane structure mediated by cholesterol. Neurobiol. Aging. 1992;13:413–419. doi: 10.1016/0197-4580(92)90116-f. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Togawa M, Hirabaru K, Mochinaga S, Narita A, Adachi M, Egashira M, Irie T, Ohno K. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genetics Metabolisml. 2013;108:76–81. doi: 10.1016/j.ymgme.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Maulik M, Westaway D, Jhamandas JH, Kar S. Role of cholesterol in APP metabolism and its significance in Alzheimer's disease pathogenesis. Mol Neurobiol. 2013;47:37–63. doi: 10.1007/s12035-012-8337-y. [DOI] [PubMed] [Google Scholar]
- McGuinness B, O'Hare J, Craig D, Bullock R, Malouf R, Passmore P. Cochrane review on 'Statins for the treatmentof dementia'. Int J Geriatr Psychiatry. 2013;28:119–126. doi: 10.1002/gps.3797. [DOI] [PubMed] [Google Scholar]
- McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez AJ. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells: Implications for intracellular cholesterol transport. J. Biol. Chem. 1995;270:5891–5900. doi: 10.1074/jbc.270.11.5891. [DOI] [PubMed] [Google Scholar]
- Mendoza-Naranjo A, Gonzalez-Billault C, Maccioni RB. Aβ1-42 stimulates actin polymerization in hippocampal neurons through Rac1 and CDC42 Rho GTPases. Journal of Cell Science. 2007;120:279–288. doi: 10.1242/jcs.03323. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Fan Q-W, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- Michikawa M, Gong J-S, Fan Q-W, Sawamura N, Yanagisawa K. A novel action of Alzheimer's amyloid β-protein (Aβ): Oligomeric Aβ promotes lipid release. J. Neurosci. 2001;18:7226–7235. doi: 10.1523/JNEUROSCI.21-18-07226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo H, Yeganehjoo H, Shah A, Mo WK, Soelaiman IN, Shen C-L. Mevalonate-suppressive dietary isoprenoids for bone health. Journal of Nutritional Biochemistry. 2012;23:1543–1551. doi: 10.1016/j.jnutbio.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Mohamed A, Saavedra L, Di Pardo A, Sipione S, Posse de Chaves E. β-amyloid inhibits protein prenylation and induces cholesterol sequestration by impairing SREBP-2 cleavage. J. Neurosci. 2012;32:6490–6500. doi: 10.1523/JNEUROSCI.0630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AM, Ferreira A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calplain activation and tau toxicity. J. Neurosci. 2009;29:4640–4651. doi: 10.1523/JNEUROSCI.0862-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. Niemann-Pick Type C disease and Alzheimer's disease: the APP-endosome connection fattens up. Am J Pathol. 2004;164:757–761. doi: 10.1016/S0002-9440(10)63163-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North P, Fleischer S. Alteration of synaptic membrane cholesterol/phospholipid ratio using a lipid transfer protein. J. Biol. Chem. 1983;258(2):1242–1253. [PubMed] [Google Scholar]
- Oh D, Han S, Seo J, Choi J, Groffen J, Kim K, Cho YS, Choi H-S, Shin H, Woo J, Won H, Park SK, Kim S-Y, Jo J, Whitcomb DJ, Cho K, Kim H, Bae YC, Heisterkamp N, Choi S-Y, Kim E. Regulation of synaptic Rac1 activity, long-term potentiation maintenance, and learning and memory by BCR and ABR Rac GTPase-activating proteins. J. Neurosci. 2010;30:14134–14144. doi: 10.1523/JNEUROSCI.1711-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Tanaka K, Dawe GS, Ittner LM, Farooqui AA. Slow excitotoxicity in Alzheimer's disease. J Alzheimers Dis. 2013;35:643–668. doi: 10.3233/JAD-121990. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, Ptok U, Bjorkhem I, von Bergmann K, Heun R. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. Journal of Psychiatric Research. 2002;36:27–32. doi: 10.1016/s0022-3956(01)00050-4. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Bryant-Thomas T, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, Girones X, Henry TL, Matsubara E, Zambon D, Wolozin B, Sano M, Cruz-Sanchez FF, Thal LJ, Petanceska S, Refolo LM. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membranes sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Passarelli MK, Winograd N. Lipid imaging with time-of-flight secondary ion mass spectrometry (ToF-SIMS) Biochim Biophys Acta. 2013;1811:976–990. doi: 10.1016/j.bbalip.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot N, Tyteca D, D'auria L, Dewachter I, Gailly P, Hendrickx A, Tasiaux B, Haylani LE, Muls N, N'Kuli F, Laquerriere A, Demoulin J-B, Campion D, Brion J-P, Courtoy PJ, Kienlen-Campard P, Octave J-N. Amyloid precursor protein controls cholesterol turnover needed for neuronal activity. EMBO Mol Med. 2013;5:608–625. doi: 10.1002/emmm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J. Neurosci. 2004;19:3151–3164. doi: 10.1111/j.0953-816X.2004.03380.x. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17:525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Popp J, Lewczuk P, Kolsch H, Meichsner S, Kornhuber J, Jessen F, Lütjohann D. Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer's disease. J. Neurochem. 2012;123:310–316. doi: 10.1111/j.1471-4159.2012.07893.x. [DOI] [PubMed] [Google Scholar]
- Popp J, Meichsner S, Kölsch H, Lewczuk P, Maier W, Kornhuber J, Jessen F, Lütjohann D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Biochem. Pharmacol. 2013;86:37–42. doi: 10.1016/j.bcp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Posse de Chaves EI. Reciprocal regulation of cholesterol and beta amyloid at the subcellular level in Alzheimer's disease. Can J Physiol Pharmacol. 2012;90:753–764. doi: 10.1139/y2012-076. [DOI] [PubMed] [Google Scholar]
- Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Ramirez DMO, Andersson S, Russel DW. Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J Comp Neurol. 2008;507:1676–1693. doi: 10.1002/cne.21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp A, Gmeiner B, Huttinger M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie. 2006;88:473–483. doi: 10.1016/j.biochi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Kindy M, LaDu MJ. Apolipoprotein E and Alzheimer's disease: The protective effects of ApoE2 and E3. J Alzheimers Dis. 2002;4:145–154. doi: 10.3233/jad-2002-4304. [DOI] [PubMed] [Google Scholar]
- Reddy S, Comai L. Lamin A, farnesylation and aging. Exp Cell Res. 2012;318:1–7. doi: 10.1016/j.yexcr.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas SN, Tang MX, Berglund L, Mayeux R. ApoE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Protein prenylation: a pivotal posttranslational process. Biochem. Biophys. Res. Commun. 2003;303:1–7. doi: 10.1016/s0006-291x(03)00323-1. [DOI] [PubMed] [Google Scholar]
- Rossello XS, Igbavboa U, Weisman GA, Sun GY, Wood WG. AP-2β regulates amyloid beta-protein stimulation of apolipprotein E transcription in astrocytes. Brain Res. 2012;1444:87–95. doi: 10.1016/j.brainres.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder F, Gallegos AM, Atshaves BP, Storey SM, McIntosh AL, Petrescu AD, Huang H, Starodub O, Chao H, Yang H, Frolov A, Kier AB. Recent advances in membrane microdomains: Rafts, caveolae, and intracellular cholesterol trafficking. Exp. Biol. Med. 2001;226:873–890. doi: 10.1177/153537020122601002. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Huang H, McIntosh AL, Atshaves BP, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Subcellular Biochemisrty. 2010;51:279–318. doi: 10.1007/978-90-481-8622-8_10. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Kojima N, Shirao T. Role of actin cytoskelton in dendritic spine morphogenesis. Neurchem. Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 2009a;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Leoni V, Kivipelto M, Besga A, Oksengard AR, Julin P, Svensson L, Wahlund L-O, Andreasen N, Winblad B, Soininen H, Björkhem I. Plasma levels of 24S-hydroxycholesterol reflect brain volumes in patients without objective cognitive impairment but not in those with Alzheimer's disease. Neurosci. Lett. 2009b;462:89–93. doi: 10.1016/j.neulet.2009.06.073. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A. Membrane domains and the "lipid raft" concept. Curr Med Chem. 2013;20:4–21. [PubMed] [Google Scholar]
- Sparks DL. Coronary artery disease, hypertension, apoE, and cholesterol: A link to Alzheimer's disease? Ann NY Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JC, III, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer's disease. Neurobiol. Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Hunsaker JC, III, Liu H, Landers T, Gross DR. Induction of Alzheimer-like β-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- Stewart R, White LR, Xue Q-L, Launer LJ. Twenty-six-year chnage in total cholesterol levels and incident dementia. Arch Neurol. 2007;64:103–107. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer's disease. Annu. Rev. Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- Tan ZS, Seshadri S, Beiser A, Wilson PWF, Kiel DP, Tocco M, D'Agostino RB, Wolf PA. Plasma total cholesterol level as a risk factor for Alzheimer disease. Arch Int Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kraats C, Killestein J, Popescu V, Rijkers E, Vrenken H, Lütjohann D, Barkhof F, Polman C, Teunissen C. Oxysterols and cholesterol precursors correlate to magnetic resonance imaging measures of neurodegeneration in multiple sclerosis. Mult Scler. 2013 doi: 10.1177/1352458513499421. in press. [DOI] [PubMed] [Google Scholar]
- Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Sem Cell Dev. Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1807–1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Govindaiah G, Liu R, De Arcangelis V, Cox CL, Xiang YK. Binding of amyloid beta peptide to beta2 adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity. FASEB J. 2010;24:3511–3521. doi: 10.1096/fj.10-156661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Durham L, Dawson H, Song P, Warner DS, Sullivan PM, Vitek MP, Laskowitz DT. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer's disease pathology following closed head injury: Evidence of pharmacogenomic interaction. Neuroscience. 2007;144:1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wang P-L, Niidome T, Akaike A, Kihara T, Sugimoto H. Rac1 inhibition negatively regulates transcriptional activity of the amyloid precursor protein gene. J. Neurosci. Res. 2009;87:2105–2114. doi: 10.1002/jnr.22039. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Steinman L, Zamvil SS. Drug Insight: using statins to treat neuroinflammatory disease. Nat Clin Pract Neurol. 2005;1:106–112. doi: 10.1038/ncpneuro0047. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston C, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Wiemer AJ, Hohl RJ, Wiemer DF. The intermediate enzymes of isoprenoid metabolism as anticancer targets. Anticancer Agents Med Chem. 2009;9:526–542. doi: 10.2174/187152009788451860. [DOI] [PubMed] [Google Scholar]
- Williamson R, Sutherland C. Neuronal membranes are key to the pathogenesis of Alzheimer's disease: The role of both raft and non-raft membrane domains. Current Alzheimer Research. 2011;8:213–221. doi: 10.2174/156720511795256008. [DOI] [PubMed] [Google Scholar]
- Wolf A, Bauer B, Hartz AMS. ABC transporters and the Alzheimer's disease enigma. Frontiers in Psychiartry. 2012;3:1–14. doi: 10.3389/fpsyt.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG, Eckert GP, Igbavboa U, Müller WE. Amyloid beta-peptide interactions with membranes and cholesterol: Causes or casualties of Alzheimer's disease. Biochim Biophys Acta. 2003;1610:281–290. doi: 10.1016/s0005-2736(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Wood WG, Gorka C, Schroeder F. Acute and chronic effects of ethanol on transbilayer membrane domains. J. Neurochem. 1989;52:1925–1930. doi: 10.1111/j.1471-4159.1989.tb07278.x. [DOI] [PubMed] [Google Scholar]
- Wood WG, Igbavboa U, Eckert GP, Johnson-Anuna LN, Müller WE. Is hypercholesterolemia a risk factor for Alzheimer's disease? Mol Neurobiol. 2005;31:185–192. doi: 10.1385/MN:31:1-3:185. [DOI] [PubMed] [Google Scholar]
- Wood WG, Igbavboa U, Eckert GP, Müller WE. Cholesterol-A Janus-Faced Molecule in the Central Nervous System, in Handbook of Neurochemistry and Molecular Neurobiology-Neural Membranes and Transport, In: lajtha A, Reith MEA, editors. Springer; New York: 2007. pp. 151–170. [Google Scholar]
- Wood WG, Igbavboa U, Müller WE, Eckert GP. Cholesterol asymmetry in synaptic plasma membranes. J. Neurochem. 2011;116:684–689. doi: 10.1111/j.1471-4159.2010.07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WG, Igbavboa U, Rao AM, Schroeder F, Avdulov NA. Cholesterol oxidation reduces Ca2+ + Mg2+-ATPase activity, interdigitation, and increases fluidity of brain synaptic plasma membranes. Brain Res. 1995;683:36–42. doi: 10.1016/0006-8993(95)00347-s. [DOI] [PubMed] [Google Scholar]
- Wood WG, Schroeder F, Igbavboa U, Avdulov NA, Chochina SV. Brain membrane cholesterol domains, aging, and amyloid beta-peptides. Neurobiol. Aging. 2002;23:685–694. doi: 10.1016/s0197-4580(02)00018-0. [DOI] [PubMed] [Google Scholar]
- Yao J, Ho D, Calingasan NY, Pipalia NH, Lin MT, Beal MF. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012;209:2501–2513. doi: 10.1084/jem.20121239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZX, Papadopoulos V. Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity. FASEB J. 2002;16:1677–1679. doi: 10.1096/fj.02-0285fje. [DOI] [PubMed] [Google Scholar]
- Yeagle PL. Cholesterol modulation of (Na++K+)-ATPase ATP hydrolyzing activity in the human erythrocyte. Biochim Biophys Acta. 1983;727:39–44. doi: 10.1016/0005-2736(83)90366-8. [DOI] [PubMed] [Google Scholar]
- Ying M, Grimmer S, Iversen T-G, van Deurs B, Sandvig K. Cholesterol loading induces a block in the exit of VSVG from the TGN. Traffic. 2003;4:772–784. doi: 10.1034/j.1600-0854.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Zhang J. Epidemiological link between low cholesterol and suicidality: a puzzle never finished. Nutr Neurosci. 2011;14:268–287. doi: 10.1179/1476830511Y.0000000021. [DOI] [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 and Alzheimer disease: clues from its structure. J. Biol. Chem. 2009;284:6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]