Abstract
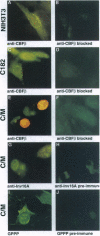
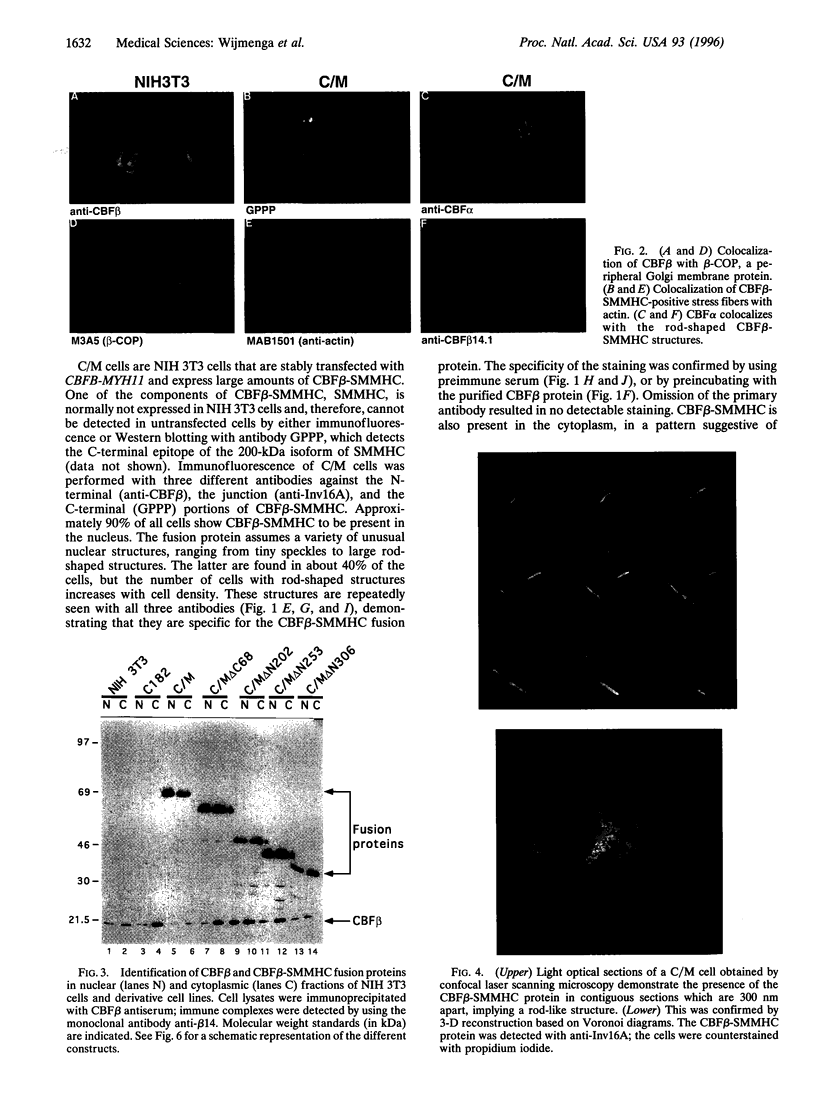
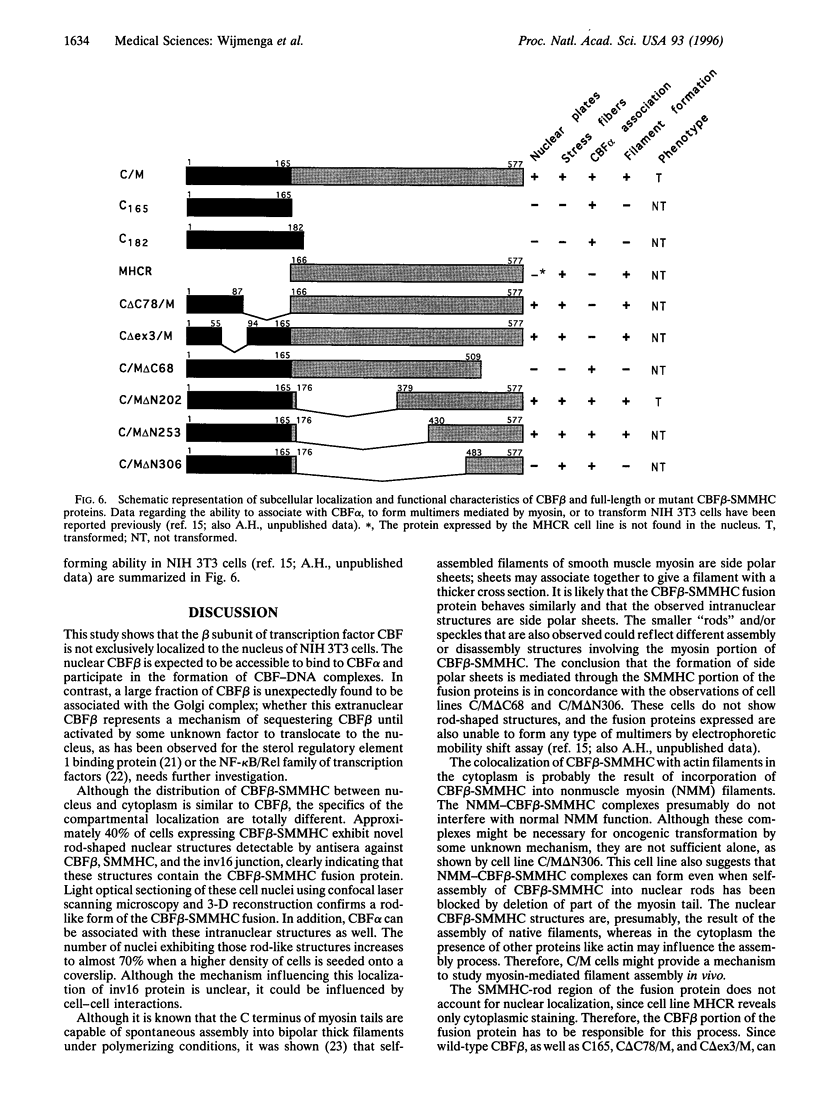
Patients with the M4Eo subtype of acute myeloid leukemia almost invariably are found to have an inversion of chromosome 16 in their leukemic cells, which results in a gene fusion between the transcription factor called core binding factor beta (CBFbeta) on 16q and a smooth muscle myosin heavy chain (SMMHC) gene on 16p. Subcellular localizations of the wild-type CBFbeta and the CBFbeta-SMMHC fusion protein were determined by immunofluorescence of NIH 3T3 cells that overexpress wild-type or fusion protein. Normal CBFbeta showed an unexpected perinuclear pattern consistent with primary localization in the Golgi complex. The CBFbeta-SMMHC fusion protein had a very different pattern. Nuclear staining included rod-like crystalline structures as long as 11 microm. The heterodimeric partner of CBFbeta, CBFalpha, formed part of this complex. Cytoplasmic staining included stress fibers that colocalized with actin, probably as a consequence of the myosin heavy chain component of the fusion protein. Deletion of different regions of the CBFbeta portion of the fusion protein showed that binding to CBFalpha was not required for nuclear translocation. However, deletion of parts of the SMMHC domain of the fusion protein involved in myosin-mediated filament formation resulted in proteins that did not form rod-like structures. These observations confirm previous indirect evidence that the CBFbeta-SMMHC fusion protein is capable of forming macromolecular nuclear aggregates and suggests possible models for the mechanism of leukemic transformation.
Full text
PDF
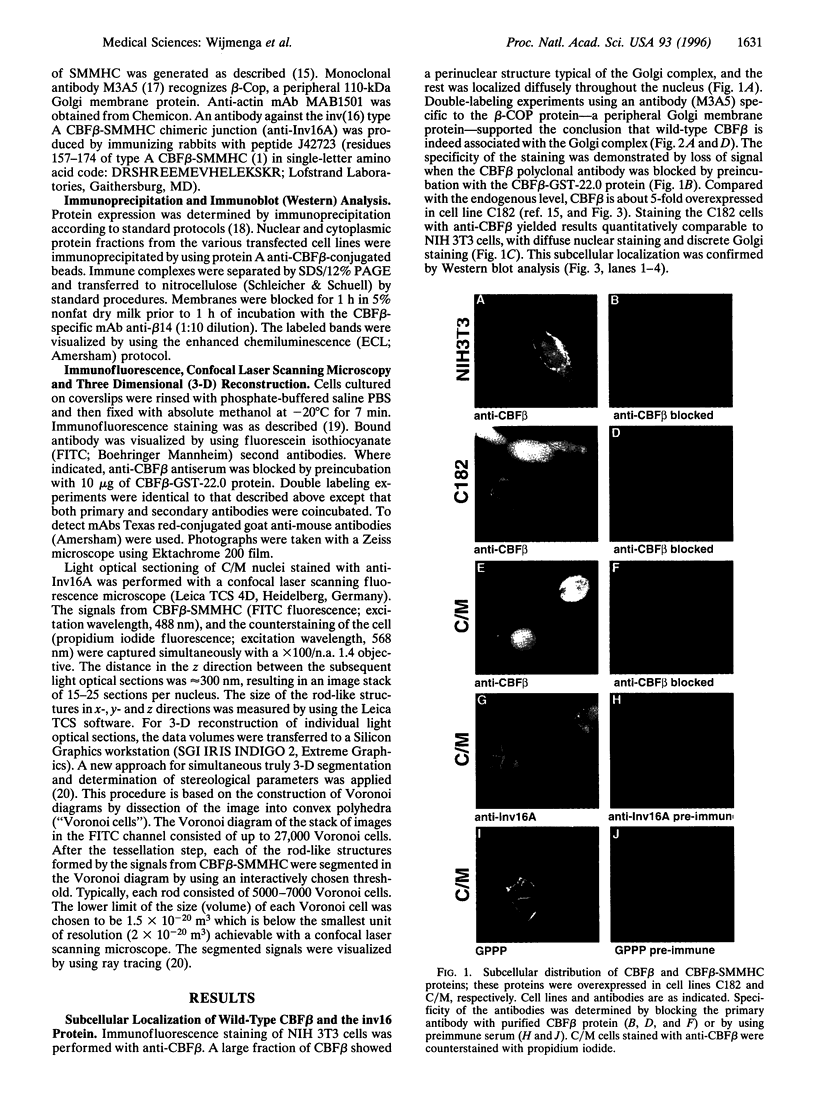


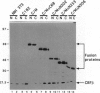
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan V. J., Kreis T. E. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986 Dec;103(6 Pt 1):2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Cameron S., Taylor D. S., TePas E. C., Speck N. A., Mathey-Prevot B. Identification of a critical regulatory site in the human interleukin-3 promoter by in vivo footprinting. Blood. 1994 May 15;83(10):2851–2859. [PubMed] [Google Scholar]
- Cross R. A., Geeves M. A., Kendrick-Jones J. A nucleation--elongation mechanism for the self-assembly of side polar sheets of smooth muscle myosin. EMBO J. 1991 Apr;10(4):747–756. doi: 10.1002/j.1460-2075.1991.tb08006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eils R., Bertin E., Saracoglu K., Rinke B., Schröck E., Parazza F., Usson Y., Robert-Nicoud M., Stelzer E. H., Chassery J. M. Application of confocal laser microscopy and three-dimensional Voronoi diagrams for volume and surface estimates of interphase chromosomes. J Microsc. 1995 Feb;177(Pt 2):150–161. doi: 10.1111/j.1365-2818.1995.tb03545.x. [DOI] [PubMed] [Google Scholar]
- Erickson P., Gao J., Chang K. S., Look T., Whisenant E., Raimondi S., Lasher R., Trujillo J., Rowley J., Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–1831. [PubMed] [Google Scholar]
- Gregory P. E., Gutmann D. H., Mitchell A., Park S., Boguski M., Jacks T., Wood D. L., Jove R., Collins F. S. Neurofibromatosis type 1 gene product (neurofibromin) associates with microtubules. Somat Cell Mol Genet. 1993 May;19(3):265–274. doi: 10.1007/BF01233074. [DOI] [PubMed] [Google Scholar]
- Hajra A., Liu P. P., Wang Q., Kelley C. A., Stacy T., Adelstein R. S., Speck N. A., Collins F. S. The leukemic core binding factor beta-smooth muscle myosin heavy chain (CBF beta-SMMHC) chimeric protein requires both CBF beta and myosin heavy chain domains for transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1926–1930. doi: 10.1073/pnas.92.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hsiang Y. H., Spencer D., Wang S., Speck N. A., Raulet D. H. The role of viral enhancer "core" motif-related sequences in regulating T cell receptor-gamma and -delta gene expression. J Immunol. 1993 May 1;150(9):3905–3916. [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- Levanon D., Negreanu V., Bernstein Y., Bar-Am I., Avivi L., Groner Y. AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics. 1994 Sep 15;23(2):425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Liu P. P., Hajra A., Wijmenga C., Collins F. S. Molecular pathogenesis of the chromosome 16 inversion in the M4Eo subtype of acute myeloid leukemia. Blood. 1995 May 1;85(9):2289–2302. [PubMed] [Google Scholar]
- Liu P., Tarlé S. A., Hajra A., Claxton D. F., Marlton P., Freedman M., Siciliano M. J., Collins F. S. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993 Aug 20;261(5124):1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- Lu J., Maruyama M., Satake M., Bae S. C., Ogawa E., Kagoshima H., Shigesada K., Ito Y. Subcellular localization of the alpha and beta subunits of the acute myeloid leukemia-linked transcription factor PEBP2/CBF. Mol Cell Biol. 1995 Mar;15(3):1651–1661. doi: 10.1128/mcb.15.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchprayoon I., Meyers S., Scott L. M., Suzow J., Hiebert S., Friedman A. D. PEBP2/CBF, the murine homolog of the human myeloid AML1 and PEBP2 beta/CBF beta proto-oncoproteins, regulates the murine myeloperoxidase and neutrophil elastase genes in immature myeloid cells. Mol Cell Biol. 1994 Aug;14(8):5558–5568. doi: 10.1128/mcb.14.8.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993 May;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Maruyama M., Kagoshima H., Inuzuka M., Lu J., Satake M., Shigesada K., Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo J. M., Pfohl J. L., Hernandez-Munain C., Wang S., Speck N. A., Krangel M. S. Indistinguishable nuclear factor binding to functional core sites of the T-cell receptor delta and murine leukemia virus enhancers. Mol Cell Biol. 1992 Nov;12(11):4817–4823. doi: 10.1128/mcb.12.11.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. W., Speck N. A. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992 Jan;12(1):89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang Q., Crute B. E., Melnikova I. N., Keller S. R., Speck N. A. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993 Jun;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Sato R., Brown M. S., Hua X., Goldstein J. L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994 Apr 8;77(1):53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Speck N. A., Dracopoli N. C., Hofker M. H., Liu P., Collins F. S. Identification of a new murine runt domain-containing gene, Cbfa3, and localization of the human homolog, CBFA3, to chromosome 1p35-pter. Genomics. 1995 Apr 10;26(3):611–614. doi: 10.1016/0888-7543(95)80185-o. [DOI] [PubMed] [Google Scholar]