Abstract
Skeletal loading enhances cortical and trabecular bone properties. How long these benefits last following loading cessation remains an unresolved, clinically-relevant question. This study investigated long-term maintenance of loading-induced cortical and trabecular bone benefits in female C57BL/6 mice and the influence of a surgically-induced menopause on the maintenance. 16-week-old animals had their right tibia extrinsically loaded 3 days/week for 4 weeks using the mouse tibial axial compression loading model. Left tibias were not loaded and served as internal controls. Animals were subsequently detrained (restricted to cage activities) for 0, 4, 8, 26 or 52 weeks, with ovariectomy (OVX) or sham-OVX surgery being performed at 0 weeks detraining. Loading increased midshaft tibia cortical bone mass, size and strength, and proximal tibia bone volume fraction. The cortical bone mass, area and thickness benefits of loading were lost by 26 weeks of detraining due to heightened medullary expansion. However, loading-induced benefits on bone total area and strength were maintained at each detraining time point. Similarly, the benefits of loading on bone volume fraction persisted at all detraining time points. The long-term benefits of loading on both cortical and trabecular bone were not influenced by a surgically-induced menopause as there were no interactions between loading and surgery. However, OVX had independent effects on cortical bone properties at early (4 and 8 weeks) detraining time points and trabecular bone properties at all detraining time points. These cumulative data indicate loading has long-term benefits on cortical bone size and strength (but not mass) and trabecular bone morphology which are not influenced by a surgically-induced menopause. This suggests skeletal loading associated with physical activity may provide long-term benefits by preparing the skeleton to offset both the cortical and trabecular bone changes associated with aging and menopause.
Keywords: growth and development, exercise, osteoporosis, physical activity, menopause
INTRODUCTION
Bone adapts to its mechanical environment with the young skeleton showing greatest plasticity to physical activity-induced mechanical loads. As the skeletal benefit of a lifetime of physical activity appears to occur mainly during the years of skeletal development,(1-3) it has been hypothesized the growing years present a ‘window of opportunity’ to induce the skeletal benefits of physical activity and prepare the skeleton to offset bone loss associated with aging.(4, 5) In order for physical activity-induced bone changes when young to have an impact later in life they need to persist into adulthood.
Numerous animal and clinical studies have demonstrated cessation of physical activity is associated with partial maintenance of the bone mass benefits of elevated loading when young; (6-9) however, the mass benefits diminish over time and do not appear to last lifelong.(10-15) In contrast, mechanisms exist for loading-induced bone size changes generated when young to last. Physical activity-induced loading of the young skeleton deposits new bone on the outer periosteal surface to increase bone size,(16) whereas bone loss during aging primarily occurs on the endocortical surface.(17) The discordant bone surface effects of loading and aging potentially enables the size benefits of loading when young to persist long-term and have lasting benefits on bone strength.
We previously used the forearm axial compression loading model in rats to demonstrate elevated skeletal loading when young with or without subsequent estrogen depletion has lifelong benefits on ulna cortical bone size and strength.(15, 18) Studying the effects of estrogen-depletion following the completion of skeletal loading when young is an important translatable question as it represents the clinical scenario of physical activity-induced skeletal changes when young followed by menopause later in life. Unfortunately, the rat ulna is limited with regard to studying the endocortical effects of mechanical loading and estrogen depletion as it has a negligible medullary cavity and, consequently endocortical surface available for bone resorption. The mouse ulna may be more suitable to exploring the endocortical effects of mechanical loading and estrogen depletion as it has a relatively larger medullary-to-cortical area ratio. However, neither the mouse or rat ulna readily allows investigation of the lasting benefits of mechanical loading on trabecular bone properties due to the principally cortical bone structure of the ulna. Exploring the lasting benefits of mechanical loading on trabecular bone properties in estrogen-replete and -deplete animals is important as the trabecular bone compartment appears more susceptible to the effects of both aging and estrogen removal.(19)
The aim of the current study was to explore the long-term maintenance of loading-induced cortical and trabecular bone benefits and the influence of estrogen depletion on the maintenance. A within-animal design was utilized whereby unilateral extrinsic skeletal loading was achieved using the mouse tibial axial compression loading model.(20, 21) The mouse tibial axial compression loading model simultaneously induces both cortical and trabecular bone adaptation, with the mouse tibia having a larger medullary-to-cortical area ratio than the ulna. Estrogen depletion was achieved by ovariectomy (OVX) to create a surgically-induced menopause.
MATERIALS AND METHODS
Animals
Female C57BL/6J mice (n=225) were purchased from Jackson Laboratories (Bar Harbor, ME) and acclimatized until 16 wk of age prior to experimentation. C57BL/6J mice were selected because of their previously demonstrated skeletal responsiveness to mechanical loading, aging and OVX.(22-25) All procedures were performed following approval of the Institutional Animal Care and Use Committee of Indiana University.
Mechanical loading
The right hindlimb of each animal was extrinsically loaded (loaded group) using the tibial axial compression loading model(20, 21) with the animal anesthetized using isoflurane inhalation (2% at 1.5 L/min for initial knockdown in a plastic container and 1.0-1.5% at 1.5 L/min via a facemask for maintenance of anesthesia). The model loads the tibia through contacts at the flexed knee and dorsiflexed foot to induce cortical and trabecular bone adaptation. Loading commenced at 16 wk of age and was introduced 3 days/wk for 4 wk using an electromechanical actuator (ElectroForce® 3200; Bose Corporation, Eden Praire, MN). The loading waveform consisted of a 2-Hz haversine waveform for 360 cycles/day with a peak load of 9N. A preliminary study in our laboratory using animals of the same genetic background, sex and age revealed these loading parameters elicited a tensile strain of 1,833 με (95% CI, 1,460 με to 2,206 με) on the medial surface of the midshaft tibia and induced lamellar bone adaptation within both cortical and trabecular bone compartments.(26) Left tibias served as internal controls and were not loaded (non-loaded group). Normal cage activity was allowed between loading sessions and throughout the study.
Surgery
One group of animals was euthanized 1 wk following completion of the loading program (0 wks detraining group). A lag time of 1 wk was implemented to allow skeletal adaptation to the final loading sessions to occur. All other animals were randomly divided into two surgical groups—OVX and sham-OVX (SHAM). Surgeries were performed under inhalation anesthesia at 1 wk following completion of the loading program (21 wks of age). A small dorsal midline incision was made and the muscle wall incised lateral to the midline and below the last rib. The periovarian fat pad was gently grasped and exteriorized, and ovary identified. In OVX animals, the fallopian tube between the fat pad and uterus was crushed. The crushed area was cut and the fat pad containing the ovary removed. The uterus was replaced and the procedure repeated on the contralateral side. The skin incision was closed with a surgical wound clip. In SHAM animals, the ovaries were exteriorized but not removed. Surgical success was assessed at necropsy by measuring uterine weight.
Micro-computed tomography assessment
Animals were euthanized at 0, 4, 8, 26 and 52 wks of detraining (animal age = 21, 25, 29, 47 and 73 wks, respectively), and the loaded and non-loaded tibias dissected free and stored in alcohol. A desktop micro-computerized tomography machine (SkyScan 1172 high-resolution μCT; SkyScan, Kontich, Belgium) was used to acquire images of the midshaft and proximal tibia at 56 kVp, 167 μA, 158 ms integration time and 11.76 μm isotropic voxel size. Beam hardening effects were reduced using a 0.5 mm aluminium filter and 20% beam hardening correction. Cortical and trabecular bone properties were derived from the midshaft and proximal tibia, respectively.
For cortical bone properties, a 0.5 mm thick volume of interest (VOI) of the tibial diaphysis immediately proximal to the midshaft was analyzed to acquire average total (Tt.Ar, mm2), cortical (Ct.Ar, mm2) and medullary area (Me.Ar, mm2), mean cortical thickness (Ct.Th, mm), and minimum (IMIN, mm4) and maximum (IMAX, mm4) second moments of area. Average areas were calculated as the VOI divided by the product of voxel height and the number of slices.(27) Polar moment of inertia (IP, mm4) was calculated as the sum of IMIN and IMAX. Bone mineral content (BMC, mg/mm) was obtained by exploiting the linear relationship between Hounsfield Units and known densities from calcium hydroxyapatite standards scanned using the same parameters as our bone samples. Cortical bone was segmented from surrounding non-mineralized material using a threshold of 427 mg/cm3.
For trabecular bone properties, a 1 mm thick VOI was analyzed beginning 0.5 mm distal to the proximal tibial growth plate. The VOI was within the secondary spongiosa and excluded cortical/subcortical bone by using an irregular anatomic contour a few pixels inside the endocortical boundary. Trabecular bone volume fraction (bone volume [BV]/total volume [TV], %), number (Tb.N, /mm), thickness (Tb.Th, mm) and separation (Tb.Sp, mm) were acquired, with a threshold of 264 mg/cm3 used to segment trabecular bone from surrounding non-mineralized material.
Mechanical properties
Mechanical properties of the tibial midshaft were acquired as previously described.(28) Bones were rehydrated overnight in saline, with 3 hours being reported as the minimum time required to restore mechanical properties following dehydration.(29) Bones were positioned anterior side up across supports with a span width of 11.2 mm and fixed with ~0.1 N static preload on an electromechanical actuator (ElectroForce® 3200; Bose Corporation, Eden Praire, MN). They were loaded in three-point bending with a crosshead speed of 0.2 mm/sec until failure at their midshaft. Force and displacement data were collected every 0.01 sec from which ultimate force (N), stiffness (N/mm) and post-yield energy to failure (mJ) were obtained.
Histomorphometry
Calcein (10 mg/kg; Sigma Chemical Co., St. Louis, MO) and alizarin (15 mg/kg; Sigma Chemical Co., St. Louis, MO) were given 12 and 5 days before euthanasia by intraperitoneal injection to permit determination of bone formation rates. The previously broken bones were embedded undecalcified in 99% methyl-methacrylate with 3% dibutyl phthalate (Sigma-Aldrich, St. Louis, MO). Transverse thick (40-50 μm) sections were removed 1 mm distal to the tibial midshaft using a diamond-embedded wire saw (Histo-saw; Delaware Diamond Knives). A lead pencil mark placed prior to mechanical testing facilitated slice localization. Sections were inspected to ensure the absence of mechanical testing artefacts and mounted unstained to assess periosteal bone formation rate. Frontal plane, thin (4 μm) sections of the proximal tibia were taken using a microtome (Reichert-Jung 2050; Reichert-Jung, Heidelberg, Germany), and mounted either unstained to enable determination of trabecular bone formation rate or stained with tartrate-resistant acid phosphatase and counterstained with hematoxylin (Sigma-Aldrich, Kit #387A-1KT, St. Louis, MO) to allow identification of trabecular osteoclasts.
Sections were analyzed using Image-Pro Plus (Version 7.0; Media Cybernetics, Inc., Bethesda, MD) on a Leica DMI6000 inverted microscope (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany). Dynamic parameters measured using the calcein and alizarin labels in the unstained midshaft and proximal tibia sections included single-label perimeter (sL.Pm), double-label area (dL.Ar) and perimeter (dL.Pm), and interlabel width (Ir.L.Wi). The following were derived from the primary data: mineralizing surface (MS/BS=[1/2sL.Pm+dL.Pm]/B.Pm; %), mineral apposition rate (MAR=mean Ir.L.Wi/interlabel period; μm/d), and bone formation rate (BFR/BS=MARxMS/BSx3.65; μm3/μm2/yr). A biological lower limit for MAR of 0.3 μm/d was used in sections lacking double labels (n=8 sections).(30) The region of interest within the proximal tibia consisted of a 1 mm2 box positioned 1 mm distal from the growth plate within the secondary spongiosa. Bone resorption was determined from stained sections of the proximal tibia by counting the number of bone-adherent, multinucleate, tartrate-resistant acid phosphatase positive cells (osteoclasts) within 1 mm2 of the secondary spongiosa and normalizing to bone surface (Oc.N/BS).
Statistics
Analyses were performed with IBM SPSS Statistics (v20.0; SPSS Inc., Chicago, IL), and were two-tailed with a level of significance set at 0.05. Data were assessed for normality and homogeneity of variance using Shapiro-Wilks and Levene tests, respectively. Load (loaded vs. non-loaded) and surgical (SHAM vs. OVX) effects on body mass in the 0 wks detraining and 4, 8, 26 and 52 wks detraining groups were assessed using paired and unpaired t-tests, respectively. Two-way, one-repeated-measure analyses of covariance (ANCOVA) were used to assess micro-CT and mechanical testing outcomes in the 4, 8, 26 and 52 wks detraining groups, with loading (loaded vs. non-loaded) and surgical (SHAM vs. OVX) groups as the within- and between-animal independent variables, respectively. Body mass was used as the covariate. Bone histomorphometric outcomes were similarly assessed in the 4, 8, 26 and 52 wks detraining groups with two-way, one-repeated-measure analyses of variance (ANOVA).
RESULTS
Animals
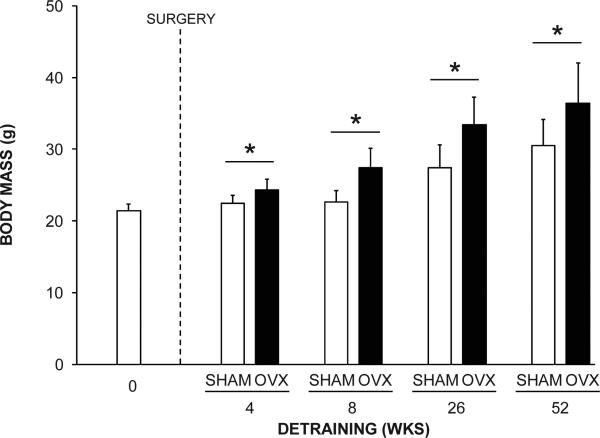
Twenty animals (8.9%) were lost due to anesthesia complications (n = 6), fractures during mechanical loading (n = 5) or unknown/natural causes (n = 9). Data from these animals were excluded from analyses. The final group sizes for the 0, 4, 8, 26 and 52 wks detraining groups were 25, 46 (SHAM, n = 22; OVX, n = 24), 45 (SHAM, n = 22; OVX, n = 23), 46 (SHAM, n = 24; OVX, n = 22) and 43 (SHAM, n = 22; OVX, n = 21), respectively. Body mass was greater (Fig. 1) and uterine weight was less (data not shown) in OVX animals compared to SHAM animals in the 4, 8, 26 and 52 wks detraining groups (all p < 0.05).
Fig. 1.
The effect of surgical intervention on body mass. OVX animals had greater body mass than SHAM animals in the 4, 8, 26 and 52 wks detraining groups (*p < 0.001). Data represent mean ± SD.
Long-term benefits of loading on cortical bone properties
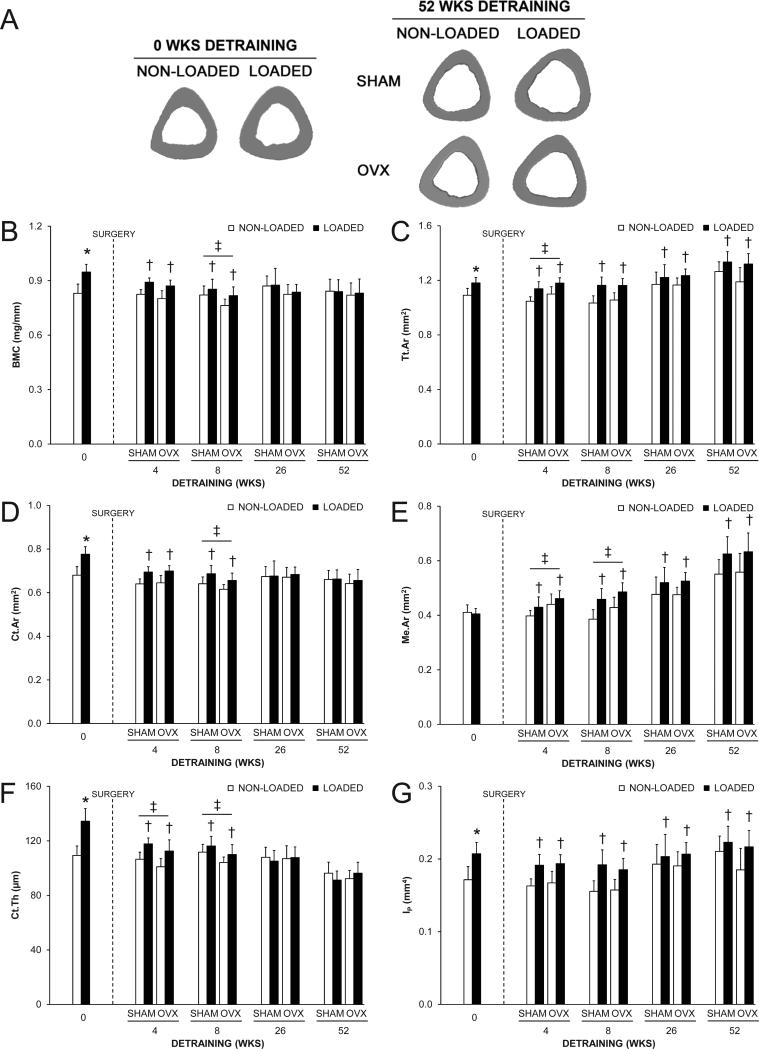
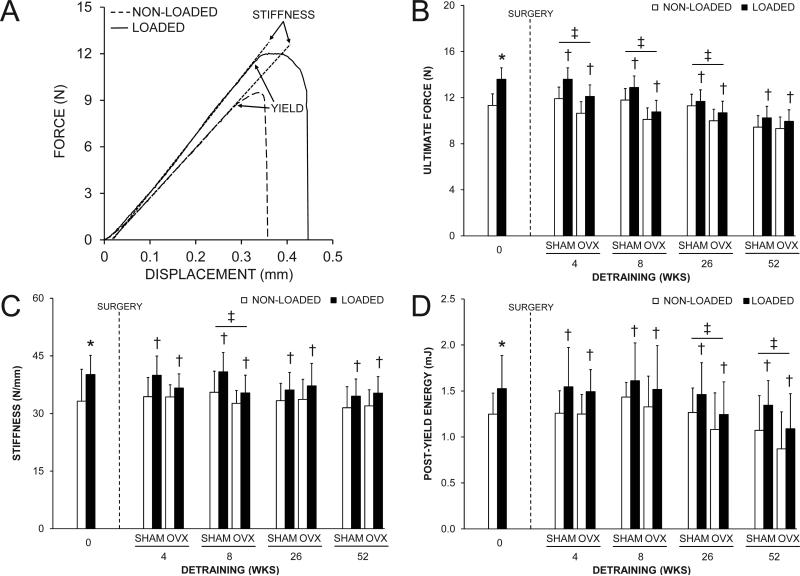
The loading program altered tibial midshaft cortical bone properties, as assessed in the 0 wks detraining group. In this group, the loaded tibia had 14.1%, 8.3% and 14.1% greater BMC, Tt.Ar and Ct.Ar compared to the contralateral non-loaded tibia, respectively (all p < 0.001, Fig. 2A-D). There was no effect of loading on Me.Ar (p = 0.52, Fig. 2E). The net result was 20.0-23.1% greater Ct.Th (Fig. 2F), IP (Fig. 2G) and mechanical properties (Fig. 3) in loaded vs. nonloaded tibias (all p < 0.001). The greater IP resulted from adaptation in orthogonal planes, as indicated by both greater IMAX and IMIN in loaded tibias (Supplemental Fig. 1). There was no effect of loading on periosteal or endocortical MS/BS, MAR or BFR/BS in the 0 wks detraining group (all p > 0.05, Fig. 4 and Supplemental Fig. 2), likely as a result of fluorescent labels being administered towards the completion of the loading program by which time accommodation to the loading stimulus had taken place.(31) Loading induced lamellar rather than woven bone formation in each animal, consistent with our preliminary study utilizing the mouse tibial axial compression model.(26)
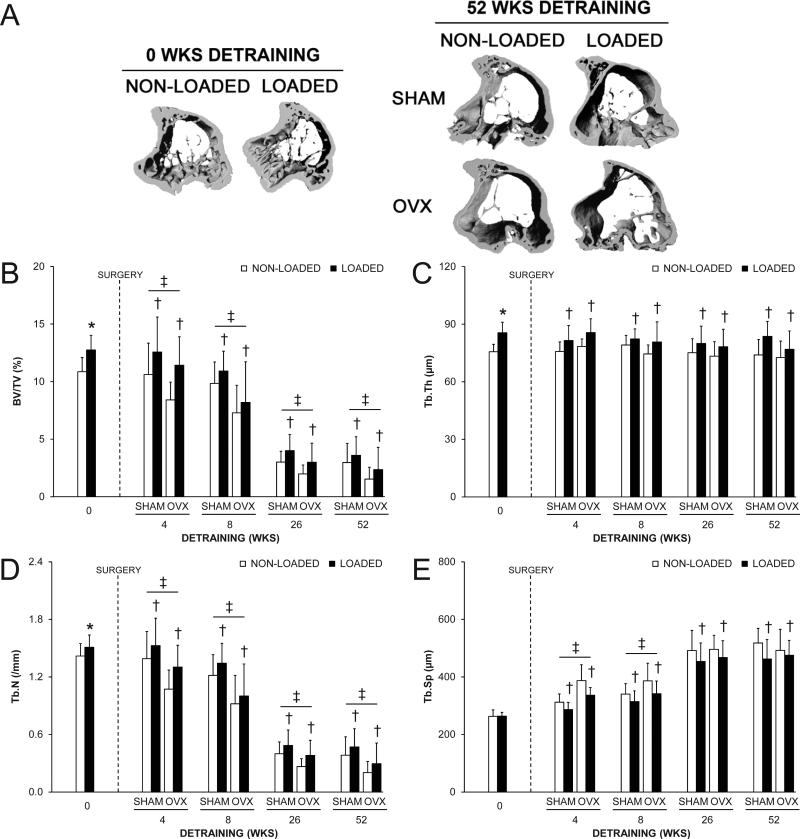
Fig. 2.
The effect of loading and surgery at select detraining time points on the midshaft tibia. A) Representative micro-CT tomographic images of the midshaft tibia in non-loaded and loaded bones from the 0 and 52 wks detraining groups. Loading increased total (Tt.Ar) and cortical (Ct.Ar) areas, and cortical thickness (Ct.Th), as evident in the 0 wks detraining group. The loading-induced increase in Tt.Ar persisted in the 52 wks detraining group in both SHAM and OVX animals. B) Bone mineral content (BMC); C) Tt.Ar; D) Ct.Ar; E) medullary area (Me.Ar); F) Ct.Th and G) polar moment of inertia (IP) at the midshaft tibia as select detraining time points. Loading increased BMC, Tt.Ar, Ct.Ar, Ct.Th and IP, as assessed in the 0 wks detraining group (*p < 0.05). There were no statistical interactions between loading and surgery in any detraining time point group. Loaded tibias had more BMC, Ct.Ar and Ct.Th in the 4 and 8 wks detraining groups and more Tt.Ar, Me.Ar and IP in each detraining time point group than non-loaded tibias (†p < 0.05 for loading main effect). OVX animals had more Tt.Ar and Me.Ar, and less Ct.Th than SHAM animals in the 4 wks detraining group, and less BMC, Ct.Ar and Ct.Th, and more Me.Ar than SHAM animals in the 8 wks detraining group, (‡p < 0.05 for surgery main effect). Data represent body mass corrected means ± SD.
Fig. 3.
The effect of loading and surgery at select detraining time points on midshaft tibia mechanical properties. A) Representative force vs. displacement curves for a pair of loaded and non-loaded tibias from the 0 wks detraining group. Loading increased: A) ultimate force (peak of the curve on the y-axis in panel A); C) stiffness (slope of the linear portion of the curve in panel A), and; D) post-yield energy to failure (area under curve between yield point and failure in panel A), as assessed in the 0 wks detraining group (*p < 0.05). There were no statistical interactions between loading and surgery in any detraining time point group for any of the properties assessed. Loaded tibias had greater ultimate force, stiffness and post-yield energy to failure than non-loaded tibias in each detraining time point group (†p < 0.05 for loading main effect). OVX animals had less ultimate force in the 4, 8 and 26 wks detraining groups, less stiffness in the 8 wks detraining group, and less post-yield energy to failure in the 26 and 52 wks detraining groups than SHAM animals (‡p < 0.05 for surgery main effect). Data represent body mass corrected means ± SD.
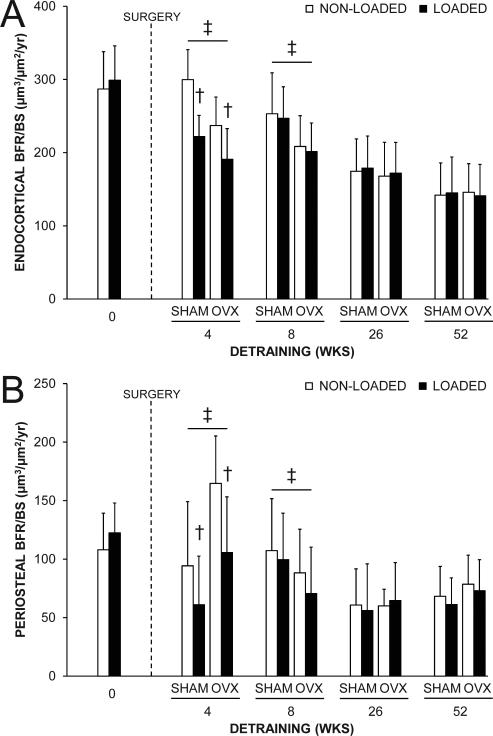
Fig. 4.
The effect of loading and surgery at select detraining time points on midshaft tibial: A) endocortical and B) periosteal bone formation rate (BFR/BS). There were no statistical interactions between loading and surgery in any detraining time point group. Loaded tibias had less endocortical and periosteal BFR/BS than non-loaded tibias in the 4 wks detraining group (†p < 0.001 for loading main effect). OVX animals had less endocortical and more periosteal BFR/BS in the 4 wks detraining group, and less endocortical and periosteal BFR/BS in the 8 wks detraining groups than SHAM animals (‡p < 0.05 for surgery main effect). Data represent means ± SD.
There were no interactions between loading and surgery in either the 4, 8, 26 or 52 wks detraining groups indicating surgery did not influence the maintenance of the cortical bone benefits of loading (all p = 0.12-0.99). Surgery altered cortical bone properties in early (4 and 8 wks) detraining groups. OVX mice in the 4 and 8 wks detraining groups had greater Me.Ar compared to SHAM mice indicating surgically-induced endocortical bone loss (all p < 0.01, Fig. 2E). Reduced endocortical MS/BS, MAR and BFR/BS contributed to the net loss of bone on this surface in the 4 and 8 wks detraining groups (all p < 0.05, Fig. 4A and Supplemental Fig. 2A,B). Endocortical bone loss in OVX animals in the 4 wks detraining group was coupled with greater periosteal MS/BS, MAR and BFR/BS (all p < 0.05, Fig. 4B and Supplemental Fig. 2C,D) resulting in greater Tt.Ar (p < 0.01, Fig. 2C). However, periosteal expansion was unable to maintain Ct.Th which was lower in OVX mice in both the 4 and 8 wks detraining groups (all p < 0.01, Fig. 2F) or BMC which was lower in OVX mice in the 8 wks detraining group (p = 0.04, Fig. 2B). The net result was lower ultimate force and energy to failure (all p ≤ 0.04, Fig. 3A,B and Supplemental Fig. 3) in tibias from OVX mice in the 4, 8 and 26 wks detaining groups. Tibias from OVX mice also had less stiffness in the 8 wks detraining group than tibias from SHAM mice (p < 0.01, Fig. 3C). In the 52 wks detraining group, the only differences in cortical bone properties between surgery groups were less energy to failure (p = 0.02, Supplemental Fig. 3) and post-yield energy to failure (p < 0.05, Fig. 3D).
Loading completed when younger had short-term benefits on tibial midshaft BMC, Ct.Ar and Ct.Th, with loaded tibias from animals in the 4 and 8 wks detraining groups having greater BMC, Ct.Ar and Ct.Th relative to contralateral non-loaded tibias (all p ≤ 0.001, Fig. 2B,D,F). However, loading benefits on BMC, Ct.Ar and Ct.Th did not persist in animals in the 26 and 52 wks detraining groups (all p = 0.09-0.81, Fig. 2B,D,F). Contributing to the eventual loss of the loading benefits on BMC, Ct.Ar and Ct.Th was a net loss of bone on the endocortical surface (evident by 7.1-18.1% greater Me.Ar in loaded tibias in each detraining time point group) (all p < 0.001, Fig. 2E) and lower periosteal MS/BS, MAR and BFR/BS in loaded tibias in the 4 wks detraining group (p < 0.001, Fig. 4B and Supplemental Fig. 2C,D). Reduced endocortical MS/BS, MAR and BFR/BS contributed to the net loss of bone on the endocortical surface in the 4 wks detraining group (all p < 0.05, Fig. 4A and Supplemental Fig. 2A,B). The lower periosteal expansion in loaded tibias did not persist in animals in the 8, 26 and 52 wks detraining groups and did not influence the maintenance of the loading benefit on Tt.Ar. Tt.Ar in loaded tibias was greater than in non-loaded tibias within animals in the 4, 8, 26 and 52 wks detraining groups (all p < 0.001, Fig. 2C), and contributed to persistent benefits of loading on IMAX, IMIN, and IP (all p < 0.001, Fig. 2G and Supplemental Fig. 1) and mechanical properties (all p ≤ 0.05, Fig. 3 and Supplemental Fig. 3).
Long-term benefits of loading on trabecular bone properties
The loading program had the expected effects on proximal tibia trabecular bone properties, with loaded tibias in the 0 wks detraining group having 17.4% greater BV/TV compared to the contralateral non-loaded tibia (all p = 0.001, Fig. 5A,B). The greater BV/TV in loaded tibias was due to greater gain in BV (+51.7% vs. non-loaded) relative to the loading-induced increase in TV (+28.4% vs. non-loaded) (Supplemental Fig. 4), and resulted in 13.1% greater Tb.Th and 6.4% greater Tb.N (all p ≤ 0.02, Fig. 5C,D). There was no effect of loading on Tb.Sp (p = 0.87, Fig. 5E), or trabecular MS/BS, MAR, BFR/BS or Oc.N/BS (all p = 0.16-0.33, Fig. 6 and Supplemental Fig. 5) in the 0 wks detraining group. The absence of a measurable loading effect on trabecular MS/BS, MAR, BFR/BS or Oc.N/BS again likely resulted from these measures being performed at a time by which accommodation to the loading stimulus had already taken place.(31)
Fig. 5.
The effect of loading and surgery at select detraining time points on proximal tibial trabecular: A) bone volume fraction (bone volume [BV]/tissue volume [TV]); B) thickness (Tb.Th); C) number (Tb.N) and D) separation (Tb.Sp). Loading increased BV/TV, Tb.Th and Tb.N (*p < 0.05). There were no statistical interactions between loading and surgery in any detraining time point group. Loaded tibias had more BV/TV, Tb.Th and Tb.N, and less Tb.Sp than non-loaded tibias in each detraining time point group (†p < 0.04 for loading main effect). OVX animals had less BV/TV and Tb.N than SHAM animals in each detraining time point group, and more Tb.Sp in the 4 and 8 wks detraining groups (‡p < 0.05 for surgery main effect). Data represent body mass corrected means ± SD.
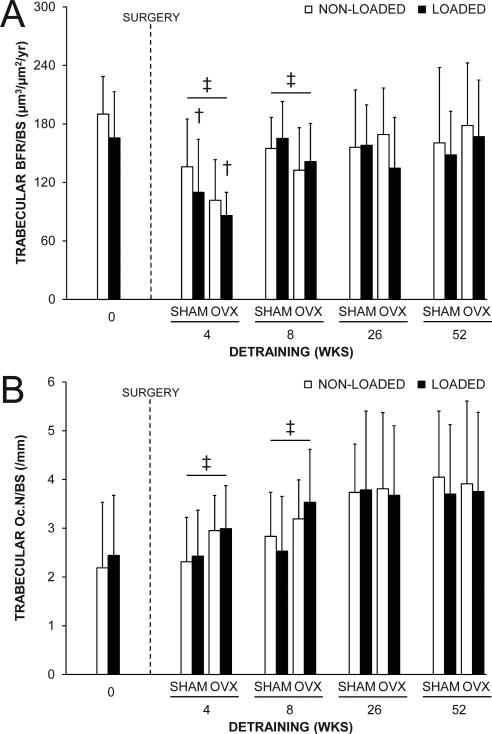
Fig. 6.
The effect of loading and surgery at select detraining time points on proximal tibial trabecular: A) bone formation rate (BFR/BS) and B) osteoclast number (Oc.N/BS). There were no statistical interactions between loading and surgery in any detraining time point group. Loaded tibias had less BFR/BS than non-loaded tibias in the 4 wks detraining group (†p = 0.04 for loading main effect). OVX animals had less BFR/BS and more Oc.N/BS than SHAM animals in the 4 and 8 wks detraining groups (‡p < 0.05 for surgery main effect). Data represent means ± SD.
There were no interactions between loading and surgery in either the 4, 8, 26 or 52 wks detraining groups indicating surgery did not influence the maintenance of the trabecular bone benefits of loading (all p = 0.20-0.94). Surgery altered trabecular bone properties in each detraining group, with BV/TV being lower in OVX mice in the 4, 8, 26 and 52 wks detraining groups (all p < 0.03, Fig. 5B). The lower BV/TV in OVX animals was due to a decrease in BV at each detraining time point and increase in TV in the 4 and 8 wks detraining groups (Supplemental Fig. 4), and resulted in less Tb.N (all p ≤ 0.05, Fig. 5D) rather than less Tb.Th (all p = 0.13-0.38, Fig. 5C). The lower BV/TV and Tb.N was caused by lower trabecular MAR and BFR/BS (p ≤ 0.05, Fig. 6A and Supplemental Fig. 5B) and increased trabecular Oc.N/BS (p < 0.05, Fig. 6B) in OVX animals at early (4 and 8 wks) detraining time points.
Loading completed when younger had long-term benefits on proximal tibia trabecular bone properties. Loaded tibias in each of the 4, 8, 26 and 52 wks detraining groups had greater BV/TV, Tb.Th and Tb.N, and decreased Tb.Sp than in their non-loaded tibias (all p < 0.05, Fig. 5), with the loading-induced increase in both BV and TV persisting in each detraining time group (all P < 0.05, Supplemental Fig. 4). There was lower trabecular BFR/BS in loaded tibias in the 4 wks detraining group (p = 0.04, Fig. 6A), but this did not persist in animals in the 8, 26 and 52 wks detraining groups and the aforementioned results indicate that the temporary decrease in trabecular BFR/BS did not influence the maintenance of the loading benefit on trabecular bone morphology. There was no effect of loading on trabecular Oc.N/BS in any detraining time point group (all p = 0.55-0.95, Fig. 6B).
DISCUSSION
The most novel findings of the current study are the long-term maintenance of loading-induced benefits on trabecular bone properties and the lack of an effect of an OVX-induced menopause on their maintenance. Unilateral extrinsic loading of the mouse tibia for 4 wks induced a 17.4% increase in BV/TV within the proximal tibia due to an increase in the thickness of preexisting trabeculae and the addition of new trabeculae. These trabecular bone benefits persisted for 52 wks after cessation of elevated skeletal loading (until animal age = 73 wks), with loaded tibias having greater BV/TV, Tb.Th and Tb.N and less Tb.Sp than contralateral non-loaded tibiae at each detraining time point assessed. OVX-induced menopause did not influence the maintenance of trabecular bone benefits, as evident by the absence of statistical interactions between loading and surgery groups. However, OVX did have independent negative effects on BV/TV and Tb.N at each assessment time point following surgery. These cumulative data suggest elevated skeletal loading when young provided long-term benefits to trabecular bone properties that were not influenced by menopause, with loading essentially preparing the skeleton to offset the trabecular bone changes associated with OVX.
The long-term maintenance of loading-induced benefits on trabecular bone properties contrasts the previous findings of Fujie et al.(32) and Iwamoto et al.(11) Both groups of investigators explored the lasting benefit of treadmill running on trabecular bone properties within the proximal tibia of rats. Although the study by Fujie et al.(32) was underpowered to detect measureable loading and detraining effects on trabecular morphology due to small group sizes (n = 5), Iwamoto et al.(11) reported the BV/TV benefit of treadmill running for 8 wks was subsequently lost with 4 wk of detraining. However, the later study was limited by the use of two-dimensional analyses of histological sections to assess trabecular bone morphology and the use of a between-animal study design to determine group differences. In contrast, the current study utilized more sensitive three-dimensional measures of trabecular morphology obtained using high-resolution micro-CT and took advantage of a unilateral skeletal adaptation model wherein the maintenance of loading benefits could be explored within-animal.
The maintenance of loading-induced benefits on trabecular bone properties is intriguing considering the concomitant loss of the cortical bone mass benefits of loading within the same bone. Elevated loading of the tibia when young enhanced midshaft tibia cortical BMC; however, the BMC benefits were no longer present 26 wks after return to habitual loading (restriction to home-cage activities). The loss of loading-induced cortical bone mass benefits resulted from a temporary decrease in periosteal bone formation combined with an increase in medullary expansion (as indicated by increased medullary area). The increase in medullary area indicates a net loss of bone on the endocortical surface, with the discordant endocortical and trabecular bone changes following return to habitual loading levels suggesting the bone cells in the two compartments are independently regulated.
The reduced periosteal bone formation and net loss of bone on the endocortical surface during detraining in loaded tibias ultimately contributed to a loss of the Ct.Ar and Ct.Th benefits of loading by 26 wks detraining. However, periosteal bone formation in loaded tibias returned to contralateral non-loaded levels by 8 wks detraining indicating loaded tibias grew radially with age following the suppression of bone formation in the initial weeks following loading program completion. The persistent radial growth in loaded tibias, despite loaded tibias initially being larger and stronger, suggests radial bone growth during aging is not entirely driven by a need to maintain bone bending strength in response to the mechanical decay associated with age-related endocortical bone loss. However, the addition of new periosteal bone during aging did maintain cortical thickness within loaded tibias at equivalent levels as in non-loaded tibias which would contribute to maintaining cortical compressive strength and the ability to resist buckling (assuming equivalent bone material properties between loaded and non-loaded tibias).
Ultimately, the persistent radial growth in loaded tibias in the current study enabled the bone size benefits of mechanical loading completed when young to persist long-term. For instance, loaded tibias had 8.0% greater midshaft tibia Tt.Ar compared to non-loaded tibias in the 52 wk detraining group, which matches the 8.1% difference observed in 0 wk detraining group (i.e. at the completion of the loading program). As cortical bone mechanical properties are proportional to the fourth power of material distance from the neutral axis,(33) the larger size of loaded tibias contributed to increased IP and ultimate force at each detraining time point. These data indicate elevated mechanical loading completed when young did not have lasting benefits on BMC, but had benefits on cortical bone size and strength that lasted for a period 13 times the length of the initial loading program.
The discordant long-term maintenance of the cortical bone mass and size benefits of mechanical loading completed when young clarifies our earlier findings. In our previous studies,(15, 18) we observed contrasting lifelong maintenance of mechanical loading benefits on bone mass when using the forearm axial compression loading model in rats. Possible reasons for our previous conflicting findings may relate to the investigation of the rat ulna, as well as the use of imaging modalities with differing resolutions to assess bone properties (dual-energy x-ray absorptiometry vs. peripheral quantitative computed tomography). The rat ulna shows a robust periosteal response to mechanical loading; however, it possesses a negligible medullary cavity and, consequently surface available for both age- and OVX-related endocortical bone loss. The current study assessed a skeletal site with a larger medullary-to-cortical area ratio than the rat ulna and utilized high-resolution micro-CT to confirm the cortical bone mass, but not size and strength benefits of mechanical loading completed when young are eventually lost with aging.
In addition to demonstrating lasting benefits of mechanical loading on bone strength, the current study observed long-term benefits on post-yield mechanical properties. Loaded tibias in the current study had greater post-yield energy to failure at each time point assessed suggesting loading completed when young increased ductility and reduced brittleness. This finding contrasts our previous observations of reduced post-yield displacement and energy in the rat ulna when assessed 92-94 weeks post loading completion.(15, 18) Reasons for the increased post-yield energy in loaded tibias in the current study and the disparate findings between our studies were not explored; however, they may relate to the age the animals were loaded (16 wks vs. 4-5 wks of age), skeletal site loaded (tibia vs. ulna), method of mechanical testing (3-point bending vs. axial compression) and length of detraining (52 wks. vs. 92-94 wks).
As with our trabecular bone findings, the lasting benefits of skeletal loading were maintained in cortical bone independent of a surgically-induced menopause. There were no statistical interactions between the loading and surgical intervention groups, indicating the cortical bone benefits of loading were equally maintained in SHAM and OVX animals. This observation supports those of Umemura et al.(34) who showed no effect of OVX on the maintenance of jump training-induced changes in tibial bone properties in rats exercised for 8 weeks and subsequently detrained for 6 months (until age = 11 months). Similarly, the current findings also confirm our previous work wherein OVX had no effect on the maintenance of loading-induced cortical bone benefits in rats extrinsically loaded for 6 wks beginning at 4 wks of age and then followed lifelong until 2 years of age.(18)
Although OVX did not influence the maintenance of the cortical bone benefits of mechanical loading completed when young, it did have independent negative effects on cortical bone properties at early time points following surgery. OVX in rodents generates a cortical bone modeling drift whereby there is elevated endocortical bone loss and periosteal apposition,(35-38) as occurs post-menopause in humans.(17) Endocortical bone loss was evident in the current study by increased midshaft tibia Me.Ar and proximal tibia TV in OVX animals within the 4 and 8 wks detraining groups, while periosteal apposition was evident by OVX animals in the 4 wks detraining group having greater midshaft tibia periosteal bone formation and Tt.Ar than SHAM animals. There was evidently more net bone lost endocortically than gained periosteally in the initial weeks following surgery as tibias from OVX animals had less BMC, Ct.Ar and Ct.Th, and subsequently mechanical properties than tibias from SHAM animals. Interestingly, there were no surgery effects on cortical bone properties in the 26 and 52 wks detraining groups suggesting that the cortical bone effects of OVX were temporary. In the end, the independent negative effect of OVX on cortical bone properties supports the trabecular bone findings that skeletal loading should be performed when young to offset bone changes associated with menopause later in life.
The current study has a number of strengths, including the: 1) use of a within-animal study design which enabled the maintenance of skeletal loading benefits to be explored while controlling for the influence of systemic factors; 2) investigation of the maintenance of loading-induced cortical and trabecular bone benefits within a single adapted element; 3) use of relatively large group sizes (n = 21-24 specimens per group) which enhanced statistical power to identify interactive effects between loading and subsequent surgery, and 4) exploration of the maintenance of loading effects for a relatively lengthy detraining period (up to 1 year). The study also has a number of limitations. The mechanical loading model utilized does not truly represent physical activity as it does not require physical exertion on the behalf of the animal. The long-term maintenance of loading effects on cortical bone may be attributable to species selection, with rodent cortical bone lacking the secondary remodeling of Haversian canals required to remodel and remove excess cortical bone after loading. The long-term maintenance of loading-induced benefits on trabecular bone may have been influenced by osteoarthritic joint changes associated with the mouse tibial axial compression loading model;(39, 40) however, any arthritis-induced lameness within the loaded leg would have potentiated loss of loading-induced benefits. The maintained loading-induced benefits on trabecular bone morphology may not contribute to enhanced mechanical properties as BV/TV declined with advancing age and was negligible (<4%) in both loaded and non-loaded tibias in the 52 wks detraining group.
In summary, this study found elevated mechanical loading of the skeleton completed when young had lasting benefits on cortical bone size and strength and trabecular bone properties that persisted independent of a surgically-induced menopause. These findings suggest that skeletal loading associated with physical activity when young may provide long-term benefits to fracture risk by preparing the skeleton to offset the cortical and trabecular bone changes associated with aging and estrogen depletion. Whether these effects translate to humans needs to be shown, but in the interim individuals should be encouraged to perform load-bearing physical activity when young to potentiate long-term bone health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Keith Condon for assistance with histological processing of specimens. This work was supported by the National Institutes of Health (R15 AR056858 and S10 RR023710).
Authors’ roles: Study design: SJW and RF. Data collection and analysis: SJW, MRG, ALH, JSR, LAG, EAG, RB. Data interpretation: SJW and RF. Drafting manuscript: SJW. Revising manuscript content: SJW and RKF. Approving final version of manuscript: SJW, MRG, ALH, JSR, LAG, EAG, RB, and RKF. SJW takes responsibility for the integrity of the data analyses.
Footnotes
Disclosures: All authors state they have no conflicts of interest
REFERENCES
- 1.Kannus P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson MK, Nordqvist A, Karlsson C. Physical activity increases bone mass during growth. Food Nutr Res. 2008:52. doi: 10.3402/fnr.v52i0.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36:250–7. doi: 10.1136/bjsm.36.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.U. S. Department of Health and Human Services . Bone Health and Osteoporosis: A Report of the Surgeon General. U. S. Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. [Google Scholar]
- 6.Duckham R, Baxter-Jones A, Johnston J, Vatanparast H, Cooper D, Kontulainen S. Does physical activity in adolescence have site and sex specific benefits on young adult bone size, content and estimated strength? J Bone Miner Res. 2013 Aug 1; doi: 10.1002/jbmr.2055. doi: 10.1002/jbmr.2055. [DOI] [PubMed] [Google Scholar]
- 7.Gunter K, Baxter-Jones AD, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23:986–93. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda A, Sogo N, Nagasawa S, Kato T, Umemura Y. Bones benefits gained by jump training are preserved after detraining in young and adult rats. J Appl Physiol. 2008;105:849–53. doi: 10.1152/japplphysiol.00902.2007. [DOI] [PubMed] [Google Scholar]
- 9.Tveit M, Rosengren BE, Nilsson JA, Ahlborg HG, Karlsson MK. Bone mass following physical activity in young years: a mean 39-year prospective controlled study in men. Osteoporos Int. 2013;24:1389–97. doi: 10.1007/s00198-012-2081-z. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson A, Olsson T, Nordstrom P. Rapid loss of bone mineral density of the femoral neck after cessation of ice hockey training: a 6-year longitudinal study in males. J Bone Miner Res. 2003;18:1964–9. doi: 10.1359/jbmr.2003.18.11.1964. [DOI] [PubMed] [Google Scholar]
- 11.Iwamoto J, Yeh JK, Aloia JF. Effect of deconditioning on cortical and cancellous bone growth in the exercise trained young rats. J Bone Miner Res. 2000;15:1842–9. doi: 10.1359/jbmr.2000.15.9.1842. [DOI] [PubMed] [Google Scholar]
- 12.Järvinen TLN, Pajamäki I, Sievänen H, et al. Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J Bone Miner Res. 2003;18:1292–9. doi: 10.1359/jbmr.2003.18.7.1292. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E. Exercise during growth and bone mineral density and fractures in old age. Lancet. 2000;355:469–70. doi: 10.1016/s0140-6736(00)82020-6. [DOI] [PubMed] [Google Scholar]
- 14.Pajamäki I, Kannus P, Vuohelainen T, et al. The bone gain induced by exercise in puberty is not preserved through a virtually life-long deconditioning: a randomized controlled experimental study in male rats. J Bone Miner Res. 2003;18:544–52. doi: 10.1359/jbmr.2003.18.3.544. [DOI] [PubMed] [Google Scholar]
- 15.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22:251–9. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 16.Bass SL, Saxon L, Daly RM, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–80. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 17.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–34. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 18.Warden SJ, Galley MR, Hurd AL, et al. Elevated mechanical loading when young provides lifelong benefits to cortical bone properties in female rats independent of a surgically induced menopause. Endocrinology. 2013;154:3178–87. doi: 10.1210/en.2013-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68:1218–25. doi: 10.1093/gerona/glt071. [DOI] [PubMed] [Google Scholar]
- 20.De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Noninvasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res. 2005;20:1085–92. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 23.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 24.Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ. Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res. 2005;20:2150–8. doi: 10.1359/JBMR.050819. [DOI] [PubMed] [Google Scholar]
- 25.Robling AG, Turner CH. Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone. 2002;31:562–9. doi: 10.1016/s8756-3282(02)00871-2. [DOI] [PubMed] [Google Scholar]
- 26.Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52:372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 28.Schriefer JL, Robling AG, Warden SJ, Fournier A, Mason J, Turner CH. A comparison of mechanical properties derived from multiple skeletal sites of mice. J Biomech. 2004;38:467–75. doi: 10.1016/j.jbiomech.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Broz JJ, Simske SJ, Greenberg AR, Luttges MW. Effects of rehydration state on the flexural properties of whole mouse long bones. J Biomech Eng. 1993;115:447–9. doi: 10.1115/1.2895510. [DOI] [PubMed] [Google Scholar]
- 30.Foldes J, Shih MS, Parfitt AM. Frequency distributions of tetracycline-based measurements: implications for the interpretation of bone formation indices in the absence of double-labeled surfaces. J Bone Miner Res. 1990;5:1063–7. doi: 10.1002/jbmr.5650051010. [DOI] [PubMed] [Google Scholar]
- 31.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accomodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Fujie H, Miyagaki J, Terrier A, Rakotomanana L, Leyvraz PF, Hayashi K. Detraining effects on the mechanical properties and morphology of rat tibiae. Biomed Mater Eng. 2004;14:219–33. [PubMed] [Google Scholar]
- 33.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 34.Umemura Y, Nagasawa S, Sogo N, Honda A. Effects of jump training on bone are preserved after detraining, regardless of estrogen secretion state in rats. J Appl Physiol. 2008;104:1116–20. doi: 10.1152/japplphysiol.00937.2007. [DOI] [PubMed] [Google Scholar]
- 35.Bagi CM, Mecham M, Weiss J, Miller SC. Comparative morphometric changes in rat cortical bone following ovariectomy and/or immobilization. Bone. 1993;14:877–83. doi: 10.1016/8756-3282(93)90318-5. [DOI] [PubMed] [Google Scholar]
- 36.Barengolts EI, Curry DJ, Bapna MS, Kukreja SC. Effects of endurance exercise on bone mass and mechanical properties in intact and ovarectomized rats. J Bone Miner Res. 1993;8:937–42. doi: 10.1002/jbmr.5650080806. [DOI] [PubMed] [Google Scholar]
- 37.Miller SC, Bowman BM, Miller MA, Bagi CM. Calcium absorption and osseous organ-, tissue-, and envelope-specific changes following ovariectomy in rats. Bone. 1991;12:439–46. doi: 10.1016/8756-3282(91)90033-f. [DOI] [PubMed] [Google Scholar]
- 38.Peng Z-Q, Tuukkanen J, Zhang HX, Jämsä T, Väänänen HK. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994;15:523–32. doi: 10.1016/8756-3282(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 39.Ko FC, Dragomir C, Plumb DA, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–78. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–47. doi: 10.1002/art.27765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.