Abstract
Hypoxia-inducible factor-1 (HIF-1) plays an important role in retinal and subretinal neovascularization (NV). Increased levels of HIF-1 cause increased expression of vascular endothelial growth factor (VEGF-A) and current therapies for ocular NV focus on neutralizing VEGF-A, but there is mounting evidence that other HIF-1-responsive gene products may also participate. In this study, we tested the effect of a designed ankyrin repeat protein (DARPin) that selectively binds and antagonizes the hypoxia-regulated gene product PDGF-BB in three models of subretinal NV (relevant to neovascular age-related macular degeneration) and compared its effects to a DARPin that selectively antagonizes VEGF-A. Daily intraperitoneal injections of 10 mg/kg of the anti-PDGF-BB DARPin or 1 mg/kg of the anti-VEGF DARPin significantly suppressed subretinal NV from laser-induced rupture of Bruch's membrane. Injections of 1 mg/kg/day of the anti-PDGF-BB DARPin had no significant effect, but when combined with 1 mg/kg/day of the anti-VEGF-A DARPin there was greater suppression than injection of the anti-VEGF-A DARPin alone. In Vldlr−/− mice which spontaneously develop subretinal NV, intraocular injection of 1.85 μg of anti-PDGF-BB or anti-VEGF-A DARPin caused significant suppression of NV and when combined there was greater suppression than with either alone. The two DARPins also showed an additive effect in Tet/opsin/VEGF double transgenic mice, a particularly severe model of subretinal NV and exudative retinal detachment. In addition, intraocular injection of 1.85 μg of anti-PDGF-BB DARPin strongly suppressed ischemia-induced retinal NV, which is relevant to proliferative diabetic retinopathy and retinopathy of prematurity. These data demonstrate that PDGF-BB is another hypoxia-regulated gene product that along with VEGF-A contributes to ocular NV and suppression of both provides an additive effect.
Keywords: Age-related macular degeneration, Diabetic retinopathy, HIF-1, DARPin
Introduction
Subretinal NV occurs in age-related macular degeneration (AMD) and other diseases of the Bruch's membrane/retinal pigmented epithelial cell complex. Subretinal NV refers to new vessels growing beneath the retina in the subretinal space regardless of the location from which the vessels originated. There are two types of subretinal NV based upon origin of the vessels, retinal angiomatous proliferation (RAP) which originates from the deep capillary bed of the retina and grows through the photoreceptor layer to reach the subretinal space [1] and choroidal NV which sprouts from choroidal vessels and extends through Bruch's membrane and the RPE to reach the subretinal space. Subretinal NV is a highly prevalent cause of visual loss.
Considerable progress has been made elucidating the pathogenesis of subretinal NV (for recent review see [2]). Studies in mouse and monkey models suggested that vascular endothelial growth factor-A (VEGF-A) plays a major role [3–5] and this has been confirmed in patients with neovascular AMD [6, 7]. Mice in which the hypoxia response element (HRE) was removed from the Vegf promoter developed markedly less subretinal NV at Bruch's membrane rupture sites than wild type mice, suggesting that hypoxia-inducible factor-1 (HIF-1) is involved [8]. This was also suggested by the strong suppression of subretinal NV by digoxin [9], which blocks HIF-1 transcriptional activation [10].
The implication of HIF-1 in the pathogenesis of subretinal NV suggests that other hypoxia-regulated gene products may also participate. Stromal derived factor-1 (SDF-1) and its receptor, CXCR4, are both induced by HIF-1, and have been shown to contribute to retinal and subretinal NV [11]. Platelet-derived growth factor-B (PDGF-B) and PDGF receptor-β, which is activated by PDGF-BB homodimers, are also hypoxia regulated and in this study, we sought to explore the role of PDGF-BB in ocular NV.
Antibodies can be generated as selective protein antagonists that are useful tools for determining if that protein participates in a disease process and can also be developed into therapeutic agents. Other agents that specifically bind to target proteins can serve a similar role. Designed ankyrin repeat proteins (DARPins) are recombinant proteins that are made up of ankyrin repeats with conserved and variable amino acid stretches. The conserved part forms a tightly packed core and the variable part lines the surface that binds the target protein. Libraries have been constructed to produce DARPins with random surface that can be screened for binding to target proteins [12, 13]. A DARPin that selectively binds VEGF-A with a Kd of 2 pM has been characterized and when injected into the vitreous cavity of rabbits, it suppresses VEGF-induced leakage [14]. It also suppresses laser-induced choroidal NV in rats after topical administration to the cornea. To gain further insights into the role of PDGF-BB, we studied the effect of a DARPin that selectively binds PDGF-BB in mouse models of ocular NV and compared its effects to those of the previously described anti-VEGF-A DARPin.
Results
Generation and characterization of DARPins that specifically bind PDGF-BB
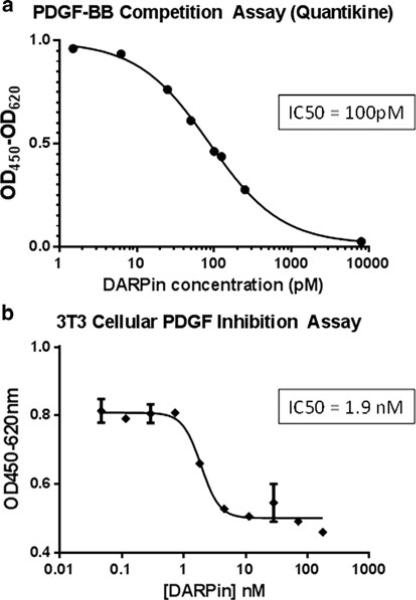
Using ribosome-display selections from naïve DARPin libraries, putative PDGF-BB binding DARPins were generated [15] and individual DARPins were screened for binding to PDGF-BB by ELISA. An anti-PDGF-BB DARPin was selected, and further characterized. The affinity of the anti-PDGF-BB DARPin to biotinylated PDGF-BB immobilized onto neutravidin coated chips was determined to be <500 pM (data not shown). No binding was detected to VEGF-A (data not shown). The apparent potency of the anti-PDGF-BB DARPin in a PDGF-BB receptor competition assay revealed an IC50 of about 100 pM (Fig. 1a). In a cellular assay, anti-PDGF-BB DARPin suppressed PDGF-BB-induced proliferation of NIH-3T3 cells with an IC50 of 1.9 nM, which reflects the limit of sensitivity of the assay (Fig. 1b).
Fig. 1.
Anti-PDGF DARPin strongly binds PDGF-BB and inhibits PDGF-BB-induced proliferation of NIH3T3 cells. a Triplicate samples consisting of 2 pM up to 8 nM of purified anti-PDGF DARPin were incubated overnight at 4 °C with 40 pM hPDGF-BB and then free PDGF-BB was assayed by ELISA. b Triplicate wells of starved NIH3T3 cells were incubated with a constant amount of hPDGF-BB (20 or 10 ng/mL) and a serial dilution of PDGF DARPin ranging from 200 to 0.05 nM. Control wells were incubated with hPDGF-BB alone. Cells were incubated for 48 h at 37 °C/5 % CO2, developed by addition of WST-1, and after 1–4 h absorbance was measured at OD450-620 nm. IC50 values were determined using the Graph Pad Prism fitting program and a non-linear fitting algorithim [log(inhibitor) vs. response with variable slope]
Anti-PDGF-BB DARPin suppresses choroidal NV and has an additive effect with an anti-VEGF-A DARPin
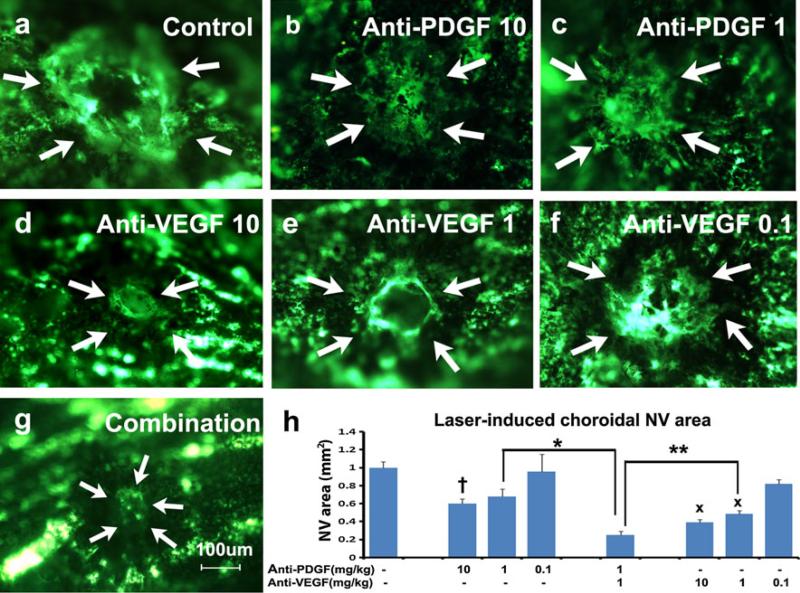
After laser-induced rupture of Bruch's membrane in each eye, 6-week old female C57BL/6 mice were treated with daily intraperitoneal injections of anti-PDGF-BB DARPin, anti-VEGF-A DARPin, both, or PBS as control. Compared to PBS-injected mice which showed large areas of choroidal NV at Bruch's membrane rupture sites (Fig. 2a), the mean area of choroidal NV was significantly less in mice that had been treated with 10 mg/kg/day of the anti-PDGF-BB DARPin (Fig. 2b and h), but not those treated with 1 mg/kg (Fig. 2c and h). There was also a significant reduction in mean area of choroidal NV compared to controls in mice that had been treated with 10 or 1 mg/kg/day of anti-VEGF-A DARPin (Fig. 2d, e, h), but not 0.1 mg/kg/day (Fig. 2f). Combination of 1 mg/kg/day of anti-PDGF-BB DARPin and 1 mg/kg/day of anti-VEGF-A DARPin caused a profound reduction in NV resulting in a mean area of choroidal NV (Fig. 2g) that was significantly less than that seen after treatment with 1 mg/kg/day of either alone (Fig. 2h).
Fig. 2.
Anti-PDGF-BB DARPin suppresses choroidal neovascularization (NV) and has an additive effect when combined with anti-VEGF-A DARPin. Female C57BL/6 mice had rupture of Bruch's membrane with laser photocoagulation at 3 locations in each eye followed by daily intraperitoneal injections of: a PBS as control, b 10 mg/kg anti-PDGFBB DARPin, c 1 mg/kg of anti-PDGF-BB DARPin, d 10 mg/kg of anti-VEGF-A DARPin, e 1 mg/kg of anti-VEGF-A DARPin, f 0.1 mg/kg of anti-VEGF-A DARPin, or g 1 mg/kg of anti-PDGF-BB DARPin + 1 mg/kg of anti-VEGF-A DARPin. After 14 days, choroidal flat mounts from fluorescein-labeled dextran-perfused mice showed that compared to PBS-treated controls which had large areas of choroidal NV (a), there was a significant reduction in mean area of choroidal NV in mice treated with 10 mg/kg of anti-PDGF-BB DARPin (b, h, †p = 0.04) but not those treated with 1 mg/kg of anti-PDGF-B DARPin (c, h). There was also significant reduction in mean area of choroidal NV compared to PBS controls in mice treated with 10 mg/kg (d, h, xp < 0.0001) or 1 mg/kg of anti-VEGF-A DARPin (e, h, xp < 0.0001), but not those treated with 0.1 mg/kg (f, h). Compared to mice treated with 1 mg/kg of anti-PDGF-BB DARP or 1 mg/kg of anti-VEGF-A DARPin, the mean area of choroidal NV was significantly reduced by combining both (g, h, *p < 0.0001, **p = 0.02). Statistical comparisons were made by ANOVA with Dunnett's correction for multiple comparisons
Intraocular injection of an anti-PDGF-BB DARPin suppresses subretinal NV in Vldlr−/− mice and has an additive effect with an anti-VEGF-A DARPin
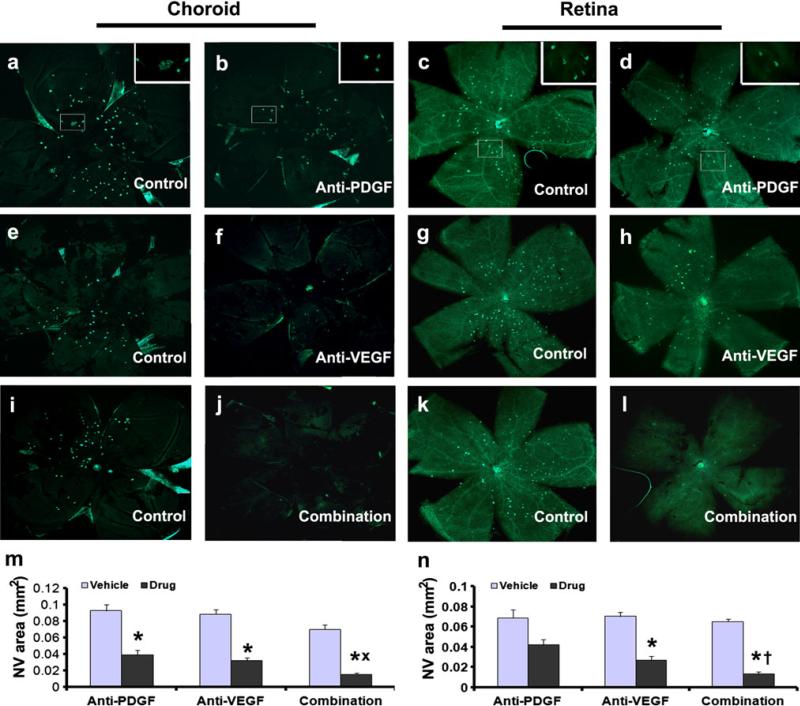
Very low density lipoprotein receptor knockout (Vldlr−/−) mice spontaneously develop NV that sprouts from the deep capillary bed of the retina and grows through the photo-receptors into the subretinal space and hence provides a model of RAP [16]. Sprouting of NV is seen around postnatal day (P) 14 and by P21 the vessels have invaded the subretinal space and retinal pigmented epithelium (RPE). The phenotype is very similar to that in rho/VEGF transgenic mice [17, 18], but due to stronger association of new vessels with the RPE in Vldlr−/− mice, portions of vessels remain adherent to the eyecup when it is separated from the retina making quantification of total amount of NV more challenging. One solution is to measure the amount of NV on both retinal and choroidal flat mounts. At P13, Vldlr−/− mice were given an intraocular injection of 1.85 μg of anti-PDGF-BB DARPin, 1.85 μg of anti-VEGF-A DARPin, or 1.85 μg of each in one eye and vehicle in the fellow eye (control). At P21, compared to fellow eye controls, the area of NV on choroidal and retinal flat mounts was significantly less in eyes injected with anti-PDGF-BB DARPin (Fig. 3a–d, m, n), anti-VEGF-A DARPin (Fig. 3e–h, m, n), or both together (Fig. 3i–l, m, n). The combination resulted in significantly less NV than either DARPin alone (Fig. 3m, n). These data confirmed that blocking PDGF-BB suppresses subretinal NV and showed that combined blockade of PDGF-BB and VEGF-A provides greater suppression than blockade of either alone.
Fig. 3.
Anti-PDGF-BB DARPin suppresses subretinal neovascularization (NV) in Vldlr−/− mice and has an additive effect when combined with anti-VEGF-A DARPin. At P13, Vldlr−/− mice (n = 5 for each group) were given intraocular injections of PBS in one eye and 1.85 μg of anti-PDGF-BB DARPin, or anti-VEGF-A DARPin, or combination of anti-PDGF-BB DARPin + anti-VEGF DARPin in the fellow eye. At P21, choroidal and retinal flat mounts were stained with the vascular stain FITC-Griffonia simplicifolia lectin to visualize NV. Compared to fellow eye controls (a, c), choroidal (b, n) and retinal flat mounts (d, n) from eyes treated with the anti-PDGF-BB DARPin showed significantly less NV. Compared to choroidal (e) and retinal flat mounts (g) from fellow eye controls, those from eyes injected with anti-VEGF-A DARPin also showed less NV (f, h, m, n). Choroidal (j) and retinal (l) flat mounts from eyes treated with both DARPins showed very little NV that was significantly less than that seen in fellow eyes (i, k) or in eyes treated with either DARPin alone (m, n). *p < 0.0001, xp = 0.0226, †p = 0.0499 by ANOVA with Dunnett's correction for multiple comparisons
Additive effect of anti-PDGF-BB and anti-VEGF-A DARPins in prevention of retinal detachment in Tet/opsin/VEGF mice
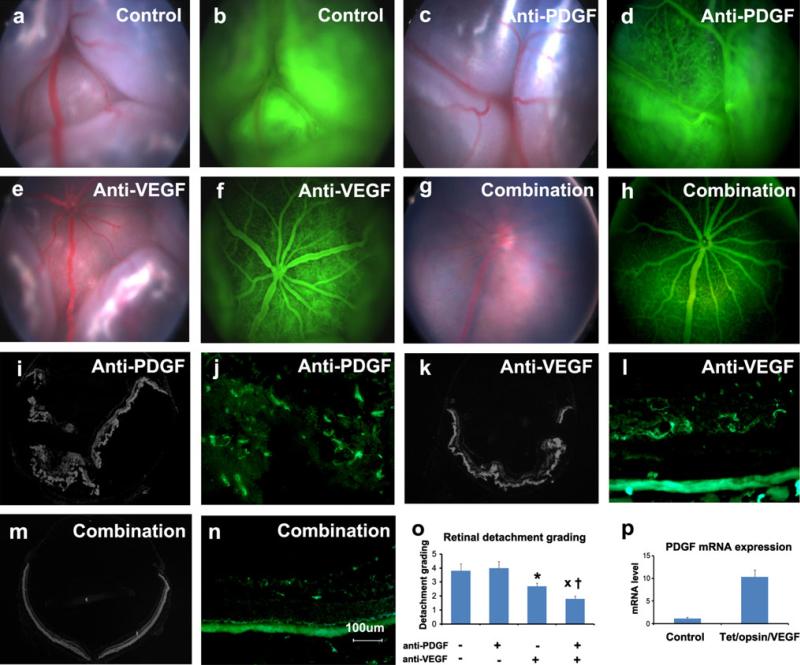
Double transgenic mice with doxycycline-inducible expression of VEGF-A (Tet/opsin/VEGF mice) in photoreceptors develop severe subretinal NV and exudative retinal detachments when treated with doxycycline [19]. This is a particularly severe model in which an intraocular injection of 25 μg of bevacizumab prevents retinal detachment in about 50 % of eyes and injection of 10 μg of ranibizumab is less effective [20]. We hypothesized that the anti-PDGF-BB DARPin would have no effect in this model that is driven by over-expression of VEGF-A in photoreceptors. Tet/opsin/VEGF mice were treated with 50 mg/kg of doxycycline for 4 days and also received intraperitoneal injections of PBS, 1 mg/kg of anti-PDGF-BB DARPin, 1 mg/kg of anti-VEGF-A DARPin, or both. Compared to PBS-treated mice (Fig. 4a, b), the incidence and severity of retinal detachment was the same in mice treated with anti-PDGF-BB DARPin alone confirming our hypothesis (Fig. 4c, d, o). Ocular sections showed total retinal detachments (Fig. 4i) and staining with a vascular marker showed vessels throughout the outer as well as inner retina indicating severe NV (Fig. 4j). Compared to controls, Tet/opsin/mice treated with anti-VEGF-A DARPin had significantly less frequent and less severe detachments (Fig. 4e, f). Ocular sections showed partial retinal detachments in most eyes (Fig. 4k) and staining with a vascular marker showed most NV limited to the region of the deep capillary bed of the retina with only a small amount in the outer retina and subretinal space (Fig. 4l). Surprisingly, mice treated with the anti-PDGF-BB + the anti-VEGF-A DARPin had less frequent and less severe retinal detachments than those treated with either alone (Fig. 4g, h, o). Ocular sections showed no detachment in most eyes (Fig. 4m) and staining with a vascular marker showed normal vessels limited to the inner retina (Fig. 4n). To explore the role of PDGF-BB in this model, Tet/opsin/VEGF double transgenic mice were treated with doxycycline or left untreated (control) for 3 days and the level of Pdgfb mRNA was measured in the retina. There was a dramatic increase in Pdgfb mRNA in the retina 3 days after the onset of VEGF expression which explains why targeting both VEGF-A and PDGF-BB provides greater benefits than targeting VEGF-A alone.
Fig. 4.
Prevention of retinal detachment in Tet/opsin/VEGF double transgenic mice is greater with both anti-PDGF-BB DARPin and anti-VEGF-A DARPin than either alone. Adult male Tet/opsin/VEGF double transgenic mice were given daily subcutanous injections of 50 mg/kg of doxcycline and intraperitoneal injections of PBS as control (a, b), 1 mg/kg anti-PDGF-BB DARPin (c, d), 1 mg/kg anti-VEGF-A DARPin (e, f), or 1 mg/kg of both DARPins (g, h). After 4 days, fundus photographs and fluorescein angiograms showed total bullous retinal detachments in eyes of mice treated with PBS (a, b) or anti-PDGF-BB DARPin (c, d), partial retinal detachments in eyes of mice treated with anti-VEGF-A DARPin (e, f), and little or no retinal detachment in eyes of mice treated with both DARPins (g, h). Ocular sections stained with Hoechst or FITC-Griffonia simplicifolia lectin confirmed total retinal detachments (i) and extensive NV thoughout the outer retina (j) in eyes of mice treated with anti-PDGF-BB DARPin, partial retinal detachments (k) and dilated vessels with NV extending from deep capillaries (l) in eyes of mice treated with anti-VEGF-A DARPin, and no retinal detachments (m) and normal vessels with little or no NV (n) in eyes of mice treated with both DARPins. Grading of the incidence and severity of leakage or retinal detachments (o) showed that eyes from mice treated with both DARPins had significantly less severe grades than those seen in eyes of mice treated with anti-VEGF-A DARPin (xp = 0.0329) or anti-PDGF-BB DARPin (†p = 0.0098) by Wilcoxin test. p Tet/Opsin/VEGF mice and C57BL/6 mice (n = 5 for each) were treated with doxycycline and after 3 days, the relative of expression of Pdgfb mRNA normalized to cyclophilin A mRNA was significantly greater in Tet/Opsin/VEGF mice (p = 0.0002 by unpaired t test)
Intraocular injection of an anti-PDGF-BB DARPin suppresses ischemia-induced retinal NV
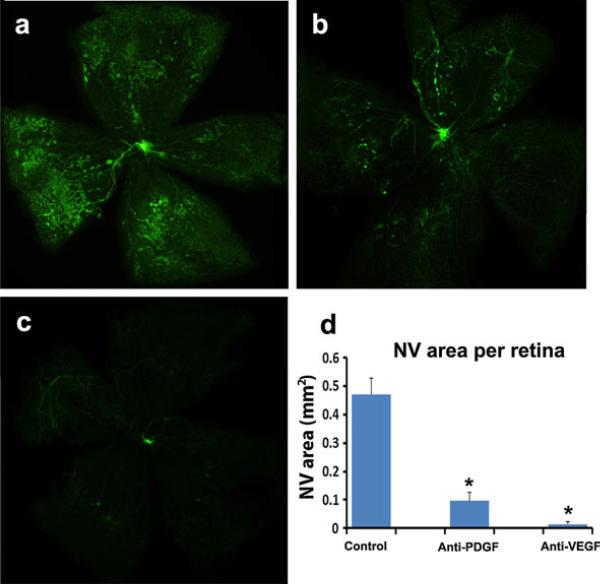
While our study focused primarily on subretinal NV which is relevant to AMD and other diseases of the Bruch's membrane/retinal pigmented epithelial cell complex, the unexpectedly strong effects of PDGF-BB antagonism alone, raised the question of whether antagonism of PDGFBB would have any effect on a different type of ocular NV, ischemia-induced retinal NV, which is relevant to proliferative diabetic retinopathy. Mice with oxygen-induced ischemic retinopathy were given an intraocular injection of PBS or PBS containing 1.85 μg of anti-PDGF-BB DARPin or 1.85 μg of anti-VEGF-A DARPin at P12. At P17, compared to eyes injected with PBS, the mean area of retinal NV on the surface of the retina was dramatically reduced in eyes injected with either anti-PDGF-BB DARPin or anti-VEGF-A DARPin (Fig. 5).
Fig. 5.
Anti-PDGF-BB DARPin suppresses ischemia-induced retinal neovascularization (NV). At P12, mice with oxygen-induced ischemic retinopathy were given an intraocular injection of PBS (n = 13) or PBS containing 1.85 μg of anti-PDGF-BB DARPin (n = 5) or 1.85 μg of anti-VEGF-A DARPin (n = 5) in one eye. At P17, compared to PBS injected eyes which showed substantial retinal NV (a), there was little retinal NV seen in anti-PDGF-BB DARPin-injected eyes (b) and almost none in anti-VEGF-A DARPin injected eyes (c). The mean area of retinal NV was significantly reduced by anti-PDGF-BB DARPin or anti-VEGF-A DARPin (d, *p < 0.0001 by ANOVA with Dunnett's correction for multiple comparisons)
Discussion
VEGF-A plays an important role in the pathogenesis of subretinal NV and several antagonists of VEGF have been demonstrated to provide benefit in patients with subretinal NV due to AMD including ranibizumab [6], bevacizumab [21], and aflibercept [7]. A VEGF-A antagonistic DARPin that selectively binds VEGF-A with an apparent IC50 of 10 pM has been shown to suppress suture-induced corneal NV in rabbits and laser-induced choroidal NV in rats after topical administration [14]. In the current study, we have extended those findings to show that systemic administration of an anti-VEGF-A DARPin reduces laser-induced choroidal NV in mice and reduces the incidence and severity of exudative retinal detachments in a particularly severe model, Tet/opsin/VEGF double transgenic mice. Intraocular injection of an anti-VEGF DARPin significantly reduced subretinal NV in Vldlr−/− mice. These data demonstrate that an anti-VEGF DARPin suppresses sub-retinal NV in several different models. In a phase I/II study in patients with diabetic macular edema (DME), 4 patients who had an intraocular injection of 0.4 mg of the currently clinically investigated anti-VEGF DARPin, MP0112, had detectable drug levels in the aqueous humor 12 weeks after the injection, and 3 of the 4 showed persistent neutralization of intraocular VEGF at 12 weeks [22]. If future studies confirm such a long duration effect for MP0112, it could reduce treatment burden for patients with neovascular AMD, DME, or macular edema secondary to retinal vein occlusion.
Another strategy to improve upon current treatments for neovascular AMD is to address other stimulators that participate in addition to VEGF-A. Like VEGF-A, PDGF-B is upregulated by HIF-1 and inducible expression of PDGF-B in photoreceptors results in NV and retinal detachment very similar to that seen in mice with inducible expression of VEGF-A in photoreceptors [19, 23]. In this study, we demonstrated that a potent PDGF-B-binding DARPin is able to suppress laser-induced choroidal NV in wild type mice and subretinal NV in Vldlr−/− knockout mice. This provides the first demonstration that blocking PDGF-BB by itself has anti-angiogenic activity in the eye. A previous report showed that daily injections of 5 mg/kg of an aptamer that binds PDGF-BB had no effect on laser-induced choroidal NV in mice, but significantly enhanced the effect of an anti-VEGF-A aptamer [24]. This difference may be because of the difference in dose, differences in pharmacokinetics, and/or some other difference between the agents that allowed for more complete blockade of PDGF-BB signaling by daily IP injections of 10 mg/kg of the anti-PDGF-BB DARPin versus daily IP injections of 5 mg/kg of the anti-PDGF-BB aptamer. Whatever the reason for the difference in findings in rodent models of laser-induced choroidal NV, the observation that the anti-PDGF-BB DARPin has anti-angiogenic activity is supported by the finding that intraocular injection of the anti-PDGF-BB DARPin suppressed subretinal NV in Vldlr−/− knockout mice. The anti-VEGF-A DARPin had a suppressive effect on choroidal NV at a 10-fold lower dose than the anti-PDGF-BB DARPin suggesting that blockade of VEGF signaling may be more effective than blockade of PDGF-BB signaling in this model. In contrast to these two models, the anti-PDGF-BB DARPin had no significant effect in Tet/opsin/VEGF mice when used alone.
In mice with laser-induced choroidal NV or Vldlr−/− mice, administration of both the anti-VEGF-A and anti-PDGF-BB DARPins was significantly more effective than either alone which is consistent with the findings of Jo et al. [24]. Surprisingly, the combination was also more effective than the anti-VEGF-A DARPin alone in Tet/opsin/VEGF mice. This finding caused us to measure PDGF-BB expression in Tet/opsin/VEGF mice and it was found that Pdgfb mRNA was significantly elevated 3 days after initiation of VEGF expression. At this time point, there is substantial subretinal NV which is the likely source of the increased production of PDGF-BB. The increased levels of PDGF-BB must contribute to disease progression, and that is why combined blockade of PDGF-BB and VEGF-A reduces progression to retinal detachment.
The suppression of subretinal NV by antagonism of PDGF-BB raised the question of whether it would also suppress ischemia-induced retinal NV. Intraocular injection of 1.85 μg of anti-VEGF DARPin caused almost complete suppression of retinal NV, but injection of 1.85 μg of anti-PDGF-BB DARPin also caused very strong suppression. These data suggest that both VEGF-A and PDGF-BB contribute to ischemia-induced retinal NV, which adds to our knowledge of the molecular mechanism of retinal NV, but the dramatic effectiveness of blocking VEGF-A alone raises doubt as to whether a PDGF-BB antagonist will have clinical utility for treatment of retinal NV.
Thus, we have shown in 3 different models that combined antagonism of VEGF-A and PDGF-BB provides greater suppression of subretinal NV than either alone. These findings are consistent with recent clinical trials indicating that combined suppression of VEGF-A and PDGF-BB may be superior to suppression of VEGF-A alone in treatment of NVAMD [25]. Taken together, all of these data suggest that PDGF-BB is a second validated molecular target for treatment of ocular NV.
Materials and methods
Ribosome display and binder screening, production, and purification
Ribosome-display selections were performed using the N2C and N3C DARPin libraries as previously described [15, 26]. Briefly, four standard selection rounds were performed on biotinylated PDGF-BB captured in an alternating manner onto Neutravidin or Streptavidin coated beads (Seradyn, resp. Invitrogen). The enriched DARPin library pools were subjected to two off-rate selection rounds using decreasing concentrations of biotinylated PDGF-BB (1–0.1 nM) in combination with up to 1 μM non-biotinylated PDGF-BB for competition. After every off-rate selection, binders were enriched using one additional standard selection round. The resulting pools of DARPins were PCR amplified and ligated in pMPAG6 [27]. Escherichia coli XL1-Blue was transformed with the resulting plasmid pool. Individual colonies were picked and grown overnight in 96-deep-well plates at 37 °C, and the resulting over-night cultures were used to inoculate expression cultures in deep-well plates, and to isolate the plasmid DNA, as has been described [26]. Expression cultures were harvested by centrifugation, lysed, the crude extracts were applied to biotinylated PDGF-BB captured onto Neutravidin-coated Maxisorp plates, and binding DARPins were detected using an anti-RGSHis antibody (Qiagen, Hilden, Germany)/Anti-mouse IgG-AP conjugate (Sigma, St. Louis, MO, USA) detection system in combination with a 4-NPP (Sigma) detection system as described [26]. The DNA sequences of DARPins giving a crude extract ELISA signal were determined by standard DNA sequencing. Once suitable DARPins were identified, they were expressed and purified at larger scale [15, 28]. The purified proteins were dialyzed (Mw cut off 3 kDa) and protein concentration was determined using the theoretical extinction coefficient of the respective DARPin at 280 nm. The purified DARPins were further characterized by SDS-PAGE and size exclusion chromatography (Superdex 200, GE Healthcare, Switzerland).
Assessment of DARPin binding to PDGF-BB
Purified DARPins (25, 12.5, 6.25, 3.12, and 1.56 nM) were first tested for binding to biotinylated PDGF-BB or VEGF-A immobilized on a neutravidin-coated chip in a ProteOn XPR36 instrument (BioRad). Suitable DARPins were selected for ELISA competition assays using a PDGF-BB Quantikine kit (R&D Systems, UK). Briefly, competition samples were prepared by mixing 60 μl 2× concentrated DARPin dilution series in PBS/0.2 %BSA with 60 μl of 40 pM hPDGF-BB (standard from the kit; diluted in RD5 K) and they were incubated overnight at 4 °C. The pre-coated wells of the ELISA plate were reconstituted with 100 μl RD1X and 100 μl of competition samples were added to wells. Plates were incubated for 2 h at RT and spun at 600 rpm. Wells were washed 4 times with wash buffer and 200 μl of conjugate was added. Plates were incubated for 2 h at RT and spun at 600 rpm. Wells were washed 4 times with wash buffer and 200 μl of substrate was added. Plates were incubated 30 min at RT and absorbance measured at 450 nm (reference: 620 nm). Data were fitted using Graphpad Prism (Graphpad, USA).
Inhibition of PDGF-BB-induced proliferation of NIH3T3 cells
NIH3T3 cells were seeded at 2,000 cells/well in a 96-well plate in growth medium (DMEM complete medium, 10 % calf serum) and incubated overnight at 37 °C/5 % CO2. The following day, the growth medium was replaced by starving medium (DMEM complete media without red phenol, 0.5 % calf serum) for 7–8 h. After discarding the starving medium, the prepared cells were supplemented with assay medium (RPMI with red phenol, 0.5 % FCS) containing constant amount of hPDGF-BB (20 or 10 ng/mL) and a serial dilution of DARPins (200–0.05 nM). As a control, samples were prepared containing only hPDGF-BB. Cells were incubated for 48 h at 37 °C/5 % CO2, developed by addition of WST-1, and after 1-4 h absorbance was measured at OD450-620 nm. IC50 values were determined using the fitting program Graph Pad Prism and a non-linear fitting algorithim [log(inhibitor) vs. response with variable slope].
Mouse model of laser-induced choroidal NV
Mice were treated in accordance with the Association for Research in Vision and Ophthalmology guideline for the use of animals in research. 6-week-old C57BL/6 female mice had laser-induced rupture of Bruch's membrane at three locations in each eye as previously described [29]. Briefly, mice were anesthesized and pupils were dilated with 1 % tropicamide. Diode laser (532 nm wavelength, 75 μm spot size, 0.1 s duration and 120 mW) with a slit lamp delivery system (OcuLight GL, Iridex, Mountain View, CA, USA) using a handheld cover slip as a contact lens to view the retina. Production of a bubble at the time of laser, which indicates rupture of Bruch's membrane, is an important factor in obtaining choroidal NV, and only burns in which a bubble was produced were included in the study. Mice were randomly divided into 8 study groups, 6 that received daily intraperitoneal injections of 0.1, 1.0 or 10.0 mg/kg of anti-PDGF-BB DARPin or anti-VEGF-A DARPin, one that received a combination of 1.0 mg/kg of each, and one that received vehicle. 14 days after rupture of Bruch's membrane, mice were anesthetized and perfused with 1 ml of PBS containing 50 ng/ml of fluorescein-labeled dextran (Sigma-Aldrich, St. Louis, MO, USA). Choroidal flat mounts were prepared as previously described [30]. Flat-mounts were examined by fluorescence microscopy (Axioskop; Carl Zeiss Meditec, Thornwood, NY, USA). Image-analysis software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA) was used to measure the area of each CNV lesion with the investigator masked with respect to treatment group.
Assessments in Vldlr−/− mice
Breeding pairs of mice homozygous for a loss-of-function mutation in the very low density lipoprotein receptor (Vldlr) gene (B6;129S7-Vldlrtm1Her/J; Vldlr−/−) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). At P13, Vldlr−/− mice were given an intraocular injection of 1 μl of PBS in one eye and in the fellow eye received 1.85 μg of anti-PDGF-BB DARPin, 1.85 μg of anti-VEGF-A DARPin, or 1.85 μg of each. At P21, the mice were euthanized and choroid and retina were dissected and stained with FITC-conjugated GSA lectin and choroidal and retinal flat mounts were examined by fluorescence microscopy. The total area of NV on the outer surface of the retina on retinal flat mounts as well as that adherent to the RPE on choroidal flat mounts was measured by image analysis with the observer masked with respect to treatment group.
Assessments in Tet/opsin/VEGF double transgenic mice
Adult Tet/opsin/VEGF double transgenic mice [19] were given daily subcutaneously injections of 50 mg/kg of doxycycline and daily intraperitoneal injections of PBS as control, 1 mg/kg of anti-PDGF-BB DARPin, 1 mg/kg of anti-VEGF-A DARPin, or 1 mg/kg of each. After 3 days, fundus photographs and fluorescein angiography were done with Micron III retinal imaging microscope (Phoenix Research Laboratories, Pleasanton, CA). For fluorescein angiography, mice were given an intraperitoneal injection of 12 μl/g of body weight of 1 % fluorescein sodium (Alcon, Fort Worth, TX, USA) and after 1 min, images were captured of the left eye and then the right eye within 30 s. Images were graded by two independent observers masked with respect to treatment group using the following grading scale: 0 = no retinal detachment and normal retinal vessels, 1 = no retinal detachment, no dilated retinal vessels, minimal fluorescein leakage, 2 = no retinal detachment, dilated retinal vessels, fluorescein leakage, 3 = partial retinal detachment, 4 = shallow total retinal detachment, 5 = severe bullous retinal detachment. Immediately after fluorescein angiography, mice were euthanized, eyes were flash frozen, and frozen sections were cut. Sections were counterstained with Hoechst (1:1,200), examined by fluorescence microscopy, and graded for retinal detachment with the observer masked with respect to treatment group.
Assessments in mice with oxygen-induced ischemic retinopathy
Oxygen-induced ischemic retinopathy [31] was induced by placing litters of C57BL/6 mice in 75 % oxygen at P7. At P12, the mice were returned to room air and given an intraocular injection of PBS or PBS containing 1.85 μg of anti-PDGF-BB DARPin or 1.85 μg of anti-VEGF-A DARPin. At P17, retinas were dissected and stained with FITC-conjugated GSA lectin and retinal flat mounts were examined by fluorescence microscopy. The total area of retinal NV on the surface of the retina was measured by image analysis with the investigator masked with regard to treatment group.
Quantitative real-time RT-PCR
Tet/opsin/VEGF and C57BL/6 mice were given a subcutaneous injection of 50 mg/kg of doxycycline for 3 days, euthanized, and retinas were dissected. Total retinal RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to manufacturer's protocol. Reverse transcriptase (SuperScript II, Life Technologies, Gaithersburg, MD, USA) was used to synthesize cDNA from 1 μg of total RNA. Real time PCR was done using SYBRGreen as fluorescent dye (Qiagen) in Rotor-Gene Q system (Qiagen) with primers specific for Pdgfb: 5′-CGG AGT CGG CAT GAA TCG-3′ (forward) and 5′-AAG GAG CGG ATG GAG TGG-3′ (reverse) and Cyclophilin A: 5′-CAG ACG CCA CTG TCG CTT T-3′ (forward) and 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′ (reverse). Cyclophilin A mRNA was used to normalize the amount of Pdgfb mRNA.
Acknowledgments
Supported by EY012609 and a grant from Molecular Partners AG.
Footnotes
Conflict of interest Daniel Snell, Savira Ekawardhani, Julia K. J. Ahlskog, Michael Baumann, and Michael T. Stumpp are employees of Molecular Partners. None of the other authors have a conflict of interest.
Ethical Standards The experiments described in this manuscript comply with the laws of the United States and the Federal Republic of Germany.
Contributor Information
Aling Dong, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Christopher Seidel, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Daniel Snell, Molecular Partners AG, Zurich, Switzerland.
Savira Ekawardhani, Molecular Partners AG, Zurich, Switzerland.
Julia K. J. Ahlskog, Molecular Partners AG, Zurich, Switzerland
Michael Baumann, Molecular Partners AG, Zurich, Switzerland.
Jikui Shen, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Takeshi Iwase, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jing Tian, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Rebecca Stevens, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sean F. Hackett, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Michael T. Stumpp, Molecular Partners AG, Zurich, Switzerland
Peter A. Campochiaro, Departments of Ophthalmology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- 1.Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter JS, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;91:311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 4.Kryzstolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NM, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 5.Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGFTRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 7.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, View 1 and View 2 Study Groups Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Vinores SA, Xiao WH, Aslam S, Shen J, Oshima Y, Nambu H, Liu H, Carmeliet P, Campochiaro PA. Implication of the hypoxia response element of the VEGF promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758. doi: 10.1002/jcp.20525. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Zhang H, Iwase T, Shen J, Semenza G, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1alpha sythesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara C, McLauer T, Aslam S, Gong YY, Xiao WH, Khu NH, Thut C, Campochiaro PA. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 12.Forrer P, Stumpp MT, Binz HK, Pluckthun A. A novel strategy to design binding molecules harnessing the modular nature of repeat proteins. FEBS Lett. 2003;539:2–6. doi: 10.1016/s0014-5793(03)00177-7. [DOI] [PubMed] [Google Scholar]
- 13.Binz HK, Stumpp MT, Forrer P, Armstutz P, Pluckthun A. Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol. 2003;332:489–503. doi: 10.1016/s0022-2836(03)00896-9. [DOI] [PubMed] [Google Scholar]
- 14.Stahl A, Stumpp MT, Schlegel A, Ekawardhani S, Lehrling C, Gottfried M, Gulotti-Georgieva M, Villemangne D, Forrer P, Agostini HT, Binz HK. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis. 2013;16:101–111. doi: 10.1007/s10456-012-9302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, Grutter MG, Pluckthun A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575–582. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- 16.Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23:518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- 18.Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998;39:180–188. [PubMed] [Google Scholar]
- 19.Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, Duh EJ, Hackett SF, Chang M, Bok D, Zack DJ, Campochiaro PA. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki K, Miki A, Matsuoka M, Muramatsu D, Hackett SF, Campochiaro PA. Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor. Ophthalmology. 2009;116:1748–1754. doi: 10.1016/j.ophtha.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CATT Research Group. Marin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Channa R, Berger BB, Heier JS, Brown DM, Fiedler U, Hepp J, Stumpp MT. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase 1/2 study. Am J Ophthalmol. 2012;155:697–704. doi: 10.1016/j.ajo.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Seo M-S, Okamoto N, Vinores MA, Vinores SA, Hackett SF, Yamada H, Yamada E, Derevjanik NL, LaRochelle W, Zack DJ, Campochiaro PA. Photoreceptor-specific expression of PDGF-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyer DS, Ophthotech Anti-PDGF in AMD Study Group Combined inhibition of platelet-derived (PDGF) and vascular endothelial (VEGF) growth factors for the treatment of neovascular age-related macular degeneration (NV-AMD). Results of a phase 1 study. Invest Ophthalmol Vis Sci. 2009 Online ARVO abstract 1260. [Google Scholar]
- 26.Steiner D, Forrer P, Pluckthun A. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J Mol Biol. 2008;382:1211–1227. doi: 10.1016/j.jmb.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 27.Kawe M, Horn U, Pluckthun A. Facile promoter deletion in Escherichia coli in response to leaky expression of very robust and benign proteins from common expression vectors. Microb Cell Fact. 2009;8:8. doi: 10.1186/1475-2859-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman T, Petit Frere C, Raba M, Weisbach M, Dohn K, Popp A, Donzeau M. Simultaneous metal chelate affinity purification and endotoxin clearance of recombinant antibody fragments. J Immunol Methods. 2006;314:67–73. doi: 10.1016/j.jim.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 31.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]