Abstract
Childhood adversity and genetic variant ADH1B-rs1229984 have each been shown to influence heavy alcohol consumption and disorders. However, little is known about how these factors jointly influence these outcomes. We assessed the main and additive interactive effects of childhood adversity (abuse, neglect, parental divorce) and the ADH1B-rs1229984 on the quantitative phenotypes “maximum drinks in a day” (Maxdrinks) and DSM-Alcohol Use Disorder (AUD) severity, adjusting for demographic variables, in an Israeli sample of adult household residents (n=1,143) evaluated between 2007–2009. Childhood adversity and absence of the protective ADH1B-rs1229984 A allele were associated with greater mean Maxdrinks [Mean Differences: 1.50; 1.13 respectively] and AUD severity [Mean Ratios: 0.71; 0.27 respectively]). In addition, childhood adversity moderated the ADH1B-rs1229984 effect on Maxdrinks (p<0.01) and AUD severity (p<0.05), in that there was a stronger effect of ADH1B-rs1229984 genotype on Maxdrinks and AUD severity among those who had experienced childhood adversity compared to those who had not. ADH1B-rs1229984 impacts alcohol metabolism. Therefore, among those at risk for greater consumption, e.g., those who experienced childhood adversity, ADH1B-rs1229984 appears to have a stronger effect on alcohol consumption and consequently on risk for AUD symptom severity. Evidence for the interaction of genetic vulnerability and early life adversity on alcohol-related phenotypes provides further insight into the complex relationships between genetic and environmental risk factors.
Key words/phrases: ADH1B, Alcohol use disorders, Alcohol consumption, childhood adversity, interaction, Israel
Introduction
Heavy alcohol consumption significantly impacts public health by increasing physical and mental health problems and related costs (Rehm et al., 2009). Alcohol use disorders (AUDs) are also associated with substantial impairment and comorbidity (Merikangas & McClair, 2012). Many factors influence the risk for these drinking phenotypes, including genetic and environmental factors (Kendler, Myers, & Prescott, 2007), which may act together to increase risk for problematic alcohol use. While the evidence for gene-environment interactions in substance use is growing (Dick & Kendler, 2012a; Xie et al., 2012; Young-Wolff, Enoch, & Prescott, 2011), much remains to be understood about the interaction of specific genetic variants with specific environmental risk factors in the etiology of harmful drinking phenotypes.
Associations between ADH1B and Alcohol-Related Phenotypes
Alcohol dehydrogenase 1B (ADH1B) encodes a key enzyme for alcohol metabolism. The A allele of rs1229984 results in the substitution of Histidine for Arginine48 (ADH1B*2), which greatly increases the activity of the ADH1B enzyme (Hurley & Edenberg, 2012). This allele has been consistently associated with a protective effect against alcoholism in Asian populations (Li, Zhao, & Gelernter, 2011), in which there is a high prevalence (~40%; (Li et al., 2011; Yokoyama et al., 2013)) of the protective ADH1B allele. Even in populations of European origin, in which the frequency of the protective allele is low (generally under 5%), a strong effect has been demonstrated (Bierut et al., 2012). Among the Jewish population in Israel, a relatively high prevalence of the ADH1B protective allele (20–41%) has been identified (Meyers et al., 2013; Neumark et al., 2004). Associations between ADH1B and alcohol phenotypes have been shown in Israeli Jews (Hasin et al., 2002; Neumark et al., 1998), including association with maximum drinks consumed in a 24-hour period (“Maxdrinks”) and AUD severity in recent work in a large household sample (Meyers et al., 2013). This large sample of Jewish Israelis presents an important opportunity to investigate whether the association of ADH1B and alcohol phenotypes is moderated by environmental factors.
Childhood Adversity
One environmental risk factor for AUDs is exposure to adverse events during childhood (Keyes, Hatzenbuehler, & Hasin, 2011). Childhood adversity can refer to a wide range of exposures including sexual, emotional and physical abuse and neglect, and parental death or separation that occur during the first 18 years of life. Despite variability in measurement and study design, most (Bensley, Spieker, et al., 1999; Kessler, Davis, & Kendler, 1997; Sartor et al., 2007) although not all (Bulik, Prescott, & Kendler, 2001; Sher et al., 1997) studies show that childhood adversities are associated with earlier onset of adolescent alcohol consumption and with AUDs in adulthood. Since the protective ADH1B variant exerts its effect by limiting alcohol consumption (Hurley & Edenberg, 2012), ADH1B may show a stronger effect among those whose risk for heavy drinking is increased by childhood adversity than among those without this increased risk.
Gene-Environment (GxE) Interactions
Studies of whether the relationship between candidate genes and alcohol use outcomes is moderated by childhood adversity (G×E interaction) have largely focused on genes affecting neuronal pathways (Young-Wolff et al., 2011). For example, in youths, the higher risk serotonin transporter promoter “s” variant together with childhood adversity (maltreatment) increases risk for early-onset alcohol use (Brody et al., 2007; Kaufman et al., 2006). Similarly, in women, the monoamine oxidase A risk allele was associated with alcoholism, but only among those experiencing childhood adversity (sexual abuse (Ducci et al., 2008)). Another study suggested that a variant in the DRD2/ANKK1 region is associated with alcohol problems among individuals exposed to stress (Madrid et al., 2001). While these studies are all important, the neuronal pathways underlying substance phenotypes remain to be elucidated, while the relationship of alcohol metabolizing liver enzymes to alcohol consumption is better understood. Therefore, investigating the effects of interaction between childhood adversity and ADH1B on heavy alcohol consumption and related alcohol disorder phenomena could more specifically address how adversity moderates direct gene effects on alcohol use and disorders.
Among the most significant challenges of GxE research is the lack of robust genetic main associations (Duncan & Keller, 2011); most studies in the literature focused on genetic variants whose relationships to alcohol phenotypes were inconsistent. Additionally, power to detect interactions is typically lower than power to detect main effects. Since continuous phenotypes offer more information than binary phenotypes by providing a range of values, and typically have more statistical power to detect genetic associations (Kuo et al., 2008; Waldman, Robinson, & Rowe, 1999), evaluating alcohol consumption and AUD with graded variables should increase power for detecting interactions, and is consistent with a general movement in psychiatry to address dimensional rather than binary traits (Ehlke, Hagman, & Cohn, 2012; Hasin et al., 2012). Studying GxE interaction using robust genetic and environmental risk factors and informative, graded phenotypes therefore offers a promising strategy.
Since childhood adversity and a lack of the protective ADH1B-rs1229984 “A” allele both increase risk for problematic drinking behaviors, we investigated whether the association between ADH1B and drinking problems was stronger among individuals who experienced childhood adversity than among those who did not. We previously demonstrated ADH1B effects on alcohol consumption and AUD severity in a household sample of Israeli Jews (Meyers et al., 2013); we began the present study in the same sample by determining the association of early childhood adversity with these two alcohol phenotypes. We then tested for additive interaction of childhood adversity and ADH1B on these phenotypes. While previous studies of ADH1B-rs1229984 have focused on the protective effects of the minor “A” allele, here we focus on the lack of the protective allele (i.e., the GG genotype – which is the higher-risk genotype) for ease of interpretation. We consider childhood adversity as the moderator of the ADH1B-rs1229984/alcohol phenotype relationships (and not the opposite) to integrate these findings into two literatures. The first is the gene-environment interaction literature, which typically examines the environmental moderation of a genetic effect (Brody et al., 2007, Kaufman et al., 2006, Madrid et al., 2001, Dick et al., 2011, van der Zwaluw CS et al., 2012). The second literature is the stress sensitization literature, which suggests that early stress may heighten sensitivity to other risk factors, including genetic predispositions (Enoch, 2011; Zhang et al., 2013). Therefore, this study examines the effect of ADH1B-rs1229984 on alcohol phenotypes in the presence or absence of childhood adversity, for interpretability in this context.
Methods
Study procedures and sample
Data were collected in 2007–2009 from 1,349 adult household residents, as described elsewhere (Hasin et al., 2002). This sample was designed to investigate environmental and genetic influences on alcohol-related traits. Because drinking among Israeli women is limited (Hasin et al., 1998; Shmulewitz et al., 2012; Spivak et al., 2007), males were oversampled. Interviewers received structured training and administered face-to-face computer-assisted interviews after obtaining written informed consent as approved by relevant IRBs (Shmulewitz et al., 2012). The overall response rate was 68.9%. Quality control included field observation, reviews of recorded interviews, and telephone verification of responses. The present analysis included 1,143 ever-drinkers (ever drank alcohol, lifetime) who provided information about childhood experiences and were genotyped for ADH1B-rs1229984. Of these, 78.3% (N=895) were male; 23.9% (N=273) were immigrants from the Former Soviet Union (FSU); 13.1% (N=177) met criteria for DSM-IV AUD; and the mean age was 41.3 (SD=12.9).
Measures
Heavy Alcohol consumption
Consistent with previous genetic studies (Saccone et al., 2009; Yang et al, 2005), we created a variable measuring maximum number of drinks in a 24-hour period (Maxdrinks) during the period of lifetime heaviest drinking. Maxdrinks ranged from 1–40. This was assessed using the Alcohol Use Disorders and Associated Disabilities Interview Schedule (AUDADIS (Grant et al., 1995; Hasin et al., 2007)) adapted for the present study. The AUDADIS measure for lifetime maximum quantity consumed has very good inter-rater reliability (intraclass correlation coefficients [ICC] of 0.70 (Hasin et al., 2007)).
Lifetime AUD severity
The AUDADIS uses 21 items to assess the 11 alcohol dependence and abuse criteria according to DSM-IV (American Psychiatric Association., 2000). As the lifetime dependence and abuse criteria were unidimensional in this sample (Shmulewitz et al., 2010), we created a quantitative measure of AUD severity by counting the number of lifetime criteria endorsed; AUD severity ranged from 0–11. Test-retest reliability of lifetime AUDADIS alcohol criterion count is excellent in general population samples (ICCs=0.86–0.89 (Hasin et al., 2007)).
ADH1B-rs1229984
A detailed description of the genotyping is available in Meyers et al., 2013 (Meyers et al., 2013). The ADH1B-rs1229984 SNP was genotyped on the Sequenom MassArray system (Sequenom, USA) as previously described (Meyers et al., 2013). No deviation from Hardy-Weinberg equilibrium was observed (χ2=2.04, p-value=0.141). Because the effects of ADH1B-rs1229984 on Maxdrinks and AUD severity remained unchanged after adjusting for ancestry (Meyers et al., 2013), no adjustment was necessary in the present interaction analyses. 8.8% of the overall sample and 9.0% of drinkers had the AA genotype, 38.9% of the overall sample and 38.5% of drinkers had the AG genotype, and 52.3% of the overall sample and 52.5% of drinkers has the GG genotype; the minor allele (A) frequency was 28.3% in the overall sample and 28.2% in drinkers (Supplemental Table 1).
Childhood adversity
Childhood adversity was defined by one or more of three experiences: (1) Neglect was assessed by a single item asking if participants were ever seriously neglected by either of their parents or any of the people who raised them before age 18 (response options were yes/no). (2) Physical abuse was assessed by a single item asking if participants were ever physically attacked, badly beaten up or injured by their parents or any of the people who raised them before age 18 (response options were yes/no). (3) Parental divorce was assessed by a single item asking participants if their parents (adoptive or biological) were divorced or permanently stopped living together before they were age 18 (response options were yes/no). The three adversity variables (abuse, neglect, parental divorce) were associated, with correlation coefficients ranging from 0.13 (for abuse and parental divorce) to 0.48 (for abuse and neglect). Exploratory factor analyses of abuse, neglect, and parental divorce indicated one underlying construct, as a single factor fit the data best (Comparative Fit index=0.96; Tucker-Lewis index=0.86; Root-mean squared error of approximation = 0.02). Factor loadings for abuse, neglect, and parental divorce were 0.82, 0.80, and 0.50, respectively. Neglect, abuse and parental divorce were reported by 3.6%, 4.8%, 11.0% of the sample, respectively; any childhood adversity was reported by 15.7% (N=180).
Analysis
Main effects
Regression models (using SAS 9.3) were used to investigate the main effects of childhood adversity and ADH1B-rs1229984 with each alcohol phenotype, controlling for sex, age, and former Soviet Union immigrant status, as drinking differs by these subgroups in Israel (Hasin et al., 2002; Shmulewitz et al., 2012; Spivak et al., 2007). Poisson regression models with overdispersion were used, as that distribution provided the best fit for the data (Meyers et al., 2013). Maxdrinks and AUD severity were also modeled using normal, Poisson (without overdispersion), negative binomial, and zero-inflated distributions; data best fit the overdispersed Poisson distribution best based on the likelihood and goodness-of-it indices (Akaike’s Information Criterion and the Bayesian Information Criterion). Furthermore, since the lowest value for Maxdrinks was 1, we re-ran analyses using a zero truncated model; results remained unchanged. Means were adjusted for demographic variables (sex, age, ethnicity), and mean differences indicating the difference in the mean trait value given the presence of the risk factor, and corresponding 95% confidence intervals (CIs), were computed on the additive scale using an identity link in SAS 9.3 (Spiegelman & Hertzmark, 2005) to remain consistent with interaction analyses. Similar means for the alcohol phenotypes were observed in the genotype groups with protective allele A (AA and AG), while phenotype means were higher in those without allele A (GG) (see Table 1). Therefore, the GG group was compared to those with genotypes AA or AG. The ADH1B-rs1229984 variable (GG vs. AA/AG) was not related to demographics (sex, age, and ethnicity).
Table 1.
Additive main effects of childhood adversity and ADH1B-rs1229984 on alcohol-related phenotypes in 1,143 ever-drinkers
| Childhood Adversity | ||||
|---|---|---|---|---|
|
| ||||
| No N=963 |
Yes N=180 |
|||
| Alcohol Outcome | Meana (95% CI) | Mean difference (95% CI)a | p-value | |
| Maxdrinks | 4.71 (4.39, 5.04) | 6.21 (5.36, 7.06) | 1.50 (0.59, 2.41) | <0.001 |
| AUD severity | 1.36 (1.25, 1.47) | 2.07 (1.76, 2.38) | 0.71 (0.38, 1.04) | <0.0001 |
| ADH1B-rs1229984 Genotype | ||||
|---|---|---|---|---|
|
| ||||
| GG N=601 |
AA or AGb N=542 |
|||
| Outcome | Meana (95% CI) | Mean difference (95% CI)a | p-value | |
| Maxdrinks | 5.48 (5.06, 5.90) | 4.36 (3.97, 4.74) | 1.13 (0.59, 1.66) | <0.0001 |
| AUD severity | 1.60 (1.45, 1.74) | 1.33 (1.20, 1.47) | 0.27 (0.09, 0.45) | <0.01 |
Maxdrinks = greatest number of drinks in a 24-hour period, during period of heaviest drinking;
AUD severity = number of DSM-IV alcohol dependence and abuse criteria endorsed;
CI = confidence interval
adjusted for demographic variables (age, sex, immigrant from former Soviet Union), using overdispersed Poisson regression with an identity link to generate values on the mean (additive) scale
Although mean comparisons of alcohol outcomes were not made between all three genotype groups, mean values (adjusted for covariates) were as follows. Maxdrinks: AA (N=102), 4.29 (95% CI=3.54, 5.03); AG (N=440), 4.37 (95% CI=3.96, 4.79). AUD severity: AA (N=102), 1.23 (95% CI=0.90, 1.57); AG (N=440), 1.35 (95%CI=1.20, 1.50); Minor allele frequency=28.2%;
Interaction
The interaction between ADH1B-rs1229984 and childhood adversity was assessed by including an interaction term (ADH1B*childhood adversity) in separate linear-Poisson regression models (Spiegelman, Hertzmark, & Wand, 2007) for each alcohol phenotype, performed using Proc GENMOD in SAS 9.3. We assessed this interaction on the additive scale, as additive interaction corresponds more closely than multiplicative interaction with the current conceptual understanding of biological interaction and causal synergism (“Modern Epidemiologic Approaches to Interaction: Applications to the Study of Genetic Interactions,” 2006; Spiegelman et al., 2007; Spiegelman & Hertzmark, 2005). The estimate for the interaction term represents the interaction contrast (IC), a “difference in differences” effect. This “difference in differences” is evaluated as follows. First, among those who experienced childhood adversity, the mean difference in the alcohol phenotypes between those with the GG genotype and those with the AA/AG genotypes is evaluated. Next, among those without childhood adversity the mean difference in the alcohol phenotypes between those with the GG genotype and those with the AA/AG genotype is evaluated. Last, the IC, i.e., the difference in these two mean differences is evaluated, to determine if the ADH1B effect is greater among those who experienced childhood adversity compared with those who did not. Likelihood ratio chi-square tests were used to assess the statistical significance of the IC and mean differences. Furthermore, as the IC indicates the difference in the slopes between those with the AA/AG genotypes and those with the GG genotype by childhood adversity status, plots of regression adjusted (for sex, age and ethnicity) mean phenotypes are presented to facilitate interpretation. No significant association was observed between childhood adversity and ADH1B-rs1229984, thus gene-environment correlation was not observed and was not a potential confounder of the results.
Results
Among the 1,143 ever-drinkers, mean Maxdrinks was 4.95 (s.d.=6.01) and AUD severity was 1.47 (s.d.=1.96), with skewed distributions (Figure S1). The two alcohol phenotypes, Maxdrinks and AUD severity, were highly correlated (r2=0.56, p-value<0.0001).
Maxdrinks, main effects
Childhood adversity showed a significant association with Maxdrinks (Table 1). The adjusted mean number of Maxdrinks was 1.50 greater in those who experienced childhood adversity compared to those who did not. In addition, ADH1B-rs1229984 was significantly associated with Maxdrinks (Table 1). The adjusted mean number of Maxdrinks was 1.13 higher in the GG group compared to the AA/AG group.
Maxdrinks, additive interaction
Childhood adversity and ADH1B-rs1229984 showed a significant additive interaction effect on Maxdrinks (p-value<0.01). Among participants who experienced childhood adversity, the adjusted mean difference in Maxdrinks between those with the GG genotype and those with the AA/AG genotype was 3.22. Among participants who did not experience childhood adversity, the adjusted mean difference in Maxdrinks by genotype was only 0.80. The interaction contrast [IC] (difference in differences) was 2.42 (Table 2, Figure 1a).
Table 2.
Additive effects of childhood adversity and ADH1B-rs1229984 on Maxdrinks in 1,143 ever-drinkers
| Childhood Adversity | ADH1B genotype | Maxdrinks | Additive Interaction | |
|---|---|---|---|---|
|
| ||||
| Meana (95%CI) | Mean Difference (95% CI)b | p-value | ||
| No (N=963) | AA/AG (N=456) | 4.28 (3.88, 4.69) | 0.80 (0.26, 1.35) | <0.01 |
| GG (N=507) | 5.09 (4.66, 5.51) | |||
| Mean Difference (95% CI)b | p-value | |||
|
| ||||
| Yes (N=180) | AA/AG (N=86) | 4.55 (3.56, 5.54) | 3.22 (1.56, 4.88) | <0.001 |
| GG (N=94) | 7.77 (6.45, 9.10) | |||
| Interaction Contrastc | p-value | |||
| 2.42 (0.68, 4.16) | <0.01 | |||
|
| ||||
| Childhood Adversity | ADH1B genotype | AUD severity | Additive Interaction | |
| Meana (95% CI) | Mean Difference (95% CI)b | p-value | ||
|
| ||||
| No (N=963) | AA/AG (N=456) | 1.27 (1.13, 1.41) | 0.17 (0.00, 0.34) | <0.10 |
| GG (N=507) | 1.44 (1.30, 1.58) | |||
| Mean Difference (95% CI)b | p-value | |||
|
| ||||
| Yes (N=180) | AA/AG (N=86) | 1.59 (1.19, 1.98) | 0.92 (0.30, 1.54) | <0.001 |
| GG (N=94) | 2.51 (2.03, 2.99) | |||
|
| ||||
| Interaction Contrastc | p-value | |||
| 0.75 (0.11, 1.39) | <0.01 | |||
Minor allele frequency=28.2%;
Maxdrinks = greatest number of drinks in a 24-hour period, during period of heaviest drinking;
AUD severity = number of DSM-IV alcohol dependence and abuse criteria;
CI = confidence interval
adjusted for age, sex, immigrant from the former Soviet Union status, using overdispersed Poisson regression with identity link function
Difference between mean for ADH1B genotype GG vs. AA/AG, calculated on the mean (additive) scale using overdispersed Poisson regression with identity link function
Difference of the mean differences; additive interaction is indicated when the IC for the childhood adversity group vs. the no adversity group is significantly greater than 0.
Figure 1.
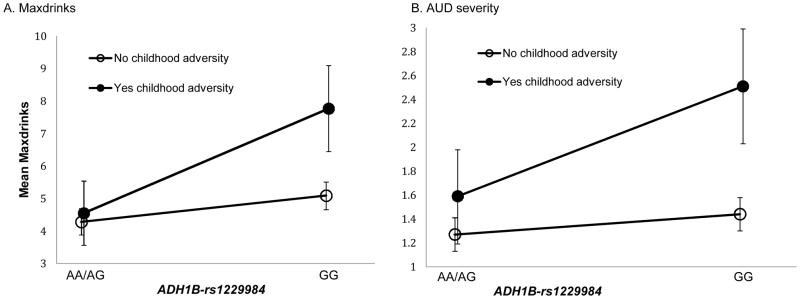
Additive interaction between childhood adversity and ADH1B-rs1229984 on (a) Maxdrinks and (b) AUD severity in 1,143 ever-drinkers
AUD severity, main effects
Childhood adversity showed a significant association with AUD severity (Table 1). The adjusted mean AUD severity (number of criteria) was 0.71 greater in those who experienced childhood adversity compared to those who did not. In addition, ADH1B-rs1229984 was significantly associated with AUD severity (Table 1). The adjusted mean AUD severity was 0.27 greater in the GG group than in the AA/AG group.
AUD severity, additive interaction
Childhood adversity and ADH1B-rs1229984 showed a significant additive interaction effect on AUD severity (p-value<0.05). Among participants who experienced childhood adversity, the adjusted mean difference in AUD severity between those with the GG genotype and those with the AA/AG genotype was 0.92. Among participants who did not experience childhood adversity, the adjusted mean difference in AUD severity by genotype was only 0.17. The interaction contrast [IC] (difference in differences) was 0.75 (Table 2, Figure 1b).
Discussion
This is the first study to demonstrate an interaction between ADH1B-rs1229984 and childhood adversity, a stressful environmental risk factor, which each show robust main effects on alcohol phenotypes (Maxdrinks and AUD severity). In an Israeli household sample, we observed that childhood adversity moderated the ADH1B-rs1229984 effect on alcohol use and problems, in that there was a stronger effect of the ADH1B-rs1229984 on heavy alcohol consumption and AUD severity among those who had experienced childhood adversity.
ADH1B x Childhood Adversity
Similar to previous studies examining interaction between childhood adversity and genes affecting neuronal processes putatively related to substance addiction (Caspi et al., 2003; Kaufman et al., 2007), we found that both ADH1B-rs1229984 (higher-risk genotype, i.e. lack of the protective allele) and childhood adversity together increase risk for problematic alcohol use beyond the influence of each risk factor individually. Specifically, ADH1B-rs1229984 has a significantly greater impact on problematic drinking among individuals who experienced childhood adversity than among those who did not. We propose this is due to the direct impact of ADH1B on alcohol metabolism and, consequently, consumption. Since the ADH1B-rs1229984 protective A allele leads to an enzyme with greater activity than the more common variant, individuals with the AA and AG genotypes may limit alcohol consumption because of subtle adverse effects that accompany the degradation of ethanol (Hurley & Edenberg, 2012). Therefore, ADH1B-rs1229984 appears to have a stronger effect on alcohol consumption, and consequently the progression to AUDs, among those with childhood adversity, a group in which alcohol consumption would likely be increased. We considered the effect of ADH1B-rs1229984 on alcohol phenotypes in the presence or absence of childhood adversity (instead of the effect of adversity in the presence or absence of the GG genotype) to be consistent with much of the gene-environment interaction literature, which considers environmental moderation of a genetic effect (Brody et al., 2007, Kaufman et al., 2006, Madrid et al., 2001, Dick et al., 2011, van der Zwaluw CS et al., 2012). However, due to the “agnostic” nature of statistical interaction analysis, results also support the alternative interpretation that ADH1B-rs1229984 moderates the effects of childhood adversity on risk for alcohol-related phenotypes in this sample.
Etiological Implications
AUDs develop via complex processes beginning with (1) drinking initiation, followed by (2) regular consumption, progressing to (3) heavier and potentially maladaptive consumption, culminating in (4) AUD criteria. Twin studies indicate that each of these steps is influenced by shared and unique environmental and genetic risk factors (Dick & Kendler, 2012b; Slutske et al., 1998). Childhood adversity increases risk for earlier age of initiation (Kilpatrick et al., 2000), most likely due to associated risk factors, such as increased access to alcohol and poor parental monitoring (Young-Wolff et al., 2011). As one transitions to regular consumption followed by heavier alcohol consumption, genes directly affecting alcohol metabolism (e.g. ADH1B) become increasingly relevant (Kendler et al., 2011). For example, ADH1B-rs1229984 can impact alcohol consumption through a physiological response to alcohol metabolism, potentially leading individuals with the protective A allele to drink less regularly or heavily, even after exposure to other strong risk factors such as childhood adversity. Furthermore, regular heavy consumption provides alcohol to the brain, where alcohol exerts its addictive effects via a cascade of biochemical actions, mainly encoded by neuronal genes (Heinz et al., 2003), which promote the progression to experiencing AUD criteria. Childhood adversity may also lead to psycho-biological changes that promote maladaptive coping strategies, such as stress-related drinking, and heighten sensitivity to genetic predispositions (Enoch, 2011), further increasing risk for heavier consumption and AUDs. Longitudinal studies should formally assess the exact roles of childhood adversity, ADH1B and other genes in this progression, as such relationships are beyond the scope of this study.
Strengths and Limitations
Study limitations are noted. First, studies of a specific gene have the advantage of providing information about that gene, but largely ignore other genes potentially involved (Dick & Kendler, 2012b). However, studying a strong candidate gene provides specific information regarding the mechanism by which a gene influences an outcome. Second, most studies of adult populations use retrospective reporting of adverse childhood events (Hardt & Rutter, 2004). However, self-reports may be unstable over time (Fergusson, Horwood, & Woodward, 2000; Polanczyk et al., 2009), resulting in underestimation of childhood adversity prevalence. Thus, our retrospective items of childhood abuse and neglect are a potential limitation of this study. However, this bias is likely insufficient to invalidate retrospective studies of easily defined major adversities (Hardt & Rutter, 2004) such as parental divorce. Furthermore, as parental divorce showed higher prevalence than abuse or neglect, we examined the association between parental divorce and both alcohol phenotypes. Parental divorce alone showed a weaker relationship to the alcohol phenotypes (β [MaxDrinks]=0.121, p-value<0.01; β [AUD severity]=0.122, p-value<0.01) than the combined abuse/neglect/parental divorce variable (β [MaxDrinks]=0.136, p-value<0.001; β [AUD severity]=0.139), p-value<0.001); Therefore, results do not appear to be driven by the effects of divorce alone. In addition, while some epidemiologic studies suggest that family history of alcohol problems may confound the relationship between childhood adversity and adult alcohol disorders (Bulik et al., 2001; Sher et al., 1997), others indicated a persistent relationship between childhood adversity and alcohol disorders after adjusting for family history of alcoholism (Young-Wolff et al., 2011). AUDADIS modules assessed history of alcohol problems in fathers and mothers, using examples of AUD diagnostic criteria including readily observable manifestations, which are mostly likely to be known to offspring (Andreasen, Endicott, Spitzer, & Winokur, 1977; Heiman, Ogburn, Gorroochurn, Keyes, & Hasin, 2008; Slutske et al., 1996). The test–retest reliability of AUDADIS family history variables is very good to excellent (Grant et al., 2003; Hasin et al., 1997). The binary parental history variable was coded as “yes” if alcohol problems were reported for either the father or mother, since the frequency of a maternal history alone or of two parents with a history of problems was very low. Prevalence of a parental history of alcohol problems was ~16%; 24% of participants who had experienced childhood adversity also reported a parental history of alcohol problems, compared with 14% of those who had not experienced childhood adversity. Secondary analyses adjusting for parental history of alcohol problems in the relationship between childhood adversity and alcohol phenotypes yielded results that were virtually unchanged. Further, a concern that associations could be confounded by parental alcohol problems is addressed in part by ADH1B-rs1229984 genotype GG not being associated with the adversity group (p=0.90), or with parental history of alcohol problems (p=0.44). The lack of association between genotype GG and parental alcoholism is most likely due to the low rates of alcohol consumption (and related problems) in the parental cohort of older Israelis (Levav et al., 1993). Finally, the interaction results described in this study should be replicated in additional well-powered samples.
Additional study strengths are noted. Since the lack of robust genetic main associations is a major challenge for GxE research in psychiatric disorders (Duncan & Keller, 2011), we investigated a genetic risk factor with such a robust effect, which yielded positive GxE results. Second, ADH1B directly affects alcohol metabolism, which is a pathway to alcohol-related phenotypes that is better understood than the genes affecting neuronal pathways that have been the main focus of previous SUDs G×E literature (Young-Wolff et al., 2011). Finally, we used graded alcohol phenotype variables (Maxdrinks and AUD severity). These typically have more statistical power to detect associations (Kuo et al., 2008; Waldman et al., 1999), and our use of them is consistent with a general movement in psychiatry to address dimensional rather than binary phenotypes (Ehlke et al., 2012; Hasin et al., 2012)., These strengths enhance the possibility of successful replication in a similarly powered sample (i.e., moderate ADH1B-rs1229984 prevalence and informative phenotypes), which has been challenging in GxE studies of SUDs (Duncan & Keller, 2011).
In conclusion, we examined the influence of a robust genetic factor that has direct effects on alcohol consumption and a well-validated environmental risk factor on informative alcohol outcomes in a large general population sample of adult Israeli Jews. This study provides evidence for the interaction between the genetic variant (ADH1B-rs1229984) and early adversity on alcohol-related phenotypes, and proposes a role for the interaction within the multi-factorial progression from initiation of alcohol use to disorder. Since individuals with pervasive environmental risks (e.g. childhood adversity) are more susceptible to the psychological, environmental and genetic factors that promote the progression to AUDs, prevention efforts should target development of healthy and effective alternative coping strategies to stress-related drinking among vulnerable individuals.
Supplementary Material
Acknowledgments
This research was funded by National Institutes of Health grants R01AA013654, R01DA018652, K05AA014223 (Hasin), K23DA016743 (Aharonovich), K01AA021511 (Keyes), T32MH13043 (Meyers), and the New York State Psychiatric Institute (Hasin, Wall). In addition, we would like to acknowledge Dr. Xiaoling Xuei for help with genotyping.
Footnotes
Authors Contribution
DH, JLM, and DS were responsible for the study concept and design, statistical analysis, and manuscript preparation. DH, EA, BS, AW, and AF were responsible for Israeli Household data collection. DH, KMK, EA, BS, AW, AF and BGF were responsible for critical manuscript editing for important intellectual content. HE and JG were responsible for all genotyping as well critical interpretation of results and manuscript editing for important intellectual content. MMW assisted with statistical analysis, interpretation, and manuscript editing. All authors critically reviewed content and approved final version for publication.
Reference List
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Archives of general psychiatry. 1977;34(10):1229–35. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Bensley LS, Spieker SJ, Van Eenwyk J, Schoder J. Self-reported abuse history and adolescent problem behaviors. II. Alcohol and drug use. The Journal of adolescent health_: official publication of the Society for Adolescent Medicine. 1999;24(3):173–80. doi: 10.1016/s1054-139x(98)00112-8. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Goate AM, Breslau N, Johnson EO, Bertelsen S, Fox L, Agrawal A, et al. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol Psychiatry. 2012;17(4):445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Philibert RA, Chen Y, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child development. n.d;80(3):645–61. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Prescott CA, Kendler KS. Features of childhood sexual abuse and the development of psychiatric and substance use disorders. The British journal of psychiatry_: the journal of mental science. 2001;179:444–9. doi: 10.1192/bjp.179.5.444. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY) 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Dick DM, Kendler KS. The impact of gene-environment interaction on alcohol use disorders. Alcohol research_: current reviews. 2012a;34(3):318–24. [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Kendler KS. The impact of gene-environment interaction on alcohol use disorders. Alcohol research_: current reviews. 2012b;34(3):318–24. [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular psychiatry. 2008;13(3):334–47. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. The American journal of psychiatry. 2011;168(10):1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlke SJ, Hagman BT, Cohn AM. Modeling the dimensionality of DSM-IV alcohol use disorder criteria in a nationally representative sample of college students. Substance use & misuse. 2012;47(10):1073–85. doi: 10.3109/10826084.2012.676698. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Woodward LJ. The stability of child abuse reports: a longitudinal study of the reporting behaviour of young adults. Psychological medicine. 2000;30(3):529–44. doi: 10.1017/s0033291799002111. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71(1):7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The Alcohol Use Disorder and Associated Disabilities Interview schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend. 1995;39(1):37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of child psychology and psychiatry, and allied disciplines. 2004;45(2):260–73. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Hatzenbuehler ML, Aharonovich EA, Alderson D. Alcohol consumption and posttraumatic stress after exposure to terrorism: effects of proximity, loss, and psychiatric history. Am J Public Health. 2007;97(12):2268–2275. doi: 10.2105/AJPH.2006.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Aharonovich E, Liu X, Mamman Z, Matseoane K, Carr L, Li TK. Alcohol and ADH2 in Israel: Ashkenazis, Sephardics, and recent Russian immigrants. Am J Psychiatry. 2002;159(8):1432–1434. doi: 10.1176/appi.ajp.159.8.1432. [DOI] [PubMed] [Google Scholar]
- Hasin D, Aharonovich E, Liu X, Mamman Z, Matseoane K, Carr Lg, et al. Alcohol dependence symptoms and alcohol dehydrogenase 2 polymorphism: Israeli Ashkenazis, Sephardics, and recent Russian immigrants. Alcohol Clin Exp Res. 2002;26(9):1315–1321. doi: 10.1097/01.ALC.0000029597.07916.A9. [DOI] [PubMed] [Google Scholar]
- Hasin D, Grant BF, Cottler L, Blaine J, Towle L, Ustun B, Sartorius N. Nosological comparisons of alcohol and drug diagnoses: a multisite, multi-instrument international study. Drug Alcohol Depend. 1997;47(3):217–226. doi: 10.1016/s0376-8716(97)00092-6. [DOI] [PubMed] [Google Scholar]
- Hasin D, Rahav G, Meydan J, Neumark Y. The drinking of earlier and more recent Russian immigrants to Israel: comparison to other Israelis. J Subst Abuse. 1998;10(4):341–353. doi: 10.1016/s0899-3289(99)00010-3. [DOI] [PubMed] [Google Scholar]
- Hasin Deborah S, Fenton MC, Beseler C, Park JY, Wall MM. Analyses related to the development of DSM-5 criteria for substance use related disorders: 2. Proposed DSM-5 criteria for alcohol, cannabis, cocaine and heroin disorders in 663 substance abuse patients. Drug and alcohol dependence. 2012;122(1–2):28–37. doi: 10.1016/j.drugalcdep.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman GA, Ogburn E, Gorroochurn P, Keyes KM, Hasin D. Evidence for a two-stage model of dependence using the NESARC and its implications for genetic association studies. Drug and alcohol dependence. 2008;92(1–3):258–66. doi: 10.1016/j.drugalcdep.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schäfer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry. 2003;36(Suppl 3):S255–8. doi: 10.1055/s-2003-45139. [DOI] [PubMed] [Google Scholar]
- Hurley TD, Edenberg HJ. Genes encoding enzymes involved in ethanol metabolism. Alcohol research_: current reviews. 2012;34(3):339–44. [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biological psychiatry. 2007;61(11):1228–34. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological psychiatry. 2006;59(8):673–80. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological medicine. 2011;41(7):1507–16. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler Kenneth S, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Archives of General Psychiatry. 2007;64(11):1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological medicine. 1997;27(5):1101–19. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology. 2011;218(1):1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of consulting and clinical psychology. 2000;68(1):19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Kalsi G, Prescott CA, Hodgkinson CA, Goldman D, Van den Oord EJ, Alexander J, et al. Association of ADH and ALDH genes with alcohol dependence in the Irish Affected Sib Pair Study of alcohol dependence (IASPSAD) sample. Alcohol Clin Exp Res. 2008;32(5):785–795. doi: 10.1111/j.1530-0277.2008.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levav I, Kohn R, Dohrenwend BP, Shrout PE, Skodol AE, Schwartz S, Link BG, et al. An epidemiological study of mental disorders in a 10-year cohort of young adults in Israel. Psychological medicine. 1993;23(3):691–707. doi: 10.1017/s0033291700025472. [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70(6):504–512. doi: 10.1016/j.biopsych.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE. Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol (Fayetteville, NY) 2001;23(2):117–22. doi: 10.1016/s0741-8329(00)00138-5. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Human genetics. 2012;131(6):779–89. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Schmulewitz Dvora Aharonovich, Efrat Waxman R, Frisch A, Weizman A, Spivak B, Edenberg HJ, Gelernter J, et al. Alcohol metabolizing genes and alcohol-related traits in an Israeli household sample. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modern Epidemiologic Approaches to Interaction: Applications to the Study of Genetic Interactions. National Academies Press (US); 2006. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK19918/ [Google Scholar]
- Neumark YD, Friedlander Y, Durst R, Leitersdorf E, Jaffe D, Ramchandani VA, O’Connor S, et al. Alcohol dehydrogenase polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res. 2004;28(1):10–14. doi: 10.1097/01.ALC.0000108667.79219.4D. [DOI] [PubMed] [Google Scholar]
- Neumark YD, Friedlander Y, Thomasson HR, Li TK. Association of the ADH2*2 allele with reduced ethanol consumption in Jewish men in Israel: a pilot study. J Stud Alcohol. 1998;59(2):133–139. doi: 10.15288/jsa.1998.59.133. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Archives of general psychiatry. 2009;66(9):978–85. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Research. 2009;69(17):6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction (Abingdon, England) 2007;102(2):216–25. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gershuny BS, Peterson L, Raskin G. The role of childhood stressors in the intergenerational transmission of alcohol use disorders. Journal of studies on alcohol. 1997;58(4):414–27. doi: 10.15288/jsa.1997.58.414. [DOI] [PubMed] [Google Scholar]
- Shmulewitz D, Keyes K, Beseler C, Aharonovich E, Aivadyan C, Spivak B, Hasin D. The dimensionality of alcohol use disorders: results from Israel. Drug Alcohol Depend. 2010;111(1–2):146–154. doi: 10.1016/j.drugalcdep.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmulewitz D, Wall MM, Keyes KM, Aharonovich E, Aivadyan C, Greenstein E, Spivak B, et al. Alcohol use disorders and perceived drinking norms: ethnic differences in israeli adults. J Stud Alcohol Drugs. 2012;73(6):981–990. doi: 10.15288/jsad.2012.73.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, et al. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of abnormal psychology. 1998;107(3):363–74. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Dunne MP, Statham DJ, et al. Reliability and reporting biases for perceived parental history of alcohol-related problems: agreement between twins and differences between discordant pairs. Journal of studies on alcohol. 1996;57(4):387–95. doi: 10.15288/jsa.1996.57.387. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer causes & control_: CCC. 2007;18(5):571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- Spiegelman Donna, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- Spivak B, Frisch A, Maman Z, Aharonovich E, Alderson D, Carr LG, Weizman A, et al. Effect of ADH1B genotype on alcohol consumption in young Israeli Jews. Alcohol Clin Exp Res. 2007;31(8):1297–1301. doi: 10.1111/j.1530-0277.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Robinson BF, Rowe DC. A logistic regression based extension of the TDT for continuous and categorical traits. Annals of human genetics. 1999;63(Pt 4):329–40. doi: 10.1046/j.1469-1809.1999.6340329.x. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Zhang H, Oslin D, Anton RF, Farrer LA, Gelernter J. Childhood adversity increases risk for nicotine dependence and interacts with α5 nicotinic acetylcholine receptor genotype specifically in males. Neuropsychopharmacology_: official publication of the American College of Neuropsychopharmacology. 2012;37(3):669–76. doi: 10.1038/npp.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Chang CC, Lin CY, Chen CL, Fann CSJ. A genome-wide scanning and fine mapping study of COGA data. BMC genetics. 2005;6(Suppl 1):S30. doi: 10.1186/1471-2156-6-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Mizukami T, Matsui T, Yokoyama T, Kimura M, Matsushita S, Higuchi S, et al. Genetic Polymorphisms of Alcohol Dehydrogenase-1B and Aldehyde Dehydrogenase-2 and Liver Cirrhosis, Chronic Calcific Pancreatitis, Diabetes Mellitus, and Hypertension Among Japanese Alcoholic Men. Alcoholism, clinical and experimental research. 2013 doi: 10.1111/acer.12108. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, Prescott CA. The influence of gene-environment interactions on alcohol consumption and alcohol use disorders: a comprehensive review. Clinical psychology review. 2011;31(5):800–16. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang F, Kranzler HR, Zhao H, Gelernter J. Profiling of childhood adversity-associated DNA methylation changes in alcoholic patients and healthy controls. PloS one. 2013;8(6):e65648. doi: 10.1371/journal.pone.0065648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


