The genus Moraxella comprises approximately 20 species of aerobic, non-motile, oxidase-positive, and gram-negative coccobacilli. Besides M. catarrhalis, a well-known pathogen, M. atlantae, M. canis, M. lacunata, M. lincolnii, M. nonliquefaciens, and M. osloensis are also occasionally isolated from clinical samples [1]. M. osloensis is a commensal microorganism in the human respiratory tract, but has also been reported as a rare causative pathogen in human infections [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. Here, we report a case of M. osloensis bacteremia in a patient with a hematologic malignancy. To our knowledge, this is the first report of M. osloensis bacteremia in Korea.
A 66-yr-old man with acute myeloid leukemia (AML) was admitted to our hospital for consolidation chemotherapy. Two months prior to this hospitalization, the patient was diagnosed with AML, not otherwise specified, according to the 2008 WHO classification system [13], and he subsequently received cytarabine and idarubicin chemotherapy. After 1 month, follow-up bone marrow examination was performed, and he achieved complete remission.
On admission, the patient presented with symptoms of an upper respiratory infection including coughing and chills. He had a temperature of 37.2℃, a blood pressure of 117/57 mmHg, a pulse of 106/min, and a respiration rate of 20 breaths/min. The results of the laboratory investigation were as follows: Hb, 11.7 g/dL; leukocyte count, 5.7×109/L; platelet count, 177×109/L; C-reactive protein level, 6.48 mg/dL; blood urea nitrogen (BUN)/creatinine, 15/1.2 mg/dL; and total protein/albumin, 6.8/3.6 g/dL. A total of four blood culture sets were collected from two separate peripheral veins and central venous catheter. There were no abnormalities in the chest radiographic images, but paranasal sinus radiographic imaging indicated a suspected maxillary sinusitis. Therefore, empirical antibiotic therapy with intravenous ampicillin-sulbactam was administered.
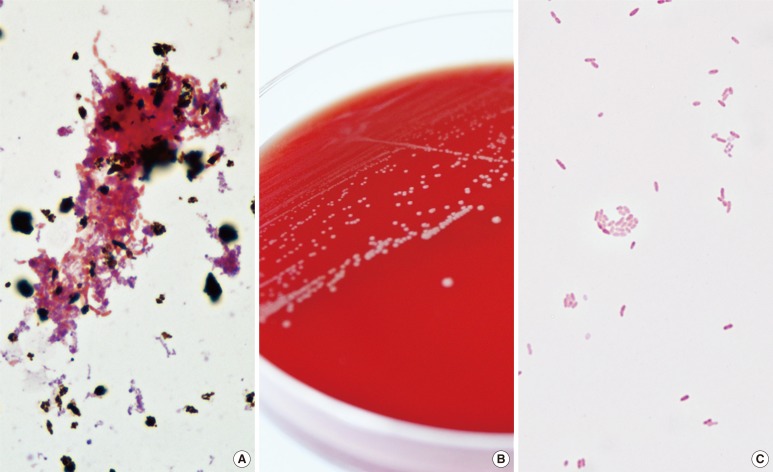
Bacterial growth was detected in an aerobic culture bottle that contained the blood from the central venous catheter after 1 day of incubation, and in the other 3 aerobic culture bottles after 2 days of incubation (Fig. 1A). The broths from positive culture bottles were inoculated onto blood agar plates (BAPs) and MacConkey agar plates (MACs) that were subsequently incubated for 24 hr at 35℃ in a 5% CO2 atmosphere. Whereas no colony was observed on the MACs, grey-white colored, non-hemolytic colonies grew on the BAPs (Fig. 1B) and gram-negative coccobacilli were observed from gram stain smear preparations (Fig. 1C). The isolates were oxidase-positive and indole-negative.
Fig. 1.
Colonial and microscopic morphology of Moraxella osloensis. (A) Gram-negative coccobacilli from positive aerobic blood culture smear preparations (Gram stain, ×1,000). (B) Grey-white colored colonies on blood agar plate. (C) Gram-negative coccobacilli from blood agar plate smear preparations (Gram stain, ×1,000).
Using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonik GmbH, Bremen, Germany) and Vitek2 GN system (bioMérieux, Marcy l'Etoile, France), the organism was suboptimally identified as M. osloensis, with a score of 1.885 in the Bruker system and with a 50% probability in the Vitek2 system.
To confirm the identity of the isolate, 16S rRNA sequence analysis was performed on the MicroSeq 500 system (Applied Biosystems, Foster City, CA, USA), using PCR and sequencing kits with universal primers designed to cover all bacteria. The sequences were analyzed with an ABI PRISM 3730 Series DNA Analyzer (Applied Biosystems). The first 500 bp of the 16S rRNA gene sequence from the isolate shared 99.6% identity with the GenBank sequence AY043376 (M. osloensis) and 99.0% identity with the GenBank sequence KC494325 (Enhydrobacter aerosaccus).
Antimicrobial susceptibility was tested with the AST-N222 card from the Vitek 2 system (bioMérieux). Using the CLSI breakpoints for other non-Enterobacteriaceae for the interpretation [14], the isolate was determined susceptible to aztreonam, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, meropenem, piperacillin, and trimethoprim-sulfamethoxazole.
Intravenous ampicillin-sulbactam treatment was maintained, and follow-up blood cultures from the peripheral veins and central venous catheter on day 3 were negative. The patient was successfully treated with a 10-day course of intravenous ampicillin-sulbactam and subsequently began consolidation chemotherapy for AML.
Despite the unique biochemical characteristics of M. osloensis, which include acetate alkalinization, ethylene glycol acidification, and desferrioxamine resistance, species-level biochemical identification is difficult because of the overall lack of biochemical differences among the Moraxella species [1]. Although only 2 reported isolates besides the present case have been identified by MALDI-TOF MS, this method suboptimally identified M. osloensis; one isolate was identified as M. osloensis with a score of <1.4 in the Bruker system [15], and the other was identified as M. osloensis without score information in the Bruker system, but as Moraxella sp. in the SARAMIS system [16]. The present isolate analyzed by Bruker system showed better score than previous cases but species-level identification for M. osloensis by MALDI-TOF MS still seem to be insufficient. At present, 16S rRNA sequence analysis is the only reliable method that can be used for species-level identification [2, 3, 4, 5, 6, 7, 8, 9]. With this method, the blood isolate in the present case was confirmed to be M. osloensis.
Although M. osloensis is a rare pathogen in humans, it can be involved in bacteremia, central venous catheter-related infections, endocarditis, endophthalmitis, meningitis, osteomyelitis, pneumonia, and septic arthritis [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. In 2010, Roh et al. [7] reported 3 cases of M. osloensis meningitis which, besides the present case, have been the only cases reported to date in Korea. The most commonly reported M. osloensis infection is bacteremia in immunocompromised patients with malignancies [2, 6, 8, 9]. Accordingly, the present case involved M. osloensis bacteremia in an immunocompromised patient who had been diagnosed as AML.
Regarding treatment, M. osloensis is susceptible to penicillin, cephalosporins, and aminoglycosides [17, 18]. The isolated strain described in this report was susceptible to the majority of antimicrobial agents, and therefore the patient was successfully treated with a 10-day course of ampicillin-sulbactam.
In conclusion, we report the first case of M. osloensis bacteremia in Korea. The present findings indicate that M. osloensis can be an opportunistic pathogen in humans, especially in immunocompromised patients such as those with hematologic malignancies.
References
- 1.Vaneechoutte M, Dijkshoorn L, Nemec A, Kämpfer P, Wauters G. Acinetobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of clinical microbiology. 10th ed. Washington, D.C: American Society for Microbiology; 2011. pp. 714–738. [Google Scholar]
- 2.Han XY, Tarrand JJ. Moraxella osloensis blood and catheter infections during anticancer chemotherapy: clinical and microbiologic studies of 10 cases. Am J Clin Pathol. 2004;121:581–587. doi: 10.1309/QBB3-AVCM-GWA3-K1XK. [DOI] [PubMed] [Google Scholar]
- 3.Shah SS, Ruth A, Coffin SE. Infection due to Moraxella osloensis: case report and review of the literature. Clin Infect Dis. 2000;30:179–181. doi: 10.1086/313595. [DOI] [PubMed] [Google Scholar]
- 4.Vuori-Holopainen E, Salo E, Saxen H, Vaara M, Tarkka E, Peltola H. Clinical "pneumococcal pneumonia" due to Moraxella osloensis: case report and a review. Scand J Infect Dis. 2001;33:625–627. doi: 10.1080/00365540110026737. [DOI] [PubMed] [Google Scholar]
- 5.Berrocal AM, Scott IU, Miller D, Flynn HW., Jr Endophthalmitis caused by Moraxella osloensis. Graefes Arch Clin Exp Ophthalmol. 2002;240:329–330. doi: 10.1007/s00417-002-0449-z. [DOI] [PubMed] [Google Scholar]
- 6.Sifri CD, Brassinga AK, Flohr T, Kinchen JM, Hazen KC, Sawyer RG, et al. Moraxella osloensis bacteremia in a kidney transplant recipient. Transpl Int. 2008;21:1011–1013. doi: 10.1111/j.1432-2277.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 7.Roh KH, Kim CK, Koh E, Kim MS, Yong D, Park SC, et al. Three cases of Moraxella osloensis meningitis: a difficult experience in species identification and determination of clinical significance. J Korean Med Sci. 2010;25:501–504. doi: 10.3346/jkms.2010.25.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dien Bard J, Lewinski M, Summanen PH, Deville JG. Sepsis with prolonged hypotension due to Moraxella osloensis in a non-immunocompromised child. J Med Microbiol. 2011;60:138–141. doi: 10.1099/jmm.0.016378-0. [DOI] [PubMed] [Google Scholar]
- 9.Hadano Y, Ito K, Suzuki J, Kawamura I, Kurai H, Ohkusu K. Moraxella osloensis: an unusual cause of central venous catheter infection in a cancer patient. Int J Gen Med. 2012;5:875–877. doi: 10.2147/IJGM.S36919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarman B, Clarridge J. Osteomyelitis caused by Moraxella osloensis. J Clin Microbiol. 1982;15:1148–1149. doi: 10.1128/jcm.15.6.1148-1149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feigin RD, San Joaquin V, Middelkamp JN. Septic arthritis due to Moraxella osloensis. J Pediatr. 1969;75:116–117. doi: 10.1016/s0022-3476(69)80109-5. [DOI] [PubMed] [Google Scholar]
- 12.Stryker TD, Stone WJ, Savage AM. Renal failure secondary to Moraxella osloensis endocarditis. Johns Hopkins Med J. 1982;150:217–219. [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-third Informational supplement, M100-S23. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 15.Gray TJ, Thomas L, Olma T, Iredell JR, Chen SC. Rapid identification of Gram-negative organisms from blood culture bottles using a modified extraction method and MALDI-TOF mass spectrometry. Diagn Microbiol Infect Dis. 2013;77:110–112. doi: 10.1016/j.diagmicrobio.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Carbonnelle E, Grohs P, Jacquier H, Day N, Tenza S, Dewailly A, et al. Robustness of two MALDI-TOF mass spectrometry systems for bacterial identification. J Microbiol Methods. 2012;89:133–136. doi: 10.1016/j.mimet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Graham DR, Band JD, Thornsberry C, Hollis DG, Weaver RE. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953-1980. Rev Infect Dis. 1990;12:423–431. doi: 10.1093/clinids/12.3.423. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal SL, Freundlich LF, Gilardi GL, Clodomar FY. In vitro antibiotic sensitivity of Moraxella species. Chemotherapy. 1978;24:360–363. doi: 10.1159/000237808. [DOI] [PubMed] [Google Scholar]