Abstract
AIM: To investigate molecular phenotypes of myocardial B19V-infection to determine the role of B19V in myocarditis and dilated cardiomyopathy (DCM).
METHODS: Endomyocardial biopsies (EMBs) from 498 B19V-positive patients with myocarditis and DCM were analyzed using molecular methods and functional experiments. EMBs were obtained from the University Hospitals of Greifswald and Tuebingen and additionally from 36 German cardiology centers. Control tissues were obtained at autopsy from 34 victims of accidents, crime or suicide. Identification of mononuclear cell infiltrates in EMBs was performed using immunohistological staining. Anti-B19V-IgM and anti-B19V-IgG were analyzed by enzyme-linked immunosorbent assay (ELISA). B19V viral loads were determined using in-house quantitative real-time polymerase chain reaction (PCR). For B19V-genotyping a new B19V-genotype-specific restriction fragment length polymorphism (RFLP)-PCR was established. B19V-genotyping was verified by direct DNA-sequencing and sequences were aligned using BLAST and BioEdit software. B19V P6-promoter and HHV6-U94-transactivator constructs were generated for cell culture experiments. Transfection experiments were conducted using human endothelial cells 1. Luciferase reporter assays were performed to determine B19V-replication activity. Statistical analysis and graphical representation were calculated using SPSS and Prism5 software.
RESULTS: The prevalence of B19V was significantly more likely to be associated with inflammatory cardiomyopathy (iCMP) compared to uninflamed DCM (59.6% vs 35.3%) (P < 0.0001). The detection of B19V-mRNA replication intermediates proved that replication of B19V was present. RFLP-PCR assays showed that B19V-genotype 1 (57.4%) and B19V-genotype 2 (36.7%) were the most prevalent viral genotypes. B19V-genotype 2 was observed more frequently in EMBs with iCMP (65.0%) compared to DCM (35%) (P = 0.049). Although there was no significant difference in gender-specific B19V-loads, women were more frequently infected with B19V-genotype 2 (44.6%) than men (36.0%) (P = 0.0448). Coinfection with B19V and other cardiotropic viruses was found in 19.2% of tissue samples and was associated with higher B19V viral load compared to B19V-monoinfected tissue (P = 0.0012). The most frequent coinfecting virus was human herpes virus 6 (HHV6, 16.5%). B19V-coinfection with HHV6 showed higher B19V-loads compared to B19V-monoinfected EMBs (P = 0.0033), suggesting that HHV6 had transactivated B19V. In vitro experiments confirmed a 2.4-fold increased B19V P6-promoter activity by the HHV6 U94-transactivator.
CONCLUSION: The finding of significantly increased B19V loads in patients with histologically proven cardiac inflammation suggests a crucial role of B19V-genotypes and reactivation of B19V-infection by HHV6-coinfection in B19V-associated iCMP. Our findings suggest that B19V-infection of the human heart can be a causative event for the development of an endothelial cell-mediated inflammatory disease and that this is related to both viral load and genotype.
Keywords: Myocarditis, Dilated cardiomyopathy, Parvovirus B19, B19V-genotypes, B19V co-infection
Core tip: Human parvovirus B19 (B19V) has recently been shown to be an emerging pathogen for inflammatory cardiomyopathy (iCMP). We showed that B19V replication intermediates could be detected in acute and ongoing myocarditis. B19V-genotypes 1 and 2 were predominant although B19V-genotype 2 was more prevalent in iCMP. Further analyses revealed that B19V-coinfection with other cardiotropic viruses does occur, most frequently with human herpes virus 6 (HHV6). In vitro experiments showed that the HHV6 U94-transactivator element could transactivate the B19V-P6-promoter. We suggest that long-term persistence of B19V DNA in the human heart occurs and that active/reactivated B19V-replication can be associated with iCMP in a viral load and genotype-dependent manner.
INTRODUCTION
It has been shown that parvovirus B19 (B19V) infection of the myocardium can cause potentially lethal acute myocarditis in infants and adults[1-3]. Acute B19V-infection of endothelial cells is accompanied by the intravascular accumulation, adhesion and penetration of inflammatory cells in to vessel walls, leading to an impairment of the myocardial micro-circulation with secondary myocyte necrosis that can mimic myocardial infarction[1,2]. B19V has been found in high copy numbers in myocardial endothelial cells of small vessels but not in myocytes[1]. We recently reported that B19V-loads greater than 500 GE/μg of isolated nucleic acid identified in endomyocardial biopsies (EMBs) argue for virus-induced myocarditis. In contrast, a low viral load detected in uninflamed hearts has been associated with a latent-type of B19V-infection[4]. As expected, high viral loads of approximately 3 × 105 GE/μg were detected in acute myocardial B19V-infection, while approximately 700 GE/μg were found to be characteristically associated with chronic myocarditis[4]. Notably, a growing number of reports suggests an association between B19V-infection and the development of chronic myocarditis, as well as isolated endothelial and/or diastolic dysfunction[5-8]. However, the frequent detection of B19V genomes in EMBs of patients clinically suspected of having myocarditis and dilated cardiomyopathy (DCM) and the potential pathogenic role of B19V remains controversial and warrants studies to differentiate viral pathogenic effects from harmless latent B19V-infection[9,10].
Infection with human parvovirus B19 (B19V) is common, with approximately 70% to 90% of adolescents having anti-B19V IgG detectable in serum[11]. B19V-infection is usually benign and in children it most commonly manifests with erythema infectiosum (fifth disease)[12]. The genome of B19V consists of a single stranded DNA molecule of approximately 5.5 kb that contains three major open reading frames coding for the two capsid proteins VP1 and VP2 and the nonstructural protein NS1. Genome diversity divides the genus erythrovirus of the parvoviridae family into three pathogenic human genotypes: PVBAu (genotype 1), Lali-like (genotype 2) and V9-like (genotype 3) viruses[13]. The three erythrovirus genotypes show different geographical and temporal distributions. Whereas B19V-genotype 1 and 2 can be detected in most populations, genotype 3 seems to be prevalent only in Ghana, France and Brazil[13-15]. Interestingly, the age distribution of B19V-genotype 1 and 2 infections is different, with B19V-genotype 1 occurring most frequently in individuals born after 1955 while B19V-genotype 2 is predominantly found in individuals older than 50 years[16,17].
Recent reports have indicated that coinfection with different cardiotropic viruses of the human heart is common[7,8,18,19]. Human herpes virus 6 (HHV6) has been identified as an important coinfecting pathogen with B19V of the myocardium and resulting in fatal myocarditis in infants[1,20]. It has been reported that HHV6 is able to transactivate human immunodeficiency virus (HIV) and human cytomegalovirus (HCMV)[19,21].
In the present study, we explored molecular phenotypes of myocardial B19V-infection in association with patient age, gender, B19V replicative mRNA intermediates, B19V genotype and B19V-coinfection to gain further insight into the pathogenesis of B19V-myocarditis as an endothelial-cell mediated inflammatory disease.
MATERIALS AND METHODS
Ethical approval
The study was approved by the ethics committee of the University Hospital of Tuebingen (297/2005). All patients gave written informed consent for EMB analysis to investigate a possible etiology for their disease.
Study population
This cardiopathological clinical and experimental study was designed as a retrospective evaluation of B19V-positive EMBs of 498 consecutive patients (341 male, 157 female, mean age 46.9 ± 15.85 years, ejection fraction < 45%, 2-3 biopsies/patient) with histologically proven myocarditis and DCM who were diagnosed at our institution between 2003 and 2010 (Table 1). In addition to the University Hospitals of Greifswald and Tuebingen, EMBs were obtained from 36 German cardiology centers. Control tissues were obtained at autopsy from 34 victims of accidents, crime or suicide (median age 29 years, kindly provided by Professor Dr. Wehner, Institute of Forensic Medicine, University of Tuebingen). In addition, myocardial tissue obtained from 57 unselected consecutive autopsies at our institute from patients (median age 67.2 years) dying of cardiovascular, cardiopulmonary or tumor-related diseases served as control tissue samples after exclusion of myocarditis and DCM.
Table 1.
Baseline characteristics of the study population n (%)
| Characteristic | Value1 | n3 |
| Age, yr | 46.9 ± 15.82 | 498 |
| Male | 341 (68.5) | 498 |
| Female | 157 (31.5) | 498 |
| Molecular findings | ||
| B19V-genotype 1 | 286 (57.4) | 498 |
| B19V-genotype 2 | 183 (36.7) | 498 |
| Endomyocardial biopsy results4 | ||
| Acute myocarditis | 25 (5.0) (3.2 × 105 GE/μg)5 | 498 |
| Inflammatory cardiomyopathy | 297 (59.6) (709 GE/μg)5 | 498 |
| Dilated cardiomyopathy | 176 (35.3) (392 GE/μg)5 | 498 |
| Uninflamed control hearts | ||
| B19V-detection | 7 (7.7) (84 GE/μg)5 | 91 |
| Age (at death), yr | 48.1 ± 20.82 | 91 |
| Male | 49 (53.9) | 91 |
| Female | 42 (46.1) | 91 |
Values are number, absolute and relative frequency of patients;
Values are expressed as mean and ± SD; 3Total number of patients;
Histopathology according to the Dallas criteria[41] supplemented by immunohistochemistry for detection of CD3-positive T-lymphocytes, CD68-positive macrophages and natural killer cells, and HLA class II expression in professional antigen-presenting immune cells as described[18];
Values are expressed as mean of B19V-genome equivalents per microgram isolated nucleic acids[4].
Immunohistochemistry and serological testing
Immunohistological staining of paraffin-embedded tissue sections was performed using an avidin-biotin-immunoperoxidase method according to the manufacturer’s protocol (Vectastain Elite ABC Kit, Vector, Burlingame, California). The following monoclonal antibodies were used for identification of mononuclear cell infiltrates: CD3 for T cells (Novocastra Laboratories, Newcastle on Tyne, United Kingdom), PGM1 (CD68) for macrophages and natural killer cells, and HLA-DR-Antigen, alpha chain (DAKO, Hamburg, Germany) to assess HLA class II expression on professional antigen-presenting immune cells. According to the World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies, EMBs were considered to have significant inflammation if immunohistochemical staining revealed the presence of focal or diffuse mononuclear infiltrates with > 14 leukocytes per mm2 (CD3+ T lymphocytes and/or CD68+ macrophages) in the myocardium, in addition to enhanced expression of HLA class II molecules[1].
Anti-B19V-IgM and anti-B19V-IgG (VP1/VP2) were analyzed by enzyme-linked immunosorbent assay (ELISA) (Parvovirus B19-IgM; B19-IgG, DxSelect™ FocusDiagnostics, Germany) according to the manufacturer’s instructions.
Nucleic acids extraction from EMBs and polymerase chain reaction amplification of viral genomes
Nucleic acids from RNAlater (Qiagen, Hilden, Germany) fixed EMBs and of controls from formalin fixed tissue were extracted as described previously[1,6]. Polymerase chain reaction (PCR) and reverse transcriptase (RT)-PCR was performed to detect parvovirus B19 (B19V), enteroviruses (EV) (including coxsackieviruses and echoviruses), adenoviruses (ADV), HCMV, Epstein-Barr virus (EBV), and human herpesvirus 6 (HHV6) as previously described[1]. B19V mRNA was detected using nucleic acids isolated from EMBs. After extensive RNase-free-DNase digestion (20 U; Qiagen, Hilden, Germany) of 30 μL nucleic acid solution for 2 h at 37 °C, the DNAse was inactivated for 15 min at 75 °C. 5 μL of the DNAse-digested samples were analyzed for removal of B19V DNA by B19V-specific PCR using primer pairs PVB3 and PVB4 and nested PCR primer pairs PVB1 and PVB2 as previously described (Table 2)[1]. RT-PCR for the detection of B19V-RNA was performed using a one-step RT-PCR reaction kit (Qiagen, Hilden, Germany) and the following primer pairs: first/RT-PCR NS-25 and NS-30 and nested PCR NS-27 and NS-32 (Table 2). RT-PCR reaction was done at 50 °C for 30 min followed by 95 °C for 15 min. PCR was for 35 cycles at 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 45 s, followed by a final extension for 5 min at 72 °C. Nested PCR was performed with an initial denaturation step at 95 °C for 2 min followed by 29 cycles at 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 45 s, followed by a final extension for 5 min at 72 °C. Five μL of each reaction was analyzed using RNase-free agarose gel electrophoresis. Sample processing (DNA/RNA-extraction, template preparation, master-mix preparation) and PCR were done in separate laboratory rooms, which are all certified for molecular diagnostics using standard precautions to prevent assay contamination.
Table 2.
Primer sequences
| No | Primer name | Sequences (5’ to 3’) | Position (numbering according M13178) | 1st, 2nd (RT/RFLP)-PCR |
| 1 | PVB1 | GCTAACTCTGTAACTTGTAC | 3221-3240 | Sense B19V-VP2-PCR (2nd PCR) |
| 2 | PVB2 | AAATATCTCCATGGGGTTGAG | 3373-3393 | As B19V-VP2-PCR (2nd PCR) |
| 3 | PVB3 | AGCATGTGGAGTGAGGGGGC | 3191-3210 | Sense B19V-VP2-PCR (1st PCR) |
| 4 | PVB4 | AAAGCATCAGGAGCTATACTTCC | 3458-3480 | As B19V-VP2-PCR (1st PCR) |
| 5 | NS-25 | AAATGCGTGGAAGTGTAGCT | 1628-1647 | Sense B19V-NS1-PCR (RT/1st PCR) |
| 6 | NS-27 | ATGCGTGGAAGTGTAGCTGT | 1630-1649 | As B19V-NS1-PCR (2nd PCR) |
| 7 | NS-30 | CCAACTAACAGTTCACGAAAC | 2172-2192 | Sense B19V-NS1-PCR (RT/1st PCR) |
| 8 | NS-32 | TAACAGTTCACGAAACTGGTC | 2168-2187 | As B19V-NS1-PCR (2nd PCR) |
| 9 | NS-38 | ATTCCACAAATTGCTGATACAC | 2498-2519 | As RFLP-PCR (1st PCR) |
| 10 | NS-40 | AATTGCTGATACACAGCTTTAG | 2490-2511 | As RFLP-PCR (2nd PCR) |
| 11 | G2170 | CAGTTTCGTGAACTGTTAGT | 2170-2189 | Sense RFLP-PCR (1st PCR) |
| 12 | G2176 | CGTGAACTGTTAGTTGGGGTTGA | 2176-2198 | Sense RFLP-PCR (2nd PCR) |
As: Antisense; RFLP: Restriction fragment length polymorphism; RT-PCR: Reverse transcriptase-polymerase chain reaction.
Quantitative real-time PCR
B19V viral load was determined using quantitative real-time PCR (qPCR) and calculated according to genome equivalents per microgram isolated myocardial nucleic acid (GE/μg) as described previously[1,4,6]. Dilutions of B19V plasmid DNA and the World Health Organization international B19V DNA standard (code 99/800) were included to standardize the assay. A qPCR of the adenosine triphosphate synthase-6 gene was performed as a control for the addition of equivalent amounts of human DNA as described previously[6]. All samples were analyzed in duplicate.
DNA sequence analysis and B19V genotype analysis
DNA fragments spanning the B19V NS1/VP1/VP2 coding region (nt 602 to 5014; 4413 nt; numbering according to GenBank accession no. AF162273) were amplified by PCR using primer pairs as described previously[22]. DNA sequencing was performed in duplicate with purified PCR products in 2 mL BigDye Terminator cycle sequencing mix (Perkin Elmer) and 15 pmol of forward and reverse primers as described previously[22]. B19V sequences were aligned using BLAST (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/ blast/blast.cgi). The reliability of alignment was checked using the BioEdit program (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Prototype B19V sequences from GenBank were used as reference sequences (GenBank accession numbers: genotype 1: AB030694, AF113323, AF162273, M13178, DQ225148, DQ225149, DQ225150 and DQ225151; genotype 2: AY064476, AY044266, AY661663 and AY661664; genotype 3: AX003421 and AY083234).
Restriction fragment length polymorphism-PCR for B19V genotyping
In order to determine B19V genotypes, a restriction fragment length polymorphism PCR (RFLP-PCR) was developed using nested PCR. The following primer pairs were used for the first PCR, G2170F and NS-38, and for the second PCR, G2176F and NS-40 (Table 2, Figure 1). Reactions were initially denatured at 94 °C for 4 min followed by 35 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 30 s, followed by a final extension for 10 min at 72 °C. Nested PCR was performed using the primer pair G2176F and NS-40 (Table 2). DNA amplicons (343 bp) were digested with the restriction enzymes HpaI and TaqI in separate reactions (New England Biolabs, Frankfurt, Germany) for 30 min at 37 °C and analyzed by agarose gel electrophoresis. The restriction recognition site for HpaI is only present in B19V-genotype 2 and B19V-genotype 3, resulting in fragment sizes of 117 and 226 bp, respectively, while the TaqI restriction site is only present in B19V-genotype 1 and B19V-genotype 2 (resulting in fragment sizes of 158 and 185 bp) (Figure 1).
Figure 1.
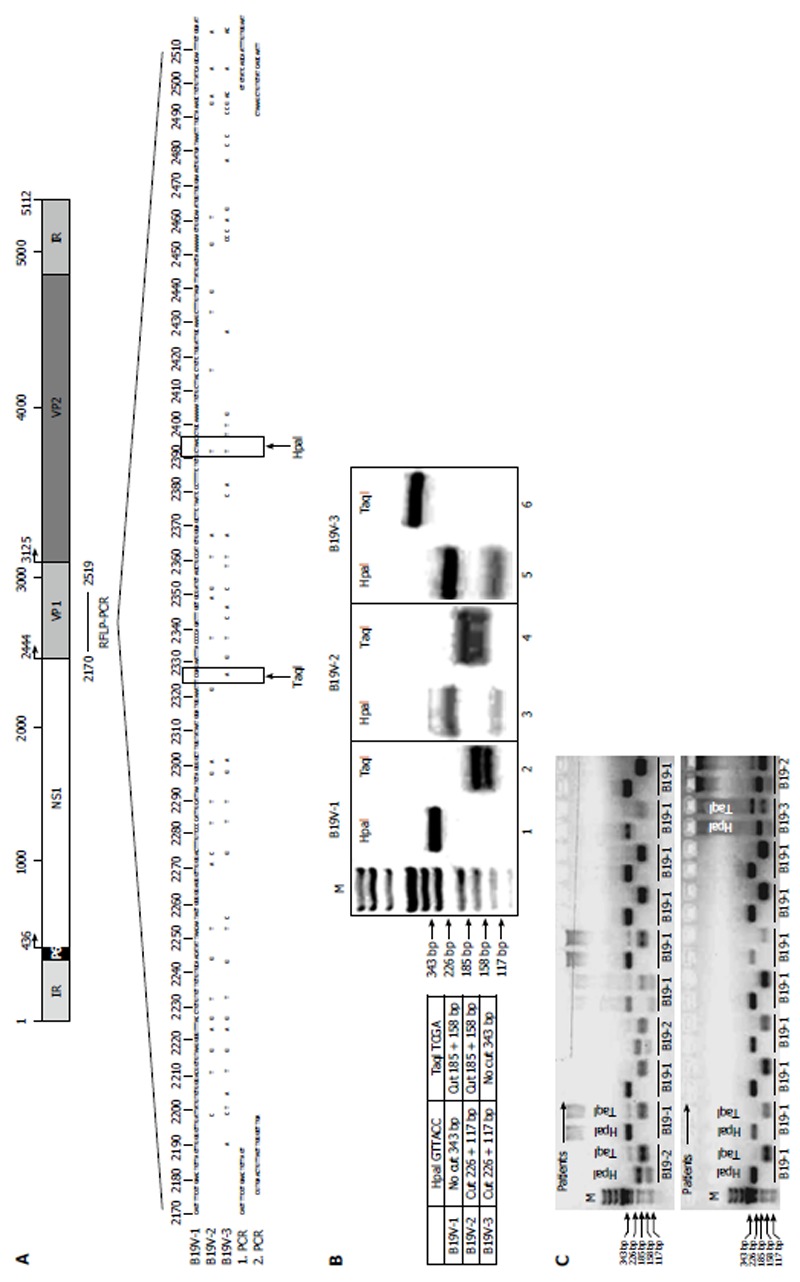
Generation of B19V-genotype 1 to 3 specific restriction fragment length polymorphism-polymerase chain reaction. A: Schematic representation of the B19V genome showing localization of the B19V-genotype-specific restriction fragment length polymorphism-polymerase chain reaction (RFLP-PCR) in the B19V NS1-VP1u region. Sequences of B19V-1, B19V-2 and B19V-3 showing the RFLP-PCR fragment and HpaI and TaqI restriction enzyme sites (lower panel). Primer positions for 1st and 2nd RFLP-PCR are indicated (1. PCR and 2. PCR, see also Table 2); B: Expected fragment size and digestion pattern after HpaI and TaqI digestions (right panel). Agarose gel electrophoresis showing respective PCR-fragments after HpaI and TaqI digestion for each B19V-genotype (left panel); C: Representative agarose gel electrophoresis of patient-specific B19V RFLP-PCRs. B19V-1: B19V-genotype 1.
Luciferase reporter gene assay
After transfection of human endothelial cells 1 cells with luciferase plasmid constructs containing the B19V P6-promoter region to a final mass of 5 μg per plasmid containing 0.2 μg of the β-galactosidase reporter pCMVbGal as an internal standard, cells were cultured for 48 h. Cells were co-transfected with an HHV6 U94 expressing vector construct (kindly provided by Dr. S. Aberle, University of Tuebingen, Germany). To measure luciferase, activity cells were harvested, lysed and measured as described previously[23].
Statistical analysis
Statistical analysis and graphical representation were performed by two-tailed T-test and non-parametric Mann-Whitney U-test using SPSS statistical software version 16.0 and GraphPad Prism, version 5 (GraphPad Software Inc, San Diego, United States). One-way ANOVA followed by post hoc testing and Tukey’s multiple comparison test were also performed. The results were expressed as mean ± SD. Values below significance level of 0.05 were considered statistically significant.
RESULTS
Study population
EMBs of 498 B19V-positive patients described in this retrospective study were obtained from 38 clinical centers in Germany between 2003 and 2010 for cardiopathological diagnosis of myocarditis and DCM. In addition, 91 uninflamed hearts without cardiac failure served as a control group. The baseline data of the patients, molecular and cardiopathological findings of the EMBs are provided in Table 1. Quantitative assessment of B19V loads has been described previously, with viral loads of more than 500 GE/μg in EMBs as a clinically relevant threshold for the maintenance of myocardial inflammation[4]. Patients were relatively young (mean age 46.9 ± 15.9 years) and approximately two thirds were men (68.5%) (Table 1). With regards to clinical history, the majority of patients had cardiac symptoms for longer than six months, consistent with chronic heart disease.
Figure 2 illustrates typical histological and immunohistological findings in acute and chronic myocarditis, as well as in uninflamed DCM in B19V-positive human hearts. As expected, acute B19V-myocarditis had a low prevalence of 5% in our study (Figure 2A and 2B, Table 1), while the majority of patients (59.6%) had chronic B19V-myocarditis (Figure 2C and 2D, Table 1). By contrast, chronic DCM without inflammation (Figure 2E and 2F) exhibiting a latent type of B19V-infection was found in 35.3% of our study population (Table 1). Only 7% of 91 uninflamed control hearts were found to harbor latent B19V genomes (Figure 2G and 2H, Table 1).
Figure 2.
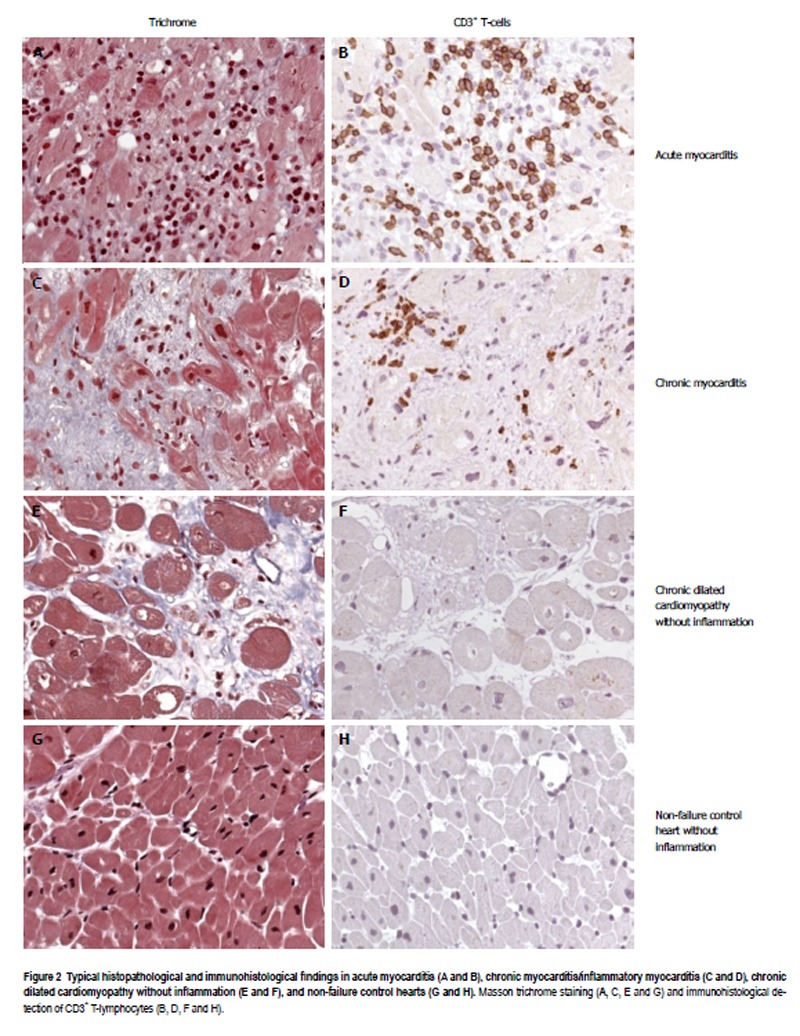
Typical histopathological and immunohistological findings in acute myocarditis (A and B), chronic myocarditis/inflammatory myocarditis (C and D), chronic dilated cardiomyopathy without inflammation (E and F), and non-failure control hearts (G and H). Masson trichrome staining (A, C, E and G) and immunohistological detection of CD3+ T-lymphocytes (B, D, F and H).
Replication activity of B19V in myocarditis
In order to determine replication activity of B19V in infected endothelial cells of the myocardium, we determined B19V RNA replication intermediates of 114 randomly selected patients of our cohorts using RT-PCR to amplify the NS1 and VP1 regions of the B19V genome (2 acute, 54 chronic myocarditis, 51 DCM and 7 control hearts). As expected, B19V replicative RNA intermediates could be confirmed in acute B19V-myocarditis as described[4] (2/2), but also in EMBs of patients with chronic myocarditis harboring viral loads greater than 500 GE/μg (16/51) (Figure 3). In contrast, B19V-mRNA intermediates were not observed in EMBs of DCM-patients with uninflamed hearts and viral loads < 100 GE/μg, indicating a latent-type of B19V-infection. In addition, B19V-mRNA intermediates were found to be absent in latent B19V-infected normal hearts without inflammation.
Figure 3.
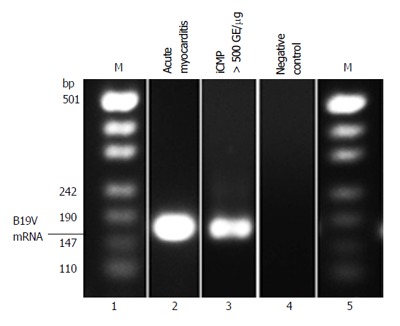
Representative B19V-specific reverse transcription-polymerase chain reaction showing B19V mRNA replication intermediates isolated from endomyocardial biopsies of patients with acute myocarditis (lane 2) and chronic myocarditis/iCMP (lane 3). iCMP: Inflammatory cardiomyopathy.
B19V-specific IgG and IgM antibodies were detected in the serum by ELISA almost exclusively in B19V-DNA positive EMBs of patients with acute myocarditis (11.4%) but not with DCM or in controls. Overall, B19V-IgG antibodies, but not IgM, were detected in the serum of 91% of patients with chronic myocarditis and in 60.9% with DCM.
Detection of different B19V genotypes in EMBs
Recent reports have shown an association between B19V-infection of myocardial endothelial cells and the development of viral myocarditis[1,4,24-26]. However, it is not clear whether B19V-genotypes modulate the outcome of the disease.
In order to determine the B19V genotype confirmed in B19V-positive EMBs, we developed a new RFLP-PCR method spanning the NS1/VP1u region (nt 2170 to 2519; Figure 1A). As shown in Figure 1B, the RFLP-PCR method is capable of discriminating between the known B19V genotypes 1 to 3 by using Hpa1 and Taq1 restriction enzyme digestions. RFLP-PCR patterns of representative patient-specific samples are shown in Figure 1C. In addition, direct sequencing and phylogenetic analysis was performed to verify the specificity of the results by RFLP-PCR. B19V-genotype 1 (286/498; 57.4%) and B19V-genotype 2 (183/498; 36.7%) were the most frequently detected genotypes in persistently infected patients, whereas twenty-five patients with acute B19V-infection showed B19V-genotype 1 (5.0%) (Table 1). In contrast, B19V-genotype 3 infection of the human heart was found to be rare in Germany and only detectable in 0.8% (4/498) of our patients.
B19V genotype-dependent correlation with inflammatory cardiomyopathy
The prevalence of B19V-genotype 1 and B19V-genotype 2 in EMBs was investigated in order to establish if a correlation exists between B19V-genotypes and the occurrence of myocarditis and DCM. B19V-genotype 1 was detected in 176/286 of EMBs (61.5%) with iCMP and in 110/286 samples (38.5%) of DCM-patients without myocardial inflammation, respectively (Figure 4A). A strong trend was observed in the more frequent prevalence of B19V-genotype 2 genomes (total 183/498) in EMBs with iCMP (119/183, 65.0%) compared to DCM (64/183, 35%; P = 0.048) (Figure 4A). We found comparable viral loads in B19V-positive EMBs for both B19V-genotype 1 (584 ± 695 GE/μg) and B19V-genotype 2 (613 ± 715 GE/μg) (P = ns; Figure 4B).
Figure 4.
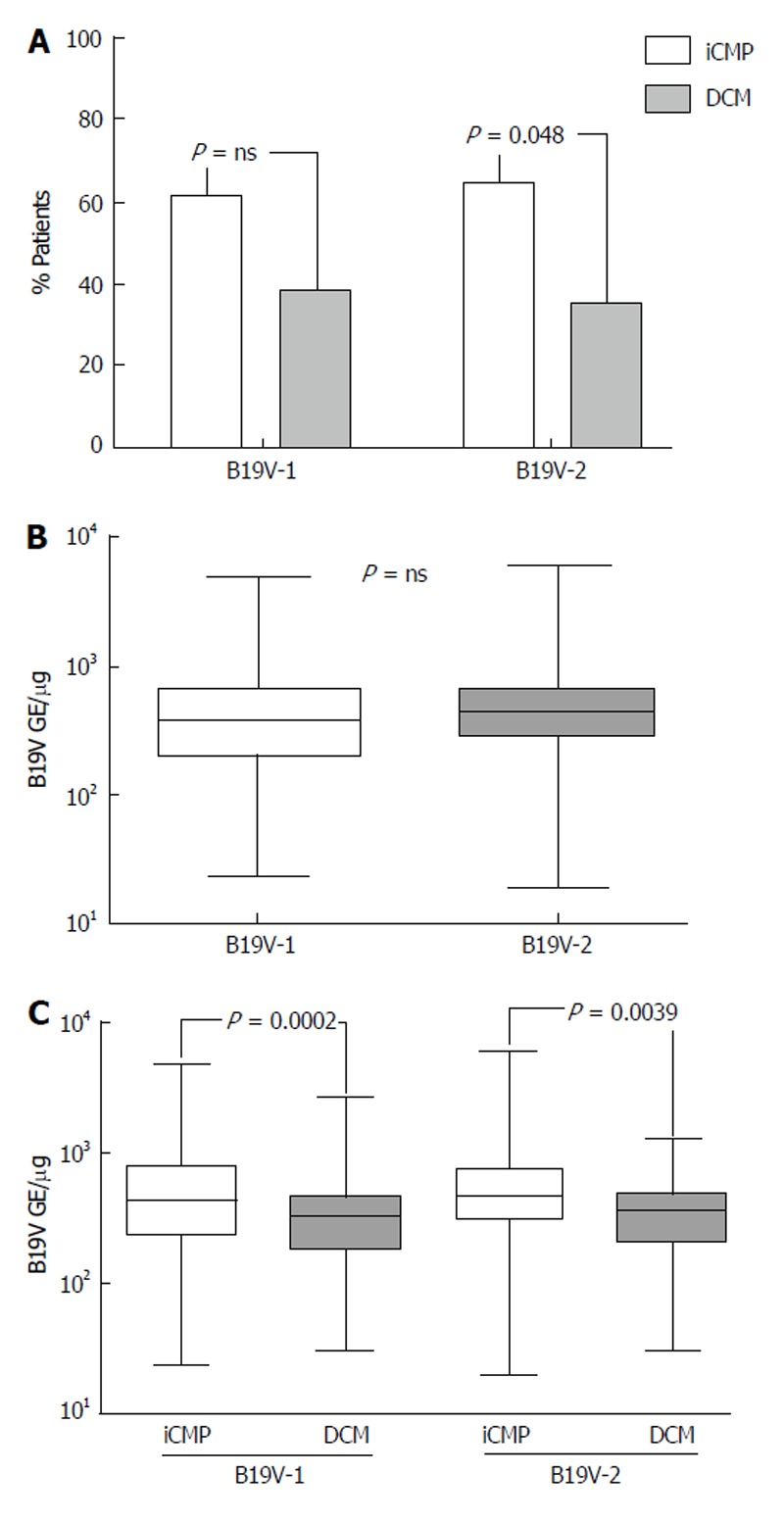
Genotype specific myocardial B19V loads of patients with chronic myocarditis. A: Prevalence of B19V-genotype 1 (B19V-1) and B19V-2 in endomyocardial biopsies of patients with myocarditis [inflammatory cardiomyopathy (iCMP), grey columns] and dilated cardiomyopathy (DCM, white columns). Patient number is given in %; B: qPCR of myocardial of B19V-1 and B19V-2 loads in endomyocardial biopsies (EMBs); C: B19V genotype-specific myocardial viral loads in EMBs of patients with chronic myocarditis (iCMP, white columns) and DCM (grey columns) determined by qPCR. One-way Anova was highly significant (P < 0.0001). P < 0.05 is statistically significant (two-tailed T-test). qPCR: Quantitative real-time polymerase chain reaction.
Determination of B19V-genotype specific viral loads in EMBs of patients with iCMP and DCM showed that B19V-genotype 1 load was significantly higher in iCMP samples (706 ± 821 GE/μg) compared to DCM (389 ± 343 GE/μg; P < 0.0002). In keeping with this finding, significantly higher viral loads were observed in B19V-genotype 2 positive EMBs with iCMP (723 ± 846 GE/μg) compared to DCM samples (408 ± 270 GE/μg; P = 0.0039) (Figure 4C).
Age-dependent distribution of B19V-genotype 1 and B19V-genotype 2
Recent publications have shown an association between age and B19V genotype prevalence[16]. B19V-genotype 2 is considered to be an “older” B19V-variant which is more often found in tissues of patients over the age of 60 years, whereas B19V-genotype 1 is more frequently observed in younger people. In accordance with recent reports, we determined a comparable age-dependent distribution of B19V-genotypes in human hearts[16,17]. B19V-genotype 1 was predominantly detected in individuals born after 1955, with an average of 43 ± 14 years (P < 0.0001), while B19V-genotype 2 was predominantly observed in individuals born before 1955, with an average age of 61 ± 12 years (Figure 5A).
Figure 5.
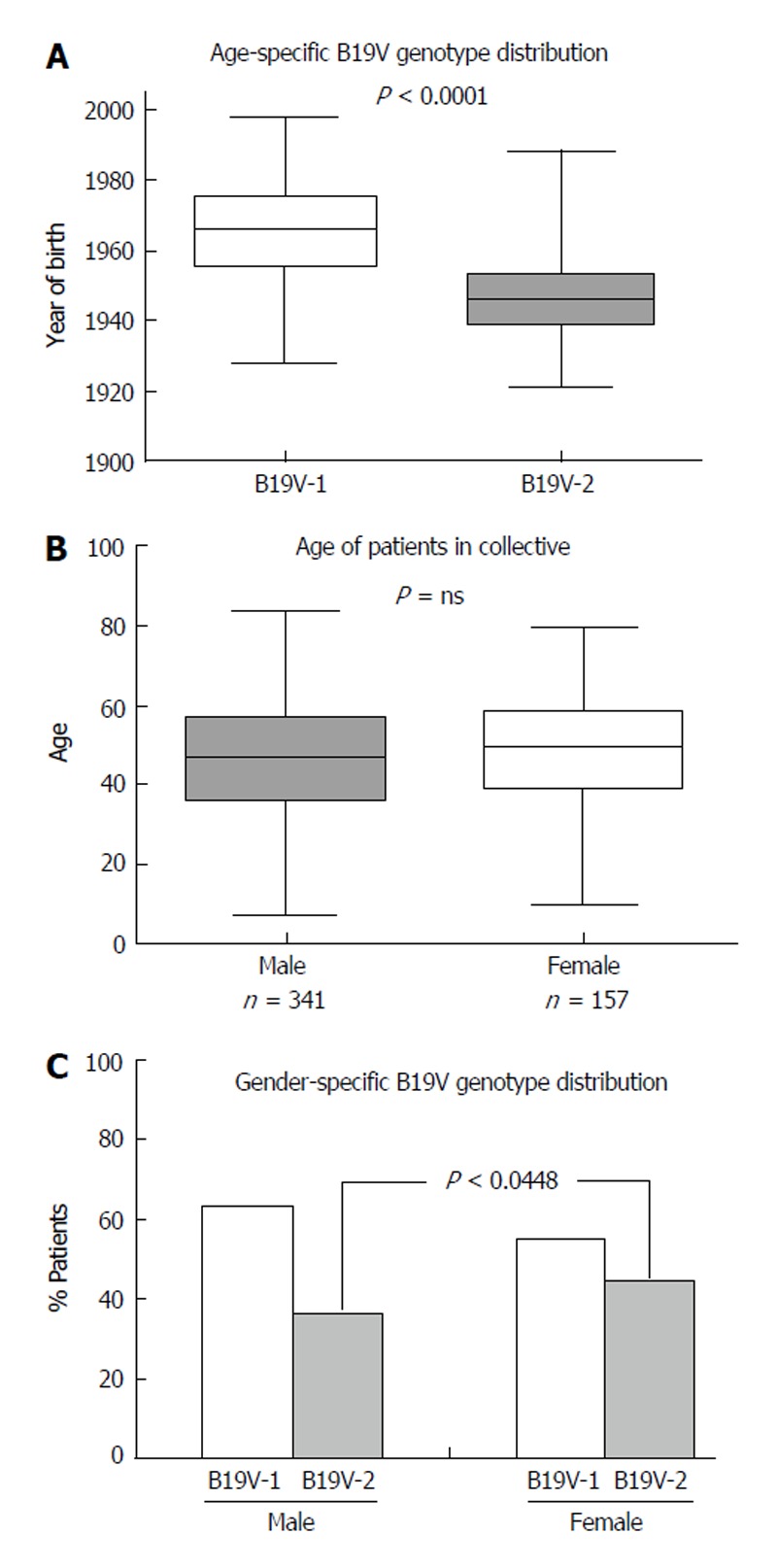
Age and gender dependent distribution of B19V-genotypes in endomyocardial biopsies of patients with myocarditis. A: Distribution of B19V-genotype 1 (B19V-1) and B19V-2 according to year of birth; B: Gender-specific mean age of our patient cohort; C: Gender-specific distribution of B19V-1 (white columns) and B19V-2 (grey columns). P < 0.05 is statistically significant (two-tailed T-test).
Gender-dependent distribution of B19V-genotype 1 and B19V-genotype 2
We next explored the role of gender in B19V-associated myocarditis. 341/498 (68.5%) EMBs were obtained from male and 157/498 (31.5%) from female patients. There was no significant difference in age between women (49 ± 16 years) and men (46 ± 16 years; P = ns) (Figure 5B). Furthermore, there was no significant difference in the occurrence of iCMP or DCM in B19V-positive male and female patients (P = ns). A comparison of the B19V-loads in EMBs from men and women showed no significant differences (men 586 ± 679 GE/μg vs women 602 ± 711 GE/μg) (P = ns). However, there was a trend towards women being more frequently infected with B19V-genotype 2 (66/148, 44.6%) compared to men (117/325; 36.0%; P = 0.0448), whereas this was not the case for B19V-genotype 1 infection of men (198/325; 60.9%) and women (81/148; 54.7%; P = ns) (Figure 5C).
Coinfection with cardiotropic viruses detected in B19V-positive EMBs
Recent reports have shown that coinfection of B19V with other cardiotropic viruses, such as HHV6, HCMV, EBV and EV, is not infrequent in viral myocarditis[18,27]. However, the impact of coinfection on B19V replication has not been determined. Hence, the frequency of B19V-coinfections in EMBs of 473/498 B19V-positive patients with iCMP and DCM was analyzed excluding the twenty-five acute cases of B19V-myocarditis (Table 1). 382/473 (80.8%) EMB samples were infected with B19V alone, while 91/473 (19.2%) were coinfected with other cardiotropic viruses (Figure 6A). The most prevalent coinfecting virus was HHV6 (78/473, 16.5%). EBV (5/473, 1.1%), EV (4/473, 0.8%) and HCMV (1/473, 0.2%) were detected less frequently (Figure 6B). 3/473 (0.6%) showed evidence of triple-infection with HHV6, EBV and/or HCMV. Only HHV6 subtype B was detected in B19V-positive EMBs. There was no statistically significant difference in the frequency of coinfection with either B19V-genotype 1 or 2 (46.2% vs 53.8%; P = ns). Determination of B19V loads in EMBs revealed a statistically significant higher viral load in B19V-coinfection in comparison to B19V-monoinfection (754 ± 967 vs 552 ± 615 GE/μg; P = 0.0012) (Figure 6C). Analysis of the prevalence of B19V genotypes showed that B19V-genotype 2 was more frequently associated with B19V coinfection in comparison to B19V-monoinfection (36.7% vs 48.3%; P = 0.023), whereas this was not the case for B19V-genotype 1 (63.3% vs 51.7%; P = ns) (Figure 6D). Interestingly, B19V loads were significantly increased in iCMP samples of B19V-coinfected in comparison to B19V-monoinfected patients with iCMP (934 ± 1122 vs 650 ± 723 GE/μg; P = 0.0157) (Figure 6E). No difference in B19V loads was found in DCM samples of B19V mono- and coinfected patients (396 ± 336 vs 371 ± 205 GE/μg; P = ns) (Figure 6E). Further analysis showed that the increased B19V load was mainly due to coinfection of B19V with HHV6 (869 ± 992 vs 552 ± 615 GE/μg; P = 0.0006) in comparison to B19V coinfections with EV or EBV (466 ± 254 and 350 ± 218 GE/μg, respectively; P = ns) (Figure 6F). Functional analysis demonstrated that the HHV6 U94 transactivator, which shows similarities to parvovirus NS1/Rep transactivator[28], is able to transactivate the P6-promoter of B19V by 2.4 fold using luciferase promoter activity assays (Figure 6G).
Figure 6.
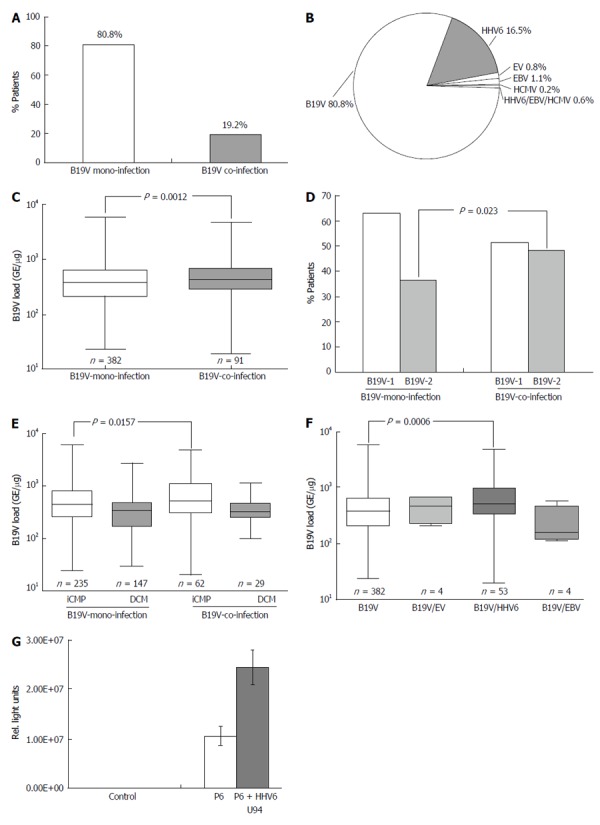
Distribution of B19V-coinfection with cardiotropic viruses. A: In endomyocardial biopsies determined by virus-specific nPCR; B: Frequency of B19V-coinfection with cardiotropic viruses; C: qPCR of B19V loads in B19V mono- and co-infection; D: Distribution of B19V-genotype 1 and 2 in B19V mono- and co-infection in endomyocardial biopsies (EMBs); E: B19V loads of B19V mono- and co-infection in EMBs of patients with iCMP and DCM. One-way Anova was highly significant (P < 0.0001); F: B19V loads of B19V mono- and co-infection with cardiotropic viruses. One-way Anova was highly significant (P = 0.0091); G: Luciferase reporter assay to determine transactivation capacity of the HHV6-U94 transactivator on the B19V P6-promoter activity. P < 0.05 is statistically significant (two-tailed T-test). HHV6: Human herpesvirus 6; EV: Enterovirus; HCMV: Human cytomegalovirus; EBV: Epstein-Barr virus; DCM: Dilated cardiomyopathy; iCMP: Inflammatory cardiomyopathy; qPCR: Quantitative real-time polymerase chain reaction.
DISCUSSION
Parvovirus B19 has been reported to be the causative agent for a wide variety of clinical conditions, ranging from asymptomatic infection to fifth disease in childhood (erythema infectiosum), hydrops fetalis and transient aplastic anemia[12,29,30]. In addition to EV, the classical causative pathogens of myocarditis[8,27], B19V has also been described to account for inflammatory cardiomyopathy (iCMP)[1,24,25,27]. However, the relatively high prevalence of B19V in EMBs has led to controversy around the role and mechanism of B19V in the pathogenesis of myocarditis[9,31].
It has been demonstrated that B19V infects endothelial cells of small myocardial blood vessels, which can result in the impairment of myocardial microcirculation[1,2,24,25], endothelial dysfunction[6] and secondary necrosis of myocardial cells[2]. A recent report by Streitz et al[32] demonstrated the presence of anti-B19V NS1-specific T-cell response in B19V-associated myocarditis, supporting the notion that B19V-infection has a pathogenic role in the development of iCMP. It has been shown that active persistent B19V-infection is responsible for triggering inflammatory response in the myocardium[18]. Recently, we demonstrated that B19V and the viral proteins of B19V play an important pathophysiological role in modulating inflammatory signaling, regulation of pro-apoptotic processes and modulation of the intracellular Ca2+-activity leading to endothelial dysfunction[26,33,34].
It is important to note that B19V DNA is more frequently found in EMBs of patients with chronic myocarditis (59.6%) compared to control cardiac tissue samples from individuals without heart failure (7.7%), a finding consistent with other reports[2,35]. Based on the assessment of B19V-myocarditis and DCM, a viral load threshold of greater than 500 B19V-GE/μg has been suggested to be of clinical significance for the maintenance of myocardial inflammation[4]. This correlates with the finding of B19V RNA replication intermediates predominantly in myocardial tissue of patients with acute and chronic myocarditis, but not in uninflamed hearts (Figure 2). However, the detection of B19V-RNA replication intermediates in EMBs is challenging because of sampling errors and RNA copy numbers that may be below limit of detection.
In order to determine the B19V-genotype distribution in EMBs of patients with iCMP/DCM, we developed a RFLP-PCR technique to discriminate between the three B19V-genotypes (Figure 3). In line with recent publications, we found that B19V-genotype 1 was the most common genotype, followed by B19V-genotype 2, while B19V-3 was rarely found[17]. This is not surprising as B19V-genotype 3 is most commonly found in Ghana, Brazil and France[13,14,36].
While B19V was detected more frequently in EMBs of patients with iCMP compared to DCM that lacked inflammatory cell infiltrates, B19V viral loads of both B19V-genotypes 1 and 2 were significantly higher in iCMP samples (approximately 700 GE/μg) compared to DCM (approximately 400 GE/μg) (Figure 4). These findings indicate that regardless of the B19V-genotype, B19V-iCMP is associated with significantly higher viral loads than B19V-DCM.
As with the age-dependent distribution of B19V-genotype 1 and 2 in various tissue samples (e.g., skin, synovium, liver, and heart)[15-17], we found that B19V-genotype 1 was predominantly detected in younger people (mean age 43 years), while B19V-genotype 2 was mostly observed in patients older than 60 years (Figure 5). This age-associated difference in frequency of genotypes is thought to be due to the reported lifelong persistence of viral genomes in humans[15,16,29]. Analysis of gender dependency of B19V genotype distribution and viral loads did not show any significant differences. However, a significantly higher proportion of women were found to be persistently infected with B19V-genotype 2 in contrast to men (P < 0.05) (Figure 5).
It can be hypothesized that B19V can be reactivated from long-term persistent or latent infection by viral and/or host-specific determinants. B19V-coinfection with other cardiotropic viruses like EV, ADV and HHV6 may contribute to the severity of B19V-myocarditis[4,7,8,20], possibly by reactivating B19V replication and thereby enhancing virus specific host immune responses, tissue inflammation and the progression to chronic heart failure[37]. This is reminiscent of the increased replication of HIV caused by coinfection with HHV6 and HHV8[21,38]. Our finding show that coinfection with B19V and other cardiotropic viruses, particularly HHV6, is not infrequent and is associated with higher B19V loads in EMBs. HHV6 cannot only infect and replicate in endothelial cells[39], but also encodes a viral transactivator[37] called U94 which is homologous to the B19V-NS1 transactivator[28]. By using promoter activity assays, we have shown that the HHV6 U94 transactivator also transactivates the B19V-P6 promoter, resulting in enhanced B19V loads in B19V/HHV6 coinfection (Figure 6).
Our data shows that B19V is capable of long-term persistence in the human heart, even lasting for decades, and that active B19V replication is associated with the development of iCMP. To establish a clinically relevant link between B19V-infection and the development of iCMP, it is important to develop molecular diagnostic techniques to determine myocardial viral loads and sequence analysis to verify the association between myocardial disease and genotype-specific B19V isolates. Notably, persistently low replicating and latent B19V-infection may be reactivated by coinfecting cardiotropic viruses, especially HHV6. Our findings together with other recently published data[1,6,18,25,26,40] argue an important role for B19V in the development of iCMP.
ACKNOWLEDGMENTS
We appreciated the excellent technical assistance of Rosa Mammato, Gerd Janke, Isabell Haussmann and Silke Aberle. We wish to thank Dr. Bernhard Renard, Robert Koch Institute, Germany, for his valuable help with the statistical analysis. The authors would like to thank Professor Joseph Torresi, Department of Infectious Diseases, Austin Hospital, The University of Melbourne, Australia, for his valuable and critical reading of the manuscript.
COMMENTS
Background
Viral and post viral myocarditis is the major cause of acute and chronic dilated cardiomyopathy (DCM). Human parvovirus B19 has been identified as a new emerging pathogenic agent in the etiology of inflammatory cardiomyopathy (iCMP). However, the role of B19V-infection in the development of chronic myocarditis is still unclear. The authors have recently demonstrated that endothelial cells but not cardiac myocytes are B19V-specific target cells in patients with B19V-associated myocarditis. Furthermore, B19V was not detected frequently in patients with unexplained isolated diastolic dysfunction. Molecular phenotypes such as patient age, gender, B19V replicative mRNA intermediates, B19V genotype and B19V-coinfection of myocardial B19V-infection contributing to the etiopathogenesis of B19V-myocarditis as an endothelial-cell mediated inflammatory disease have not been described so far.
Research frontiers
The identification of various viral nucleic acids by polymerase chain reaction in the myocardium of patients with suspected myocarditis led to the hypothesis that acute and chronic myocarditis may develop as a result of infection, not only with enteroviruses (Coxsackievirus B3), but also with other cardiotropic viruses, such as parvovirus B19. In this regard, the research hotspot is how molecular phenotypes can contribute to the pathogenic role of myocardial B19V-infection in myocarditis and DCM.
Innovations and breakthroughs
The study results showed that B19V RNA replication intermediates could be determined mainly in acute and ongoing myocarditis, indicating active replication of B19V. B19V-genotypes 1 and 2 were predominant with a more frequent prevalence of B19V-genotype 2 in iCMP. Furthermore, B19V-coinfections with other cardiotropic viruses can occur, most frequently with human herpes virus 6 (HHV6). Functional experiments showed that the HHV6 U94-transactivator could transactivate the B19V-P6-promoter. From these findings it is suggested that long-term persistence of B19V DNA in the human heart occurs and that active/reactivated B19V-replication can be associated with iCMP in a viral load and genotype-dependent manner.
Applications
To establish a clinically relevant link between B19V-infection and the development of iCMP, it is important to pursue molecular diagnostic techniques to determine myocardial B19V loads and to verify genotype-specific B19V isolates. Notably, persistent low replicating and even latent B19V-infection may be reactivated by coinfecting cardiotropic viruses, especially HHV6.
Terminology
Parvovirus B19 was assumed to be an agent of human disease when its association with erythema infectiosum (fifth disease), hydrops fetalis and transient aplastic anemia was demonstrated in the 1980s. During the last few years, a growing number of reports have been published demonstrating an association between B19V and many other clinical diseases, like arthritis, myocarditis, various vasculitis syndromes, hepatitis and neurological disorders. A growing number of reports have suggested an association between B19V infection with acute and chronic cardiac diseases. Myocarditis is the term used to indicate infectious, toxic or autoimmune processes causing inflammation of the heart and represents a non-ischemic inflammatory heart disease with a highly variable clinical outcome. In most cases this disease is self-limiting; however, it may also lead to acute heart failure, resulting in early death or heart transplantation. Myocarditis can also mimic acute myocardial infarction. ICMP was included as a subtype of the specific cardiomyopathies and defined “as myocarditis in association with cardiac dysfunction” by the World Health Organization.
Peer review
The paper deals with the interesting subject of molecular phenotypes of human parvovirus B19 in patients with myocarditis. Both the specific virus, which is a cause of important pathologies, as well as myocarditis, an entity that can affect great portions of a population, among them young, otherwise healthy individuals, are very interesting subjects with an impact on the general practice of internists, cardiologists, general physicians, pathologists, biologists and genetic scientists. The paper deals with the aforementioned interesting subject in a thorough way.
Footnotes
P- Reviewers: Robert KI, Tagarakis G S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Bültmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M, Kandolf R. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol. 2003;34:92–95. doi: 10.1053/hupa.2003.48. [DOI] [PubMed] [Google Scholar]
- 2.Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R. Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol. 2004;193:101–107. doi: 10.1007/s00430-003-0190-1. [DOI] [PubMed] [Google Scholar]
- 3.Mahfoud F, Gärtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, Kandolf R, Böhm M, Kindermann I. Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J. 2011;32:897–903. doi: 10.1093/eurheartj/ehq493. [DOI] [PubMed] [Google Scholar]
- 4.Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N Engl J Med. 2010;362:1248–1249. doi: 10.1056/NEJMc0911362. [DOI] [PubMed] [Google Scholar]
- 5.Ruppert V, Meyer T, Balbach A, Richter A, Müller HH, Maisch B, Pankuweit S. Genotype-specific effects on left ventricular function in parvovirus B19-positive patients with dilated cardiomyopathy. J Med Virol. 2011;83:1818–1825. doi: 10.1002/jmv.22187. [DOI] [PubMed] [Google Scholar]
- 6.Tschöpe C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kühl U, Kandolf R, et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111:879–886. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 7.Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 8.Kandolf R, Bültmann B, Klingel K, Bock CT. [Molecular mechanisms and consequences of cardiac viral infections] Pathologe. 2008;29 Suppl 2:112–117. doi: 10.1007/s00292-008-1027-x. [DOI] [PubMed] [Google Scholar]
- 9.Schenk T, Enders M, Pollak S, Hahn R, Huzly D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol. 2009;47:106–110. doi: 10.1128/JCM.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koepsell SA, Anderson DR, Radio SJ. Parvovirus B19 is a bystander in adult myocarditis. Cardiovasc Pathol. 2012;21:476–481. doi: 10.1016/j.carpath.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BJ, Buckley MM. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol. 1988;25:151–153. doi: 10.1099/00222615-25-2-151. [DOI] [PubMed] [Google Scholar]
- 12.Brown KE, Young NS. Human parvovirus B19 infections in infants and children. Adv Pediatr Infect Dis. 1997;13:101–126. [PubMed] [Google Scholar]
- 13.Servant A, Laperche S, Lallemand F, Marinho V, De Saint Maur G, Meritet JF, Garbarg-Chenon A. Genetic diversity within human erythroviruses: identification of three genotypes. J Virol. 2002;76:9124–9134. doi: 10.1128/JVI.76.18.9124-9134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanabani S, Neto WK, Pereira J, Sabino EC. Sequence variability of human erythroviruses present in bone marrow of Brazilian patients with various parvovirus B19-related hematological symptoms. J Clin Microbiol. 2006;44:604–606. doi: 10.1128/JCM.44.2.604-606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekman A, Hokynar K, Kakkola L, Kantola K, Hedman L, Bondén H, Gessner M, Aberham C, Norja P, Miettinen S, et al. Biological and immunological relations among human parvovirus B19 genotypes 1 to 3. J Virol. 2007;81:6927–6935. doi: 10.1128/JVI.02713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis-Hübinger AM, et al. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci USA. 2006;103:7450–7453. doi: 10.1073/pnas.0602259103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kühl U, Lassner D, Pauschinger M, Gross UM, Seeberg B, Noutsias M, Poller W, Schultheiss HP. Prevalence of erythrovirus genotypes in the myocardium of patients with dilated cardiomyopathy. J Med Virol. 2008;80:1243–1251. doi: 10.1002/jmv.21187. [DOI] [PubMed] [Google Scholar]
- 18.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 19.Pozzuto T, von Kietzell K, Bock T, Schmidt-Lucke C, Poller W, Zobel T, Lassner D, Zeichhardt H, Weger S, Fechner H. Transactivation of human parvovirus B19 gene expression in endothelial cells by adenoviral helper functions. Virology. 2011;411:50–64. doi: 10.1016/j.virol.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Rohayem J, Dinger J, Fischer R, Klingel K, Kandolf R, Rethwilm A. Fatal myocarditis associated with acute parvovirus B19 and human herpesvirus 6 coinfection. J Clin Microbiol. 2001;39:4585–4587. doi: 10.1128/JCM.39.12.4585-4587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caselli E, Galvan M, Cassai E, Caruso A, Sighinolfi L, Di Luca D. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005;106:2790–2797. doi: 10.1182/blood-2005-04-1390. [DOI] [PubMed] [Google Scholar]
- 22.Toan NL, Duechting A, Kremsner PG, Song le H, Ebinger M, Aberle S, Binh VQ, Duy DN, Torresi J, Kandolf R, et al. Phylogenetic analysis of human parvovirus B19, indicating two subgroups of genotype 1 in Vietnamese patients. J Gen Virol. 2006;87:2941–2949. doi: 10.1099/vir.0.82037-0. [DOI] [PubMed] [Google Scholar]
- 23.Bock CT, Kubicka S, Manns MP, Trautwein C. Two control elements in the hepatitis B virus S-promoter are important for full promoter activity mediated by CCAAT-binding factor. Hepatology. 1999;29:1236–1247. doi: 10.1002/hep.510290426. [DOI] [PubMed] [Google Scholar]
- 24.Bültmann BD, Klingel K, Sotlar K, Bock CT, Kandolf R. Parvovirus B19: a pathogen responsible for more than hematologic disorders. Virchows Arch. 2003;442:8–17. doi: 10.1007/s00428-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 25.Bock CT, Klingel K, Aberle S, Duechting A, Lupescu A, Lang F, Kandolf R. Human parvovirus B19: a new emerging pathogen of inflammatory cardiomyopathy. J Vet Med B Infect Dis Vet Public Health. 2005;52:340–343. doi: 10.1111/j.1439-0450.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 26.Duechting A, Tschöpe C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock CT. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol. 2008;82:7942–7952. doi: 10.1128/JVI.00891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandolf R. [Virus etiology of inflammatory cardiomyopathy] Dtsch Med Wochenschr. 2004;129:2187–2192. doi: 10.1055/s-2004-831863. [DOI] [PubMed] [Google Scholar]
- 28.Rapp JC, Krug LT, Inoue N, Dambaugh TR, Pellett PE. U94, the human herpesvirus 6 homolog of the parvovirus nonstructural gene, is highly conserved among isolates and is expressed at low mRNA levels as a spliced transcript. Virology. 2000;268:504–516. doi: 10.1006/viro.1999.0163. [DOI] [PubMed] [Google Scholar]
- 29.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 31.Lindner J, Noutsias M, Lassner D, Wenzel J, Schultheiss HP, Kuehl U, Modrow S. Adaptive immune responses against parvovirus B19 in patients with myocardial disease. J Clin Virol. 2009;44:27–32. doi: 10.1016/j.jcv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Streitz M, Noutsias M, Volkmer R, Rohde M, Brestrich G, Block A, Klippert K, Kotsch K, Ay B, Hummel M, et al. NS1 specific CD8+ T-cells with effector function and TRBV11 dominance in a patient with parvovirus B19 associated inflammatory cardiomyopathy. PLoS One. 2008;3:e2361. doi: 10.1371/journal.pone.0002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupescu A, Bock CT, Lang PA, Aberle S, Kaiser H, Kandolf R, Lang F. Phospholipase A2 activity-dependent stimulation of Ca2+ entry by human parvovirus B19 capsid protein VP1. J Virol. 2006;80:11370–11380. doi: 10.1128/JVI.01041-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupescu A, Geiger C, Zahir N, Aberle S, Lang PA, Kramer S, Wesselborg S, Kandolf R, Foller M, Lang F, et al. Inhibition of Na+/H+ exchanger activity by parvovirus B19 protein NS1. Cell Physiol Biochem. 2009;23:211–220. doi: 10.1159/000204110. [DOI] [PubMed] [Google Scholar]
- 35.Donoso Mantke O, Nitsche A, Meyer R, Klingel K, Niedrig M. Analysing myocardial tissue from explanted hearts of heart transplant recipients and multi-organ donors for the presence of parvovirus B19 DNA. J Clin Virol. 2004;31:32–39. doi: 10.1016/j.jcv.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Parsyan A, Szmaragd C, Allain JP, Candotti D. Identification and genetic diversity of two human parvovirus B19 genotype 3 subtypes. J Gen Virol. 2007;88:428–431. doi: 10.1099/vir.0.82496-0. [DOI] [PubMed] [Google Scholar]
- 37.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 38.Isegawa Y, Katahira J, Yamanishi K, Sugimoto N. Reactivation of latent human immunodeficiency virus 1 by human herpesvirus 6 infection. Acta Virol. 2007;51:13–20. [PubMed] [Google Scholar]
- 39.Caruso A, Rotola A, Comar M, Favilli F, Galvan M, Tosetti M, Campello C, Caselli E, Alessandri G, Grassi M, et al. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J Med Virol. 2002;67:528–533. doi: 10.1002/jmv.10133. [DOI] [PubMed] [Google Scholar]
- 40.Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 41.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]


