Abstract
AIM: To study the prognostic value of carbohydrate antigen 125 (CA125) and whether it adds prognostic information to N-terminal pro-brain natriuretic peptide (NT-proBNP) in stable heart failure (HF) patients.
METHODS: The predictive value of CA125 was retrospectively assessed in 156 patients with stable HF remitted to the outpatient HF unit for monitoring from 2009 to 2011. Patients were included in the study if they had a previous documented episode of HF and received HF treatment. CA125 and NT-proBNP concentrations were measured. The independent association between NT-proBNP or CA125 and mortality was assessed with Cox regression analysis, and their combined predictive ability was tested by the integrated discrimination improvement (IDI) index.
RESULTS: The mean age of the 156 patients was 72 ± 12 years. During follow-up (17 ± 8 mo), 27 patients died, 1 received an urgent heart transplantation and 106 required hospitalization for HF. Higher CA125 values were correlated with outcomes: 58 ± 85 KU/L if hospitalized vs 34 ± 61 KU/L if not (P < 0.05), and 94 ± 121 KU/L in those who died or needed urgent heart transplantation vs 45 ± 78 KU/L in survivors (P < 0.01). After adjusting for propensity scores, the highest risk was observed when both biomarkers were elevated vs not elevated (HR = 8.95, 95%CI: 3.11-25.73; P < 0.001) and intermediate when only NT-proBNP was elevated vs not elevated (HR = 4.15, 95%CI: 1.41-12.24; P < 0.01). Moreover, when CA125 was added to the clinical model with NT-proBNP, a 4% (P < 0.05) improvement in the IDI was found.
CONCLUSION: CA125 > 60 KU/L identified patients in stable HF with poor survival. Circulating CA125 level adds prognostic value to NT-proBNP level in predicting HF outcomes.
Keywords: Heart failure, Prognosis, Carbohydrate antigen 125, Brain natriuretic peptides, Survival
Core tip: Increased carbohydrate antigen 125 (CA125) has prognostic implications in acute heart failure (HF). The aim of this study was to assess the prognostic value of increased CA125 and whether it adds prognostic information to N-terminal pro-brain natriuretic peptide (NT-proBNP) in stable HF patients. Higher CA125 values correlated with outcomes. The highest risk was observed when both biomarkers CA125 and NT-proBNP were elevated vs not elevated (HR = 8.95, 95%CI: 3.11-25.73; P < 0.001). CA125 > 60 KU/L identified patients in stable HF with very poor survival. Circulating CA125 level adds prognostic value to NT-proBNP level in predicting HF outcomes.
INTRODUCTION
Heart failure (HF) is a disease with high mortality[1] and an estimated prevalence up to 3% in the European population[2]. This prevalence increases exponentially with age, especially in patients older than 75 years. As the main cause of hospitalization, in patients over 65, HF is also associated with high costs. Despite recent advances in treatment, mortality is still high (12% per year) in stable patients, and there is a high rate of hospital readmissions due to worsening HF[3,4]. Even though a high number of clinical parameters have been associated with poor outcome in patients with stable HF, the assessment of prognosis is still a challenge[5,6]. Multiple biomarkers have been suggested to identify patients with worse prognosis, such as some interleukins, natriuretic peptides, endothelin, ST2, and several fibrosis markers[7-11]. Although most of these effectively select patients with high risk of death, only circulating levels of natriuretic peptides have proven useful in clinical practice. Natriuretic peptides are released when ventricular wall stress and ventricular end-diastolic pressure increase. Thus, an elevated level of either brain natriuretic peptide (BNP) or the amino terminal portion of N-terminal pro-BNP (NT-proBNP) in peripheral blood is associated with decompensated HF. The negative and positive predictive values of these peptides to diagnose HF have been widely studied[12-14]. Moreover, they are useful in the assessment of prognosis after a HF admission[8]. However, certain limitations affect the more extensive use of natriuretic peptides[15].
Carbohydrate antigen 125 (CA125) is a biomarker previously used in the detection and monitoring of some cancers, especially ovarian cancer[16]. It is a high-molecular-weight glycoprotein synthesized by epithelial cells of the serosa when there is inflammation and increased interstitial fluid; therefore, its level is elevated in the presence of pleural or pericardial effusion and ascites. Elevated CA125 has also been found in patients with HF, with or without fluid retention[16-19]. Although its release mechanisms are not yet well understood, they correlate with increased left ventricular end-diastolic pressure, higher BNP level, and worse New York Heart Association (NYHA) functional class[20,21]. Moreover, previous studies of BNP and CA125 in acute HF have demonstrated an additive prognostic value of CA125; therefore, the determination of both biomarkers would improve risk stratification[22]. The advantages of CA125 determination with respect to natriuretic peptides are its higher stability in the circulation and lower cost[23]. The usefulness of CA125 measurement to assess prognosis in patients admitted with acute decompensated HF has been previously demonstrated[19-22]. However, information is lacking on its value in assessing the prognosis of patients with stable chronic HF. The aim of this study was to analyze the prognostic implications of increased CA125 concentration in peripheral blood and whether it adds prognostic information to NT-proBNP in stable HF patients.
MATERIALS AND METHODS
Study population
The population was a prospective cohort of 156 patients diagnosed with HF and remitted to the outpatient HF unit for monitoring from 2009 to 2011. Patients were included in the study if they had a previous documented episode of HF and received HF treatment. The diagnosis of HF was made following the Clinical Practice Guidelines of the European Society of Cardiology[24]. HF treatment included angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) if ACEIs were contraindicated, beta blockers, diuretics, and aldosterone antagonists, with individualized assessment of treatment indication and optimized doses per recommendations of the Clinical Practice Guidelines of the European Society of Cardiology[24].
We excluded patients in unstable HF (NYHA functional class IV), patients with hemodynamic instability, or those diagnosed with cancer or systemic diseases that could shorten life expectancy. Patients with valve heart disease waiting for surgery repair were also excluded from the study. A 12-lead electrocardiogram (ECG) chest radiography and echocardiogram were performed in all patients to establish the HF etiology. Left ventricular systolic dysfunction was considered when ejection fraction was < 50%[25] and ventricular hypertrophy when ventricular septum or posterior left ventricular wall thickness was > 11 mm. Ischemic heart disease was diagnosed if pathologic Q waves were observed on the ECG or significant coronary lesions on the coronary angiography. Valve heart disease was diagnosed when a biological or mechanic valve prosthesis had been implanted or if the valve dysfunction was considered significant by echocardiography.
The hospital ethics committee approved the study, patients gave informed consent to participate, and the protocol conformed to the principles outlined in the Declaration of Helsinki.
Laboratory measurements
To obtain routine laboratory determinations and NT-proBNP concentrations, fasting serum blood samples were obtained during the first visit to the HF unit. CA125 and NT-proBNP concentrations were measured with commercial electrochemiluminescence assays in a Modular E170 analyzer (both from Roche Diagnostics, Basel, Switzerland). The CA125 assay used a pair of monoclonal anti-CA125 antibodies, one labeled with biotin, the other linked to ruthenium chelates. The analyzer had a sensitivity of 0.60 KU/L, and the total imprecision at different concentrations was ≤ 2.5%, expressed as a coefficient of variation.
Statistical analysis
During follow-up, mortality or need for urgent heart transplantation and new hospital admissions due to worsening HF were assessed. The primary end-point was all-cause mortality, which included need for urgent heart transplantation. The secondary end-point was worsening HF requiring hospital admission.
Continuous variables are expressed as mean ± SD and categorical variables as percentages. Continuous variables were compared using Student’s t test. The χ2 or Fisher exact test was used for categorical variables. The Kruskal-Wallis test was used when necessary. Variables without homogeneous distribution were analyzed with the nonparametric Mann-Whitney test. To normalize CA125 and NT-proBNP measurements, the neperian logarithm of CA125 and NT-proBNP values was used, creating two new variables: ln-CA125 and ln-NT-proBNP. Without a clear cut-off for NT-proBNP in the literature, 3100 ng/L was selected because it was close to the mean NT-proBNP value of our population and it had the best cut-off according to area under the curve (AUC) calculation, with a sensitivity of 62% and a specificity of 82% to predict mortality. A CA125 cut-off of 60 KU/L was used, in keeping with the best predictor of mortality identified by a previous study in patients with acute HF[22]. Based on these data, a new dichotomous variable was created for a CA125 value of 60 KU/L. Also, both variables were categorized for clinical practice and better interpretation.
To avoid overfitting multivariate models, two propensity score models[26,27] were used to adjust for confounding factors. Propensity score analysis was performed using logistic regression for computing probability to fall in NT-proBNP 3100 ng/L or CA125 60 KU/L categories. This analysis was based on seven variables associated to end-points with the objective of eliminating differences in baseline patient characteristics that might affect event comparison. The AUC was 0.75 (0.66-0.84); P < 0.001 for NT-proBNP categories and 0.70 (0.61-0.80); P < 0.001 for CA125. Variables included in the propensity score were left atrial diameter, age, atrial fibrillation, left ventricular ejection fraction, estimated glomerular filtrate rate (eGFR), hemoglobin and interventricular septum thickness.
The independent associations between CA125 and NT-proBNP and survival were assessed with the Cox regression analysis. The models were adjusted for the two propensity scores performed previously.
Two Cox models were compared to test improvement in risk stratification: Cox-model 1 (adjusted model with NT-proBNP 3100 ng/L) vs Cox-model 3 (adjusted model with 4 groups according to the cut-off concentrations of CA125 and NT-proBNP) (Table 1): The four groups in Cox model 3 were patients with both markers below the cut-off values, with only one elevated level (assessed for each biomarker), and with both markers above the cut-offs. The increase in the prognostic utility of NT-proBNP and NT-proBNP combined with CA125 when adding sequentially to the model was evaluated by the integrated discrimination improvement (IDI) index. When two nested models are compared, IDI quantifies the increment in the predicted probabilities for the subset of patients experiencing the event and the decrease for those not experiencing the event. In simpler terms, it reflects an improvement in the average of the true positive rate without sacrificing its average true negative rate[28]. The proportionality assumption for the hazard function over time was tested by means of the Schoenfeld residuals. The discriminative ability of the final model was assessed by Harrell’s C-statistic, and the calibration ability was assessed by the Gronnesby and Borgan test[29]. A two-sided P < 0.05 was considered statistically significant for all analyses. All analyses were performed using SPSS version 19.0 (Statistical Package for the Social Sciences, Chicago, Illinois) and R (R: A Language and Environment for Statistical Computing at http://www.R-project.org).
Table 1.
Baseline characteristics by carbohydrate antigen 125 and N-terminal pro-brain natriuretic peptide class
| Variable | CA125 < 60 | CA125 > 60 | CA125 < 60 | CA125 > 60 | P |
| NT-proBNP < 3100 | NT-proBNP < 3100 | NT-proBNP > 3100 | NT-proBNP > 3100 | ||
| (n = 95, 61%) | (n = 17, 11%) | (n = 24, 15%) | (n = 20, 13%) | ||
| Male | 55 (58%) | 11 (65%) | 19 (79%) | 14 (70%) | 0.240 |
| Age | 71 ± 12 | 69 ± 10 | 76 ± 12 | 73 ± 15 | 0.309 |
| Hypertension | 73 (77%) | 10 (59%) | 18 (75%) | 14 (70%) | 0.471 |
| Diabetes | 38 (40%) | 7 (41%) | 11 (46%) | 8 (40%) | 0.964 |
| Dyslipidemia | 46 (48%) | 5 (29%) | 11 (46%) | 7 (35%) | 0.406 |
| Atrial fibrillation | 47 (49%) | 9 (53%) | 17 (71%) | 11 (55%) | 0.316 |
| NYHA class II | 85 (89%) | 7 (41%) | 13 (54%) | 11 (55%) | < 0.001 |
| NYHA class III | 10 (11%) | 10 (59%) | 11 (46%) | 9 (45%) | < 0.001 |
| HF Etiology | |||||
| Hypertension | 26 (27%) | 1 (6%) | 5 (21%) | 3 (15%) | 0.212 |
| Ischemic heart disease | 30 (32%) | 7 (41%) | 8 (33%) | 9 (45%) | 0.641 |
| Dilated cardiomyopathy | 8 (8%) | 2 (12%) | 1 (4%) | 2 (10%) | 0.768 |
| Valve heart disease | 15 (16%) | 5 (29%) | 4 (17%) | 5 (25%) | 0.439 |
| Congenital | 0 (0%) | 1 (6%) | 1 (4%) | 1 (5%) | 0.058 |
| Others | 16 (17%) | 1 (6%) | 5 (21%) | 0 (0%) | 0.110 |
| Systolic BP (mmHg) | 129 ± 18 | 118 ± 20 | 119 ± 21 | 114 ± 12 | 0.001 |
| Diastolic BP (mmHg) | 74 ± 12 | 67 ± 8 | 73 ± 14 | 71 ± 9 | 0.156 |
| LVEF (%) | 51 ± 16 | 46 ± 16 | 46 ± 22 | 38 ± 18 | 0.015 |
| LVDD (mm) | 54 ± 8 | 52 ± 9 | 56 ± 13 | 57 ± 11 | 0.290 |
| LAD (mm) | 49 ± 9 | 49 ± 9 | 51 ± 9 | 54 ± 12 | 0.184 |
| IVS (mm) | 12 ± 3 | 12 ± 2 | 13 ± 3 | 11 ± 3 | 0.158 |
| LVPW (mm) | 11 ± 2 | 10 ± 2 | 11 ± 2 | 10 ± 2 | 0.461 |
| Na+ (mEq/dL) | 140 ± 3 | 138 ± 4 | 140 ± 4 | 139 ± 5 | 0.227 |
| K+ (mEq/dL) | 4.2 ± 0.5 | 4.3 ± 0.6 | 4.4 ± 0.7 | 4.2 ± 0.5 | 0.453 |
| GF (mL/min per 1.73 m2) | 61 ± 20 | 62 ± 16 | 49 ± 22 | 53 ± 20 | 0.029 |
| Hemoglobin (g/dL) | 131 ± 20 | 118 ± 25 | 126 ± 21 | 129 ± 24 | 0.111 |
LVEF: Left ventricular ejection fraction; NYHA: New York Heart Association; HF: Heart failure; LVDD: Left ventricular diastolic diameter; LAD: Left auricular diameter; IVS: Interventricular septum; LVPW: Left ventricular posterior wall; BP: Blood pressure; Na: Sodium; K: Potassium; GF: Glomerular filtration; CA125: Carbohydrate antigen 125; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
RESULTS
Baseline clinical characteristics
The mean age of the studied population was 72 ± 12 years; 63% were male. Clinical characteristics of the studied population and HF treatments according to the specified 4 groups are shown in Table 1. Mean ejection fraction was 48% ± 17%, and half (77) of the patients had preserved ejection fraction. Ischemic heart disease was the most frequent etiology of HF, followed by hypertension and valve disease. The percentage of patients with HFrEF treated with ACEI/ARB was 85%, with beta-blockers 76% and with mineral corticoid receptor antagonists 56%, while in the HFpEF group the percentage of patients treated with ACEI/ARB was 69%, with beta-blockers 42% and with mineral corticoid receptor antagonists 39%. The percentage of patients treated with furosemide was similar in both groups, 84%.
Outcomes
No patient was lost during the mean follow-up of 17 ± 8 mo (range 2 to 32 mo). Monitoring of patients was blinded to CA125 values. During follow-up, 27 patients died (17%), and 1 had progressive HF deterioration requiring urgent heart transplantation at one year. All 27 deaths were due to cardiovascular causes, 23 (85%) of them to progressive HF (3 of which required hospital admission for decompensated HF) and 3 to cerebrovascular accident. The remaining patient had a diagnosis of ischemic heart disease and was lost to sudden death while being treated with chemotherapy for rectal cancer that appeared during follow-up. Mortality was similar for women (19%) and men (18%). There was a high incidence of worsening, new HF episodes: 106 patients required an admission for decompensated HF (68%), and nearly half (50 patients) were admitted more than once for HF during follow-up.
Prognostic implications of CA125
Older age, increased ventricular septum thickness and left atrial diameter, low eGFR and hemoglobin concentration, higher CA125 and NT-proBNP levels, the presence of atrial fibrillation, and worse NYHA functional class were all associated with mortality or need for urgent heart transplantation by univariate analyses (Table 2). Hospitalization for worsening HF was associated with low eGFR, higher CA125 and NT-proBNP values, presence of atrial fibrillation, and worse NYHA functional class by univariate analyses (Table 3). Patients who died and those who required admission for decompensated HF had significantly lower eGFR, had higher CA125 and NT-proBNP, and more frequently had atrial fibrillation and NYHA functional class III. In univariate analyses, ln-CA125 was positively associated with ln-NT-proBNP (β = 0.39, P = 0.0001) and negatively with left ventricular ejection fraction (β = -0.23, P = 0.003) and sodium concentration (β = -0.24, P = 0.003). There was no relationship between ln-CA125 and eGFR or hemoglobin. The receiver operating characteristic curve analysis showed similar values for CA125 and NT-proBNP; the AUC for mortality prediction was 0.699 (95%CI: 0.59-0.80) for CA125 and 0.710 (95%CI: 0.59-0.82) for NT-proBNP.
Table 2.
Univariate analysis, variables significantly associated with mortality or need for urgent cardiac transplantation
| Variable | Deaths | Alive | P |
| Age | 78 ± 12 | 70 ± 12 | 0.004 |
| LVEF (%) | 53 ± 16 | 47 ± 17 | 0.08 |
| IVS (mm) | 13.7 ± 3.4 | 11.9 ± 2.5 | 0.005 |
| LA (mm) | 54 ± 12 | 49 ± 9 | 0.01 |
| GF (MDRD) | 46 ± 18 | 61 ± 19 | 0.0001 |
| Hemoglobin (g/L) | 121 ± 21 | 130 ± 21 | 0.02 |
| Na+ (mEq/mL) | 140 ± 4 | 139 ± 3 | 0.54 |
| K+ (mEq/mL) | 4.3 ± 0.6 | 4.2 ± 0.5 | 0.54 |
| Systolic BP (mmHg) | 120 ± 19 | 125 ± 19 | 0.24 |
| Diastolic BP (mmHg) | 70 ± 12 | 73 ± 12 | 0.25 |
| CA125 (KU/L) | 94 ± 121 | 45 ± 78 | 0.01 |
| NT-proBNP (pg/dL) | 6613 ± 8437 | 2326 ± 2823 | 0.02 |
| AF (%) | 71 | 50 | 0.05 |
| NYHA class III (%) | 70 | 30 | 0.03 |
LVEF: Left ventricular ejection fraction; IVS: Interventricular septum; LA: Left atrial; GF: Glomerular filtration; Na: Sodium; K: Potassium; BP: Blood pressure; CA125: Carbohydrate antigen 125; NT-proBNP: N-terminal pro-B-type natriuretic peptide; AF: Atrial fibrillation; NYHA: New York Heart Association; MDRD: Modification of diet in renal disease.
Table 3.
Univariate analysis, variables significantly associated with need for hospitalization for decompensated heart failure
| Hospitalization | No hospitalization | P | |
| LVEF (%) | 48 ± 18 | 49 ± 17 | 0.7 |
| IVS (mm) | 12 ± 3 | 11 ± 3 | 0.14 |
| LA (mm) | 50 ± 9 | 48 ± 10 | 0.15 |
| GF (MDRD) | 56 ± 19 | 64 ± 21 | 0.01 |
| Hemoglobin (g/L) | 127 ± 22 | 132 ± 20 | 0.16 |
| Na+ (mEq/mL) | 139 ± 3.7 | 140 ± 3.6 | 0.4 |
| K+ (mEq/mL) | 4.2 ± 0.5 | 4.3 ± 0.6 | 0.3 |
| Systolic BP (mmHg) | 124 ± 18 | 125 ± 20 | 0.9 |
| Diastolic BP (mmHg) | 73 ± 11 | 72 ± 13 | 0.7 |
| CA125 (KU/L) | 58 ± 85 | 34 ± 61 | 0.01 |
| NT-proBNP (pg/dL) | 3431 ± 4792 | 2031 ± 3234 | 0.03 |
| AF (%) | 61 | 39 | 0.01 |
| NYHA class III (%) | 97 | 3 | 0.0001 |
LVEF: Left ventricular ejection fraction; IVS: Interventricular septum; LA: Left atrial; GF: Glomerular filtration; Na: Sodium; K: Potassium; BP: Blood pressure; CA125: Carbohydrate antigen 125; NT-proBNP: N-terminal pro-B-type natriuretic peptide; AF: Atrial fibrillation; NYHA: New York Heart Association.
The relationship between CA125 and outcomes was evaluated in multivariate Cox proportional analyses. Cox-model 1 identified NT-proBNP ≥ 3100 ng/L and Cox-model 2 identified CA125 ≥ 60 KU/L as independent predictors of mortality (Table 4). Two Cox models were compared to test improvement in risk stratification: Cox-model 1 (adjusted model with NT-proBNP ≥ 3100 ng/L) vs Cox-model 3 (adjusted model with 4 groups according to the cut-off concentrations of CA125 and NT-proBNP). Cox-model 1 had good discriminative ability (Harrell’s C-statistic = 0.774), and Cox-model 3 had slightly better discriminative ability (Harrell’s C-statistic = 0.777) and good calibration ability (P = 0.39, Gronnesby and Borgan test). However, the C-statistic is not sensitive to changes when a new factor is included in a model. Therefore, when we compared Cox-model 1 vs Cox-model 3 by the IDI index, the discrimination slope of the Cox-model 3 was 4 percentage points higher than model 1 (P < 0.05). Survival curves adjusted by propensity scores from Cox-model 3 (Figure 1) showed risk stratification when HF patients were classified into the 4 groups defined by NT-ProBNP and CA125 cut-offs. Patients with both biomarkers elevated presented the worst survival.
Table 4.
Multivariate analysis for global mortality
| HR | 95%CI | Sig | |
| Cox-model 1 variable | |||
| NT-proBNP (3100 ng/L) | 4.95 | 2.11-11.62 | < 0.001 |
| Cox-model 2 variable | |||
| CA125 (60 KU/L) | 3.32 | 1.50-7.37 | 0.003 |
| Cox-model 2 variable | |||
| CA125 < 60 NT-proBNP < 3100 | 1 | ||
| CA125 > 60 NT-proBNP < 3100 | 2.49 | 0.65-9.53 | 0.18 |
| CA125 < 60 NT-proBNP > 3100 | 4.15 | 1.41-12.24 | < 0.01 |
| CA125 > 60 NT-proBNP > 3100 | 8.95 | 3.11-25.73 | < 0.001 |
Adjusted by propensity scores of NT-proBNP > 3100 ng/L and CA125 > 60 KU/L categories. CA125: Carbohydrate antigen 125; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
Figure 1.
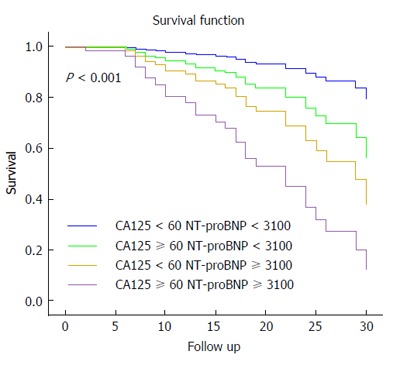
Kaplan-Meier survival curves in the 4 groups defined by N-terminal pro-brain natriuretic peptide < or ≥ 3100 ng/L and carbohydrate antigen 125 < or ≥ 60 KU/L. Patients with both biomarkers elevated presented the worst survival. CA125: Carbohydrate antigen 125; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
DISCUSSION
In our study, a concentration of CA125 > 60 KU/L was identified as an independent predictor of mortality or of the need for urgent heart transplantation at mid-term follow-up. This biomarker also added prognostic information beyond that provided by NT-proBNP concentration. Patients with NT-proBNP ≥ 3100 ng/L and CA125 ≥ 60 KU/L had extremely low survival during follow-up.
CA125 and HF
CA125 is a cancer marker that can be synthesized in mesothelial cells from the peritoneum and pleura[30]. CA125 concentration is increased in patients with pericardial, pleural, and peritoneal effusions[30]. Increased CA125 concentration in patients with HF is believed to be multifactorial and secondary to the increased end-diastolic and pulmonary venocapillary pressure that causes interstitial pulmonary edema[31], an inflammatory substrate present in HF. In addition, the appearance of pleural effusion and signs of congestion have been associated with increased CA125[32].
The good correlation of CA125 with natriuretic peptides suggests a similar release mechanism related to an increase in intracavitary pressures and stress of both atrial and ventricular walls. It has also been suggested that mesothelial cells can secrete CA125 in response to activation of cytokines. Thus, the activation of either TNF-alpha or interleukin-6 has been associated with elevated CA125, and the increase of these cytokines correlates with the severity of HF[7,18]. It has been suggested that increased CA125 can be a sign of both congestion and inflammation; both parameters have been associated with poor prognosis in HF patients. Furthermore, due to the long half-life of CA125 (about 12 d) it can be used as an indirect marker of fluid retention in the weeks preceding blood extraction, in both acute and chronic HF. Especially in patients with stable HF, CA125 ≥ 60 KU/L can be an early sign of congestion that would identify patients with worse prognosis.
Previous studies
Numerous studies have examined the value of natriuretic peptides to assess the prognosis of HF[33-36], most of them performed in the emergency room or in patients with acute decompensated HF requiring hospitalization. Few of the studies focus on stable chronic HF[37,38]. Earlier studies in acute HF patients associated increased CA125 with worse NYHA functional class, greater left atrial size, higher BNP, and poor prognosis at 6 mo follow-up[19,20,22]. A recent study by Ordu et al[37] reports that increased CA125 and NT-proBNP were similar in their capacity to predict prognosis in patients with stable HF[38]. Although our population differed from theirs, which included only patients with systolic dysfunction and elevated NT-proBNP, the CA125 concentrations were similar in both studies. Our patients were better controlled, with higher proportions receiving ACEI/ARB, beta-blockers and spironolactone therapy. Both studies report a significant correlation between CA125 and NT-proBNP. However, Ordu et al[37] used a higher NT-proBNP cut-off value for patient stratification than previous studies[11,15]. It is possible that with this high cut-off value the addition of CA125 did not improve upon the predictive value obtained with only NT-proBNP. However, lower NT-proBNP is frequently found in stable HF patients. In our study, CA125 ≥ 60 KU/L added prognostic information to that obtained from the ≥ 3100 ng/L NT-proBNP cut-off. In fact, when both biomarkers were elevated, the prognosis was very poor. In another recent study performed in stable patients with left ventricular dysfunction, a value of CA125 ≥ 60 KU/L was associated with an increased risk of cardiovascular death and hospitalization for HF[22]. Our study did not select patients according to the degree of left ventricular dysfunction; all patients diagnosed with HF were included. A high level of CA125 allowed the identification of patients with worse prognosis independently of their ejection fraction. This is important because almost half of all patients diagnosed with HF in the majority of epidemiological studies have preserved ejection fraction[39]. Furthermore, new biomarkers are under development to improve diagnosis and prognosis assessment in HF. Recently, experimental studies have suggested that changes in circulating microRNAs can be used as a biomarker of disease[40,41]. However, these new molecular markers are still under investigation, On the contrary, more than 10 years of experience with natriuretic peptides and CA125 have already been reported[12,20].
Although the value of natriuretic peptides for the diagnosis of HF has been widely demonstrated, its usefulness to assess prognosis in some cases remains controversial. Thus, in advanced HF, there is controversy whether BNP concentration helps identify patients requiring heart transplantation[42,43]. Similarly, in asymptomatic individuals with HF and those in pre-HF stages, NT-proBNP failed to predict the patients who presented with clinical HF during follow-up[44]. Other limitations to the use of natriuretic peptides exist, particularly with NT-proBNP since cut-off points for assessing prognosis have not been clearly established. Therefore, the addition of CA125 may help to improve prognosis assessment in patients with stable HF.
Limitations
In this study the inclusion criterion was the diagnosis of HF. The predictive value of CA125 possibly could have been even greater if left ventricular dysfunction had been a selection criterion. Although the cohort was relatively small, mortality and hospitalization rates were similar to previous studies analyzing stable HF patients. When the study population was divided according to CA125 and NT-proBNP values, some subgroups were quite small. Nonetheless, increased CA125 concentration was effective in identifying stable HF patients at high risk of death and new admissions for worsening HF, and despite having a relatively small sample size the results are consistent with those obtained in previous studies in acute HF.
In conclusion, CA125 is an excellent marker of prognosis in patients with stable HF. CA125 ≥ 60 KU/L identified patients at high risk of death or need for urgent heart transplantation. CA125 added prognostic information to the predictive value of NT-proBNP. Its easy determination and low cost may encourage its expanded use.
ACKNOWLEDGMENTS
The authors are especially grateful to Elaine Lilly, Ph.D. for English language revision of the manuscript.
COMMENTS
Background
Heart failure (HF) is the final phase of many heart diseases. The estimated prevalence is up to 3% in the European population. This prevalence increases exponentially with age. As the main cause of hospitalization, in patients over 65, HF is also associated with high costs. Despite recent advances in treatment, mortality is still high (12% per year) in stable patients, and there is a high rate of hospital readmissions due to worsening HF. Even though a high number of clinical parameters have been associated with poor outcome in patients with stable HF, the assessment of prognosis is still a challenge.
Research frontiers
Several biomarkers have been used to assess the prognosis of patients with HF. Although the natriuretic peptides are considered the most widely used biomarkers, they still have some limitations. Increased concentrations of natriuretic peptides have been associated with worse prognosis in patients hospitalized for worsening HF. However, its utility to identify patients at high risk of death in stable HF has been less studied. Furthermore, although new molecular biomarkers are under development to improve prognosis assessment in HF, they are not ready for clinical use. On the other hand, more than 10 years of experience with natriuretic peptides and carbohydrate antigen 125 (CA125) have already been reported.
Innovations and breakthroughs
This study provides the results of a clinical investigation demonstrating that the combination of two well-known biomarkers, CA125 and N-terminal pro-brain natriuretic peptide (NT-proBNP), is useful to select patients with stable HF and poor outcome. CA125 concentration, when added to NT-proBNP, provides relevant prognostic information.
Applications
CA125 concentration can be routinely added to NT-proBNP assessment in stable HF patients, at a low cost, to identify patients at high risk of death or a worsening HF episode.
Terminology
CA125 is a biomarker previously used in the detection and monitoring of some cancers. It is a high-molecular-weight glycoprotein synthesized by epithelial cells of the serosa when there is inflammation and increased interstitial fluid. Elevated CA125 has also been found in patients with HF, with or without fluid retention.
Peer review
The authors report on the prognostic valve of CA125 in patients with stable chronic HF. This biomarker has proven its validity in previous studies, in this manuscript has additive value, due to the combination of the new biomarker with the established brain natriuretic peptide-value.
Footnotes
P- Reviewers: Deshpande SR, Ghanem A, Panduranga P, Santulli G S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
References
- 1.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56:362–368. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 2.Santulli G. Epidemiology of Cardiovascular Disease in the 21 century: update numbers and update facts. J Cardiovas Dis. 2013;1:1–2. [Google Scholar]
- 3.Cleland JG, Khand A, Clark A. The heart failure epidemic: exactly how big is it? Eur Heart J. 2001;22:623–626. doi: 10.1053/euhj.2000.2493. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Santulli G. Coronary heart disease risk factors and mortality. JAMA. 2012;307:1137; author reply 1138. doi: 10.1001/jama.2012.323. [DOI] [PubMed] [Google Scholar]
- 6.Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, Deswal A, Bozkurt B. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161:567–573. doi: 10.1016/j.ahj.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orús J, Roig E, Perez-Villa F, Paré C, Azqueta M, Filella X, Heras M, Sanz G. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. 2000;19:419–425. doi: 10.1016/s1053-2498(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 8.Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 9.Bartunek J. Biomarkers for coronary artery disease: mission impossible? Biomark Med. 2010;4:339–340. doi: 10.2217/bmm.10.62. [DOI] [PubMed] [Google Scholar]
- 10.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayes-Genis A, de Antonio M, Galán A, Sanz H, Urrutia A, Cabanes R, Cano L, González B, Díez C, Pascual T, et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012;14:32–38. doi: 10.1093/eurjhf/hfr156. [DOI] [PubMed] [Google Scholar]
- 12.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 13.Bayés-Genís A, Santaló-Bel M, Zapico-Muñiz E, López L, Cotes C, Bellido J, Leta R, Casan P, Ordóñez-Llanos J. N-terminal probrain natriuretic peptide (NT-proBNP) in the emergency diagnosis and in-hospital monitoring of patients with dyspnoea and ventricular dysfunction. Eur J Heart Fail. 2004;6:301–308. doi: 10.1016/j.ejheart.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Maisel A, Hollander JE, Guss D, McCullough P, Nowak R, Green G, Saltzberg M, Ellison SR, Bhalla MA, Bhalla V, et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44:1328–1333. doi: 10.1016/j.jacc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien TJ, Tanimoto H, Konishi I, Gee M. More than 15 years of CA 125: what is known about the antigen, its structure and its function. Int J Biol Markers. 1998;13:188–195. doi: 10.1177/172460089801300403. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz MB, Nikolaou M, Cohen Solal A. Tumour biomarkers in heart failure: is there a role for CA-125? Eur J Heart Fail. 2011;13:579–583. doi: 10.1093/eurjhf/hfr022. [DOI] [PubMed] [Google Scholar]
- 18.Kosar F, Aksoy Y, Ozguntekin G, Ozerol I, Varol E. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur J Heart Fail. 2006;8:270–274. doi: 10.1016/j.ejheart.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Núñez J, Núñez E, Consuegra L, Sanchis J, Bodí V, Martínez-Brotons A, Bertomeu-González V, Robles R, Bosch MJ, Fácila L, et al. Carbohydrate antigen 125: an emerging prognostic risk factor in acute heart failure? Heart. 2007;93:716–721. doi: 10.1136/hrt.2006.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Aloia A, Faggiano P, Aurigemma G, Bontempi L, Ruggeri G, Metra M, Nodari S, Dei Cas L. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J Am Coll Cardiol. 2003;41:1805–1811. doi: 10.1016/s0735-1097(03)00311-5. [DOI] [PubMed] [Google Scholar]
- 21.Duman D, Palit F, Simsek E, Bilgehan K. Serum carbohydrate antigen 125 levels in advanced heart failure: relation to B-type natriuretic peptide and left atrial volume. Eur J Heart Fail. 2008;10:556–559. doi: 10.1016/j.ejheart.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Núñez J, Sanchis J, Bodí V, Fonarow GC, Núñez E, Bertomeu-González V, Miñana G, Consuegra L, Bosch MJ, Carratalá A, et al. Improvement in risk stratification with the combination of the tumour marker antigen carbohydrate 125 and brain natriuretic peptide in patients with acute heart failure. Eur Heart J. 2010;31:1752–1763. doi: 10.1093/eurheartj/ehq142. [DOI] [PubMed] [Google Scholar]
- 23.Vizzardi E, Nodari S, D’Aloia A, Chiari E, Faggiano P, Metra M, Dei Cas L. CA 125 tumoral marker plasma levels relate to systolic and diastolic ventricular function and to the clinical status of patients with chronic heart failure. Echocardiography. 2008;25:955–960. doi: 10.1111/j.1540-8175.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen PK, Schnittger I, Heidenreich PA. A comparison of echocardiographic measures of diastolic function for predicting all-cause mortality in a predominantly male population. Am Heart J. 2011;161:530–537. doi: 10.1016/j.ahj.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Pattanayak CW, Rubin DB, Zell ER. [Propensity score methods for creating covariate balance in observational studies] Rev Esp Cardiol. 2011;64:897–903. doi: 10.1016/j.recesp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Núñez E, Steyerberg EW, Núñez J. [Regression modeling strategies] Rev Esp Cardiol. 2011;64:501–507. doi: 10.1016/j.recesp.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207-212. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 29.May S, Hosmer DW. Hosmer and Lemeshow type goodness-of-fit statistics for the Cox proportional hazards model. Advances in Survival Analysis. 2003;23:383–394. [Google Scholar]
- 30.Epiney M, Bertossa C, Weil A, Campana A, Bischof P. CA125 production by the peritoneum: in-vitro and in-vivo studies. Hum Reprod. 2000;15:1261–1265. doi: 10.1093/humrep/15.6.1261. [DOI] [PubMed] [Google Scholar]
- 31.Sevinc A, Buyukberber S, Sari R, Kiroglu Y, Turk HM, Ates M. Elevated serum CA-125 levels in hemodialysis patients with peritoneal, pleural, or pericardial fluids. Gynecol Oncol. 2000;77:254–257. doi: 10.1006/gyno.2000.5776. [DOI] [PubMed] [Google Scholar]
- 32.Turk HM, Pekdemir H, Buyukberber S, Sevinc A, Camci C, Kocabas R, Tarakcioglu M, Buyukberber NM. Serum CA 125 levels in patients with chronic heart failure and accompanying pleural fluid. Tumour Biol. 2003;24:172–175. doi: 10.1159/000074425. [DOI] [PubMed] [Google Scholar]
- 33.Pascual-Figal DA, Domingo M, Casas T, Gich I, Ordoñez-Llanos J, Martínez P, Cinca J, Valdés M, Januzzi JL, Bayes-Genis A. Usefulness of clinical and NT-proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J. 2008;29:1011–1018. doi: 10.1093/eurheartj/ehn023. [DOI] [PubMed] [Google Scholar]
- 34.Tang WH. Biomarkers of risk stratification in congestive heart failure: North American view. Biomark Med. 2009;3:443–452. doi: 10.2217/bmm.09.52. [DOI] [PubMed] [Google Scholar]
- 35.Bayes-Genis A, Vazquez R, Puig T, Fernandez-Palomeque C, Fabregat J, Bardají A, Pascual-Figal D, Ordoñez-Llanos J, Valdes M, Gabarrús A, et al. Left atrial enlargement and NT-proBNP as predictors of sudden cardiac death in patients with heart failure. Eur J Heart Fail. 2007;9:802–807. doi: 10.1016/j.ejheart.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 37.Ordu S, Ozhan H, Alemdar R, Aydin M, Caglar O, Yuksel H, Kandis H. Carbohydrate antigen-125 and N-terminal pro-brain natriuretic peptide levels: compared in heart-failure prognostication. Tex Heart Inst J. 2012;39:30–35. [PMC free article] [PubMed] [Google Scholar]
- 38.Vizzardi E, D’Aloia A, Pezzali N, Bugatti S, Curnis A, Dei Cas L. Long-term prognostic value of CA 125 serum levels in mild to moderate heart failure patients. J Card Fail. 2012;18:68–73. doi: 10.1016/j.cardfail.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG, van Rooij E. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15:650–659. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- 41.Santulli G, Campanile A, Spinelli L, Assante di Panzillo E, Ciccarelli M, Trimarco B, Iaccarino G. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1125–1130. doi: 10.1016/j.amjcard.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Pirracchio R, Salem R, Mebazaa A. Use of B-type natriuretic peptide in critically ill patients. Biomark Med. 2009;3:541–547. doi: 10.2217/bmm.09.45. [DOI] [PubMed] [Google Scholar]
- 43.Vidal B, Roig E, Pérez-Villa F, Orús J, Pérez J, Jiménez V, Leivas A, Cuppoletti A, Roqué M, Sanz G. [Prognostic value of cytokines and neurohormones in severe heart failure] Rev Esp Cardiol. 2002;55:481–486. doi: 10.1016/s0300-8932(02)76639-6. [DOI] [PubMed] [Google Scholar]
- 44.McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, Rodeheffer RJ, Burnett JC. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–2147. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


