Abstract
The optimal vascular access for elderly patients remains a challenge due to the difficulty balancing the benefits and risks in a population with increased comorbidity and decreased survival.
Age is commonly associated with failure to mature in fistula and decreased rates of primary and secondary patency in both fistula and grafts. In the elderly, at 1 and 2-years, primary patency rates range from 43% to 74% and 29% to 67%, respectively. Secondary patency rates at 1 and 2-years range from 56% to 82% and 44% to 67% respectively. Cumulative fistula survival is no better than grafts survival when primary failures are included. Several observational studies consistently demonstrate a lower adjusted mortality among those using a fistula compared to a catheter (1–3)(1–3) however catheter use in the elderly is increasing in most countries with the exception of Japan.
Both guidelines and quality initiatives do not acknowledge the trade-offs involved in managing the elderly patients with multiple chronic conditions and limited life expectancy or the value that patients place on achieving these outcomes(4)(4). The framework for choice of vascular access presented in this article considers: 1) likelihood of disease progression before death 2) patient life expectancy, 3) risks and benefits by vascular access type and 4) patient preference. Future studies evaluating the timing and type of vascular access with careful assessments of complications, functionality, cost benefit, and patients’ preference will provide relevant information to individualize and optimize care to improve morbidity, mortality, and quality of life in the elderly patient.
Keywords: vascular access, elderly, hemodialysis
Introduction
There are a rapidly growing number of elderly patients among the incident hemodialysis population with a high prevalence of comorbidities, shortened life expectancy and reduced quality of life. The establishment and maintenance of the optimal vascular access both in terms of timing and type of access for elderly patients presents major challenges, particularly in view of the heterogeneity of outcomes in this population. Recent studies highlight the high rate of primary failure in native fistulae identifying patient characteristics associated with reduced fistula use (1;5;6), yet there are no standard patient eligibility criteria to guide fistulae placement. Older age has been associated with lower rates of fistula use (5;7), which is partially attributable to a decrease in referral for fistula as well as increased rates of failure to mature (FTM) among those who do get a fistula (6). Clinical guidelines and recommendations are rarely age specific and do not address issues in the context of the older patient despite these notable differences in comorbidities, patient preference, and pathophysiology in the natural history of chronic kidney disease (CKD) in older vs. younger individuals. Recommendations to consider the fistula as the access of choice and for all patients to be referred when their eGFR is < 15 mL/min/1.73 m2 or when expected to start dialysis in 6 months may not be the most rational or economic approach, and is certainly not reflected in the current practice (8). This article will review the current literature on vascular access in the elderly, addressing both the benefits and risks of each access type while highlighting some of the current challenges and strategies, and future areas of investigation.
Epidemiology of vascular access in the elderly
There has been a marked growth in the rate of dialysis initiation in the elderly. Elderly patients account for an increasing fraction of patients on renal replacement worldwide, reaching 25–30% in most end stage kidney disease (ESKD) registries (9;10). In the United States(US), the proportion of patients > 65 years of age starting dialysis has increased by nearly 10% annually, representing an overall increase of 57% between 1996 and 2003 (10). In Canada, from 1990 until 2001, the dialysis incident rate among patients age 75 and older increased 74% (11). However, it should be noted that since 2005 incidence rates have slowly declined among patients 65-to-75 and 75 and older age groups (11). Similarly in Australia, rates of ESKD were increasing among patients >65 years from 2000–2005, but have now plateaued or declined in all age groups except those ≥85 years, which are still increasing (12). Currently 11% of patients on hemodialysis in Australia are ≥75 years or older (12).
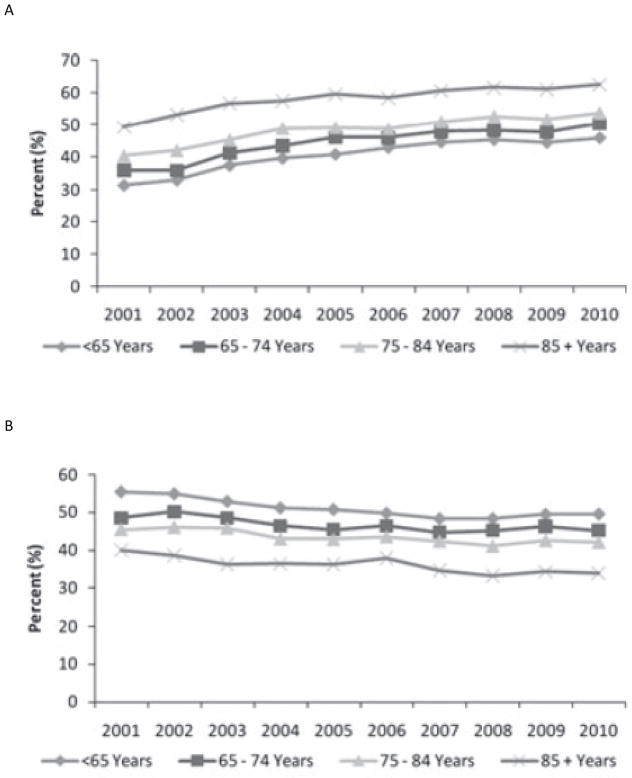
Central venous catheters are used more frequently in the elderly versus younger hemodialysis patients in Europe, Australia and North America but are rarely used in the elderly in Japan (13). The prevalent use of catheters in 2005–2007, among those ≥ 75 years old was 24% in Europe, 9% in Australia, 28% in North America and <1% in Japan. Use of arteriovenous (AV) grafts in those ≥ 75 years old varied from 7.1% in Europe to 23% in North America. Figure 1 illustrates the percentage change in prevalent vascular access use by age group from 2001–2010 in Canada (11). Catheters use increased from 36% to 50% in those between 65–74 years, 40% to 53% in 75–84 years and 49% to 62% in those > 85 years. Similar results were reported from the United States, where two-thirds of patients >65 years old were still using a catheter 3 months after starting dialysis (14). In Canada, prevalent AV graft use declined significantly in all age categories while fistula use remained stable although used less frequently in the elderly (11) (15). In Australia, prevalent catheter use in patients age ≥75 years is 13%, with 77% fistula use and 10% grafts (12). Incident catheter use is high among the elderly in Canada and the United States. In Canada, 79% of patients age 75–84 years and 88% of those > 85 years started dialysis with a catheter (11). While incident catheter use in Australia is high among patients ≥75 years (58%), it does not differ greatly from younger patients (61% age 55–74, 60% age 25–55 years) (12).
Figure 1.
Prevalent adult (>=18) hemodialysis patients by vascular access type and age group, Canada, 2001 to 2010. A) Central venous catheter B) Arteriovenous fistula C) Arteriovenous graft
Arteriovenous fistula outcomes in the elderly
Increased age has been associated with the non-maturing fistula with a more than doubling of the risk in those age >65 years (OR 2.23; 95% CI 1.25 to 3.96)(6). The association between increasing age and greater risk for FTM can be attributed to the need for adequate vessels, which deteriorate with the normal aging process and are damaged by concurrent disease; this finding is supported by other studies (16). Further, there is a wide range of reported fistula patency rates for fistulae among elderly patients, in part due to the differing classification of the elderly by age, differing comorbidities, inconsistent use of standard definitions for patency, the effect of the era, individual facility practice patterns, and the location of the fistula.
In the elderly, at 1 and 2-years, primary patency rates range from 43% to 74% and 29% to 67%, respectively. Secondary patency rates at 1 and 2-years range from 56% to 82% and 44% to 67% respectively (17–23). A recent observational study compared fistula and patient survival in dialysis-dependent patients older and younger than 70 years of age (24). Cumulative fistula survival at 12 months was 68% in the younger patients, but only 39% in those over age 70.
A meta-analysis of 13 cohort studies, reported primary and secondary patency rates for radiocephalic and brachiocephalic fistula comparing the elderly to the non-elderly (25). There was an increased primary failure rate and reduced patency in elderly patients at all time points compared to non-elderly adults for both types of access. There was a significantly higher rate of fistula failure in the elderly at 12 months; odds ratio (OR) 1.54; p=0.001 and 24 months OR 1.36; p=0.01 compared to the non-elderly. The radiocephalic fistula primary failure rate in the elderly vs. non-elderly was estimated at OR 1.79 (95% CI, 1.14 to 2.82).
Other studies have reported conflicting findings for the effects of patient’s age on the outcome of fistula (22;26;27). In a retrospective study, Lok studied the effect of age (>65 years vs. ≤ 65years) on outcomes in radiocephalic (52%), brachiocephalic (42.2%) and basilic vein transposed AVF (5.6%) (21). The one-year AVF cumulative survival was 75.1% (>65) and 79.7% (≤65 group); the five-year survival was 64.7% (>65) and 71.4% (≤ 65). Swindlehurst also reported similar rates of fistula patency in the elderly ( > 65years) compared to the non-elderly at 25 months follow up; primary patency 70% vs. 68% respectively, assisted primary patency rate 73% vs. 68% and secondary patency 73% and 79% respectively (22).
Physicians also need to determine the ideal location for fistula creation as this may influence the maturation and patency of the fistula. In a secondary analysis of the meta-analysis described above, brachiocephalic fistula were found to have a statistically significant 12% higher 12-month patency rate compared to the radiocephalic fistula (25). In another study the risk of primary failure for elderly males and females was 69% and 78% for forearm fistulas and 40% and 39% for upper arm fistulas (28). The data on location of the access is limited by observational designs and small sample size.
Arteriovenous graft outcomes in the elderly
Overall, prosthetic grafts are deemed as secondary or tertiary choices for access due to the lower primary and secondary patency rates and increased association with morbidity and mortality. However, grafts are considered the first choice of access for the elderly with comorbidities, particularly in the United States (8;13). Grafts are considered viable options in patients with failed fistula, exhausted, unsuitable, or damaged veins, late nephrologist referral, and need for urgent cannulation with avoidance of central venous catheter (29).
The literature is lacking on outcomes of grafts in the elderly, over the last decade. Using data from the 1990’s, Staramos reported the cumulative primary patency at 1 and 2-years was 81% and 65% among 67 elderly patients over the age of 70 years (23). Further, the secondary patency was reported to be 65% and 58% at 1 and 2-years respectively. Overall, the rate of surgical revisions among patients was 0.55 per graft-year. Berardinelli reported lower cumulative patency rates at 1 and 2-years (30). The 1 and 2-years total patency rate among elderly patients with synthetic grafts was 44.2% and 38.6%. It should be noted that these patency rates were based on only eight patients.
Fistula versus graft outcomes in the elderly
In the overall ESKD population, cumulative fistula survival is no better than that obtained with grafts when primary failures are included in access survival analysis (29;31). Chan compared access survival by access type among those > 65 years using data from the United States Renal Data System (32). Use of a fistula vs. a graft was not associated with increased patency among non- diabetic elderly (OR 1.48, 95% CI 0.95–2.3) or diabetic elderly (OR 1.49, 95% CI 0.76–2.89). Interestingly, during the first 18 months after access creation, graft survival may actually be superior to that obtained with fistulas (33). This last observation is particularly relevant in patients whose expected survival is unlikely to exceed 1 to 2 years. In this patient subpopulation, placing a graft first may dramatically lengthen the proportion of the patient’s lifespan with freedom from catheter dependence and its potential complications (29).
Vascular access complications in the elderly
Complications related to vascular access have not been consistently reported in prospective trials by age category. Dialysis-associated steal syndrome is an uncommon but recognized complication access. Recent studies have found no significant differences in the mean age among patients with steal syndrome compared to those with no steal syndrome (34;35). However, age greater than 65 years has been recognized as a potential risk factor for dialysis-associated steal syndrome (36). Risks of bleeding and cannulation injury have not been reported by age specific rates, identifying a significant gap in the literature.
Patient outcomes by access type in the elderly
Several observational studies, examining only patients who have initiated dialysis, consistently demonstrate a lower adjusted mortality among those using a fistula compared to a catheter (1–3) with the adjusted mortality risk associated with graft use falling between the fistula and the catheter. Similar studies have been done comparing outcomes in the elderly by vascular access type. In a study from the Netherlands, the adjusted hazard ratio (HR) for in those ≥ 65 years using a catheter vs. a fistula was 1.54 (95% CI 1.13–2.12 ) for all cause mortality, HR 1.60 (95% CI 0.62–4.19 ) for infection-related mortality, and HR 1.67 (95% CI 1.04–2.68) for cardiovascular mortality (37). Using Medicare data from the 1990s, patients starting dialysis with a fistula had a lower likelihood of death compared to graft, HR 1.16, 95% CI 1.08–1.24 and catheter (HR 1.70, 95% CI 1.59–1.81) although direct comparison between fistula and grafts did not result in a significant difference (38). Others have demonstrated a modification of this effect when separating out unplanned catheters from planned catheters, likely addressing confounding from late referral or an acute event precipitating dialysis start (39).
Challenges to the selection of access type in the elderly
There are however, several unique challenges to selecting the optimal vascular access in the elderly that are not addressed in the observational trials reported above. Significant work has recently been published addressing an individualized approach to care versus a disease based model or worse, a guideline-based model (29;40–42;42;43). Quality improvement initiatives in ESKD care advocate for quality benchmarks but fail to identify patients who may not benefit from “standard of care” applicable to a “standard” patient. Unfortunately, the elderly are often excluded when the “standards” are initially developed and as such may not have direct applicability. Both guidelines and quality initiatives tend not to acknowledge the trade-offs involved in managing patients with multiple chronic conditions and limited life expectancy or the value that patients place on achieving these outcomes (4). The framework for choice of vascular access presented below will consider the: 1) likelihood of kidney disease progression before death 2) patient life expectancy, 3) the risks and benefits by vascular access type and 4) the patient preference.
The likelihood of kidney disease progression before death: Timing of vascular access placement
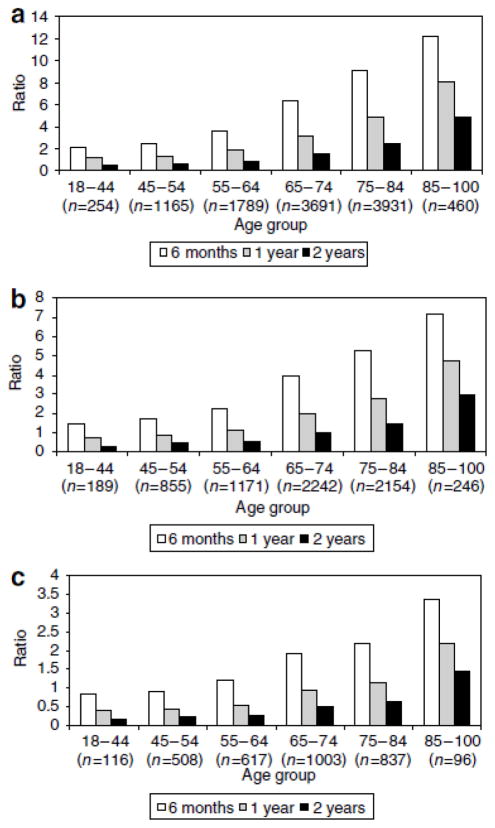
Pre-dialysis access decisions involve an expanded and more complex set of considerations compared with access decision for patients already on dialysis. As discussed above, the presence of a functioning graft or fistula is associated with improved outcomes among patients who begin dialysis; however, it does not address the complications and costs associated with access creation in patients who will never start dialysis. Patients who do not survive to the point of needing dialysis or decide not to go on dialysis cannot benefit from access placement. In an individual patient, the expected time before initiation of dialysis is usually unknown and clinical practice guidelines on this topic are largely opinion-based and quite variable. Current guidelines recommend patient referral for surgical creation of a vascular access when eGFR = 15–20 ml/minute/1.73 m2 or expected dialysis start within the next 12 months (44;45). Recently, the Canadian guidelines were modified to indicate that in the elderly (and other stable) patients with non-progressive CKD who are not expected to need to initiate HD, these task targets may or may not be relevant (46). Older patients lose renal function at slower rates than younger patients, have lower incidents of progression to end-stage kidney disease, and have shorter survival (47). O’Hare reported that among 85 to 100 year olds with an eGFR < 15 ml/min/1.73 m2 only 1 in 4 patients started dialysis within 6 months and only 1 in 3 started dialysis within a year (40). Recent studies have found that up to two-thirds of elderly patients who had undergone AVF placement die before their AVF was ever used for dialysis either because they did not start dialysis or their AVF did not reach maturity (24). In a theoretical model, the ratio of unnecessary to necessary permanent access surgeries, at different referral eGFR thresholds and survival estimates was always higher in older than younger patients (40). Figure 2 (reproduced with permission) illustrates that among patients referred for access at an eGFR < 15 ml/min/1.73m2, with a 6 months survival, three accesses would have to be created for every access used. This rate improves with a longer duration of life. These finding are important because unnecessary surgeries are more costly and carry a risk to patients with no benefit during the timeframe expected.
Figure 2.
Ratio of unnecessary to necessary permanent access surgeries at different theoretical referral eGFR thresholds by age and length of follow-up. (a) Referral threshold eGFRo25 ml/min/1.73m2. (b) Referral threshold eGFRo20 ml/min/1.73m2. (c) Referral threshold eGFRo15 ml/min/1.73m2. Reproduced with permission from reference 40.
Life expectancy and trade off with vascular access
Although life expectancy generally decreases with age, it can vary widely across individuals within a given age group. For example, in US patients aged 65–79 years at initiation of chronic dialysis therapy, median survival was approximately 2 years, but with an interquartile range of 8.3 months and more than 4 years (48). Several studies have identified risk factors associated with mortality among elderly patients on dialysis, and these need to be considered when deciding on the type of vascular access (49). The net benefit of different access strategies will vary between individuals as a function of life expectancy and quality of life. Patients whose life expectancy is less than 3–6 months will not benefit from fistula placement due the maturation time. A life expectancy of > 1 year would be required for elderly patients who require a longer time to maturation or who will require additional procedures and/or second access to justify fistula placement. It was estimated that an AV-graft survival was actually superior to that of a fistula for the first 18 months after creation suggesting that patients with a life expectancy of less that this do not experience the benefit of the longer patency expected from fistula placement (33). The need for prolonged catheter use during fistula maturation may be considered a harm if the patient will not live long enough to reap the benefits of a functioning fistula. The advantages and disadvantages of each form of access may also vary depending on the timing of the access placement relative to dialysis initiation. Tamura et al estimated the remaining lifetime absolute risk reduction in vascular access-related bacteremia attributable to the use of a preferred vs. non preferred access (fistula vs. AV-graft and AV-graft vs. catheter, respectively) for patients with different life expectancies (41). The results, presented in Table 1, (reproduced with permission) show that fistulae confer very modest reduction in risk of bacteremia compared with AV-graft, when measured from the time of first use. For example, among patients in the oldest age group with life expectancy in the 25th percentile, more than 200 fistulas would be needed to prevent one episode of AV-graft related bacteremia. The benefits of the fistula are more apparent among elderly patients with a longer life span. Importantly, any advantages of fistulas over AV-grafts disappear when they lag between access creation and access use is factored in. The number of grafts required to prevent a catheter-related bacteremia was much less given the high rate of catheters related bacteremia, however, the relative benefits of AV-grafts vs. catheter declines with age and life expectancy. One may consider delaying AV-graft creation until dialysis starts, due to the shortened time between AV- graft creation and use, however this practice resulted in an increased risk of catheter-related bacteremia when compared to those who had the AV-graft inserted before dialysis initiation (50). Another consideration in elderly patients is the length of time needed to achieve fistula maturation especially when weighed against the patient’s life expectancy. In a European study, fistula maturation required a longer time until functional permanent access was achieved compared to the catheter group among elderly patients (51). Additionally, the fistula group required multiple temporary catheter placements associated with an increased rate of infection and central vein thrombosis. This suggests that AVF placement may contribute to complications and infections if sufficient time is not provided for AVF to mature prior to the start of dialysis.
Table 1.
Number needed to treat with preferred access type to prevent one episode of vascular access–related bacteremia due to non-preferred access (reproduced with permission from reference 41)
| Treatment strategy to prevent bacteremia | 65–69 years
|
70–74 years
|
75–79 years
|
80–84 years
|
85–89 years
|
≥90 years
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of life expectancy
| ||||||||||||||||||
| 75th | 50th | 25th | 75th | 50th | 25th | 75th | 50th | 25th | 75th | 50th | 25th | 75th | 50th | 25th | 75th | 50th | 25th | |
| AVF vs. AVG Model 1 | 9 | 17 | 48 | 10 | 20 | 62 | 11 | 21 | 82 | 14 | 33 | 110 | 18 | 47 | 167 | 24 | 67 | 219 |
| AVF vs. AVG Model 2 | 27 | — | — | 35 | — | — | 62 | — | — | — | — | — | — | — | — | — | — | — |
| AVG vs. CVC Model 1 | <1 | 1 | 4 | <1 | 1 | 5 | <1 | 2 | 6 | 1 | 2 | 8 | 1 | 4 | 12 | 2 | 5 | 16 |
| AVG vs. CVC Model 3 | <1 | 1 | 4 | <1 | 2 | 5 | <1 | 2 | 7 | 1 | 3 | 9 | 1 | 4 | 15 | 2 | 5 | 21 |
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter.
‘—’ indicates life expectancy is shorter than time required to achieve benefit from intervention.
Model 1: Assumes both access types are functional at the start of dialysis.
Model 2: Assumes patients with AVF dialyze via CVC for 3 months while AVF matures and that patients with an AVG dialyze for 0.5 months with a CVC until the AVG is ready for use.
Model 3: Assumes patients with AVG dialyze via CVC for 0.5 months until the AVG is ready for use.
Patient preferences and decision-making
A patient with CKD nearing or on dialysis is faced with many different, difficult decisions, with each choice potentially encumbered by decision conflict, one of which might be deciding among a fistula, AV-graft, and catheter(52;53). Decision-making processes are individual to the patient, as those on dialysis do not always make decisions about vascular access based on the favorable morbidity and mortality statistics, but some patients live “one day at a time” and prefer the convenience of a CVC, which includes avoiding needles during cannulation, better appearance, and less bleeding (54–57). In a recent survey, satisfaction was highest in those among elderly patients on hemodialysis using catheters (58). In order to optimize the type of vascular access in this population we need to understand the barriers to doing so minimizing concerns with regard to what is important to patients and acknowledge and address their concerns.
Knowledge transfer to nephrologists, surgeons, administrators, and patients is critical in helping patients make their choice of vascular access. Poor personal experience with a fistula, testimonials from other patients, or even observation of struggles of other patients with a fistula can deter patients from choosing this access (57). Timing of knowledge transfer can be a challenge, as individuals are overwhelmed with their diagnosis and information at the start of dialysis. Thus, information on vascular access should be presented early as once patients are established in their dialysis modality and have a CVC as their vascular access, they are more inclined to maintain the status quo rather than consider a fistula or graft if appropriate, especially if they have not experienced (or downplay) complications with their CVC (8;53;55;57). Recent work has also solidified the importance of factoring in patient preferences in creating patient-care guidelines (59). Nephrologists and their team have a major role in shaping patient preference with their knowledge and experience of vascular access and patient outcomes; their role should be to advocate for the appropriate access for the individual patient. Meanwhile, patients will synthesize this information with their own values and beliefs to formulate a personal, informed choice on vascular access.
Concerns with vascular access-related procedures in the elderly
Vascular access (VA) procedures and complications represent an important cause of morbidity and mortality in the hemodialysis population (60). The elderly patient does require more frequent endovascular interventions to assist with the maturation process and/or maintaining patency of a surgically created vascular access (21). The growing trend of performing endovascular procedures in an out-patient setting brings in a unique set of challenges in an elderly ESKD pateint.
Elderly patients with ESKD patients have a high prevalence of co-morbid and disability increasing the risk related to conscious sedation that is generally used to perform these procedures. A thorough assessment of an elderly patient from suitability and safety of providing conscious sedation becomes a crucial component of the planning process. Assessments of a patient’s body habitus and condition, ability to lay supine and still during the procedure, cardiopulmonary status, oncologic and other medical history, and various psychosocial and ethical issues are critical to both the anesthesia care of these patients and the outcome of the procedure. Diseases common in the elderly patient such as arthritis, chronic obstructive pulmonary disease, pleural effusions from congestive heart failure, obstructive sleep apnea, and inability to cooperate because of dementia or language differences can make a seemingly straightforward procedure like placement of a tunneled catheter a very difficult problem in terms of sedation. Elderly patients may or may not have a supportive family or community network. The ability to be safely transported home following a procedure (including access creation) is often a limiting factor. The consequences of conscious sedation on ESKD patient with cognitive impairment (48;61) is an important component of preoperative assessment for an elective procedure. All elderly patients may not be suitable for procedures performed in an out-patient setting.
The frailty and malnutrition commonly seen in an elderly ESKD patient often leads to thin skin and easy bruising tendencies, causing a relatively higher incidence of skin tears, hematomas and minor complications that are often not documented or reported in studies. The role of anti-platelet drugs, often used to treat comorbid conditions, has not been studied, but can potentially increase the risk of bleeding complications.
Elderly ESKD patients have a higher incidence of peripheral vascular disease related to atherosclerosis, systolic hypertension, and arterial stiffness (32). The interventionalist should avoid being overzealous and aggressive while performing endovascular procedures as it can lead to ischemic steal syndrome (62).
Finally, performing procedures on elderly ESKD population who have a “Do Not Resuscitate” order creates an ethical dilemma. There is a potential risk of an adverse catastrophic cardiovascular event either from the medications used for sedation or from manipulating wires and catheters close to the heart during the procedure, which often times is performed electively. Should these procedures be avoided in such situations? Should the DNR-status be rescinded before the procedure? These ethical issues are evolving as the elderly ESKD population is growing and the nephrology community needs to consider these concerns in future.
Biological Considerations in the Elderly
While progression of CKD and timing of initiation of hemodialysis are important factors in placement of vascular access in elderly patients, additional considerations must include the biology of the elderly patients’ vascular beds in regards to pathophysiology such as calcification, oxidative stress and inflammation, and endothelial function. Indeed, the prevalence of arterial calcification has been shown to worsen with progression of CKD (63;64) and age (65–67). Recent studies have shown that arterial vascular disease and calcification plays a major role in the development of arterial steal syndrome, which includes symptoms such as cold hand, numbness, and hand pain (68;69). Furthermore, these arterial vascular changes may also play an important role in the development of arterial inflow problems after creation of a fistula and eventual fistula maturation (62;70;71). Venous calcificationhas recently been described to be present in one-third of veins used to create a new vascular access at the time of surgery (72)., and, thus, may be an additional factor in reduced fistula maturation in the elderly.
Aging has been shown to result in chronic and progressive low-grade systemic inflammation and oxidative stress (73–76), which is exacerbated by progression of CKD (77;78). Inflammation and oxidative stress markers within vein tissue have been shown to be associated with development of neointimal hyperplasia in the fistula and with fistula dysfunction. Thus, the chronic elevation in systemic inflammation and oxidative stress may be an important consideration in fistula maturation in an elderly population.
Finally, endothelial function has been shown through brachial artery flow mediated vasodilatation to be reduced in the elderly population (79). Endothelial function is important because it represents the ability of a vessel to produce nitric oxide and other beneficial mediators that promote vasodilation and inhibit vascular stenosis in response to vascular injury. On histology, in vein specimens collected at the time of vascular access surgery, severe pre-existing venous and arterial changes have been recently reported, likely resulting from poor endothelial function from the effects of uremia of the progressive CKD process (80–83). Thus, elderly patients receiving new vascular access may have poorer endothelial function placing them at higher risk for AVF maturation failure and vascular access dysfunction after placement.
Pragmatic Approach to Vascular Access in the Elderly
Clearly, determining an optimal vascular access for the elderly ESKD patient is challenging and dependent on multiple factors. The main considerations are life expectancy, complications from each vascular access, patient preference, and most importantly, the overall quality of life of the patient. While a “patient first” approach has been advocated within the context of the Fistula First Initiative, individualization is most important in the elderly population
For example, a fistula would be considered the most appropriate access in an elderly patient with minimal comorbidities, managed in a pre-dialysis clinic with an expected dialysis start of more than 6months. Even so, timing of the fistula creation must be considered in view of the lack of evidence for early initiation of dialysis (84) but the need for extra time for fistula maturation to avoid catheter use. In another situation, an AV- graft would also be considered appropriate in an elderly patient, with multiple comorbidities and a life expectancy of < 1–2 year. Lastly, a catheter is the least preferred option but would be an appropriate option in a patient with multiple comorbidities and a minimal life expectancy.
Allon and Lok have developed an algorithm to aid in decision making for vascular access (29); we emphasize however, that all of these decisions are dependent on the access to care, time to surgical creation, expertise of the surgeon and surgical outcomes, facility practice patterns, availability of procedures to assist with maturation, and the rates of complications including catheter related bacteremia.
Future Opportunities
We now recognize that decision making and care of the elderly patient is critically important on many levels and certainly requires more study regarding the optimal vascular access. The elderly patient has a very unique set of considerations and challenges including: comorbid conditions and vascular biology, rate of kidney decline, timing and initiation of dialysis, social issues, and life expectancy. Studies evaluating markers to determine linear progression of CKD and biological markers assessing vasculature health in the elderly are urgently needed. Future studies need to evaluate outcomes in the elderly focusing on the timing of initiation of vascular access care and the type of vascular access placement with careful assessments of complications, functionality, cost benefit, and, most importantly, the patients’ preference and quality of life. Ultimately, this information will provide relevant information to individualize and optimize care to improve morbidity, mortality, and quality of life in this elderly patient population.
Reference List
- 1.Moist LM, Trpeski L, Na Y, Lok CE. Increased hemodialysis catheter use in Canada and associated mortality risk: data from the Canadian Organ Replacement Registry 2001–2004. Clin J Am Soc Nephrol. 2008;3(6):1726–1732. doi: 10.2215/CJN.01240308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004;15(2):477–486. doi: 10.1097/01.asn.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 3.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16(5):1449–1455. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 4.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 5.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 7.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis. 2009;53(3):475–491. doi: 10.1053/j.ajkd.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 8.Xi W, Macnab J, Lok CE, Lee TC, Maya ID, Mokrzycki MH, et al. Who should be referred for a fistula? A survey of nephrologists. Nephrol Dial Transplant. 2010;25(8):2644–2651. doi: 10.1093/ndt/gfq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ERA-EDTA Registry: ERA-EDTA Registry 2005 Annual Report. Amsterdam, The Netherlands: Academis Medical Center, Department of Medical Informatics; Jun, 2007. 2005. [Google Scholar]
- 10.Bethesda M. U.S. Renal Data System. USRDS 2009 Annual Data Report Atlas of Chronic Kidney Disease and End-Stage Renal DIsease in the United States. National Institue of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 11.Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2000 to 2009. 2011. [Google Scholar]
- 12.Polkinghorne KR, Dent H, Gulyani A, Hurst K, McDonald SP. Australia and New Zealand Dialysis and Transplant Registry. 2011. Haemodialysis In ANZDATA Registry Report 2011; pp. 5–1pp. 5–41. [Google Scholar]
- 13.Canaud B, Tong L, Tentori F, Akiba T, Karaboyas A, Gillespie B, et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2011;6(7):1651–1662. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasse H, Speckman RA, Frankenfield DL, Rocco MV, McClellan WM. Predictors of delayed transition from central venous catheter use to permanent vascular access among ESRD patients. Am J Kidney Dis. 2007;49(2):276–283. doi: 10.1053/j.ajkd.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canadian Institute for Health Information, 2010 Annual Report - Treatment of End-Stage Organ Failure in Canada 1999 to 2008-CORR. Ottawa, Ont: CIHI; 2010. 2010. [Google Scholar]
- 16.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis. 2003;42(5):1000–1012. doi: 10.1016/j.ajkd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Golledge J, Smith CJ, Emery J, Farrington K, Thompson HH. Outcome of primary radiocephalic fistula for haemodialysis. Br J Surg. 1999;86(2):211–216. doi: 10.1046/j.1365-2168.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- 18.Burt CG, Little JA, Mosquera DA. The effect of age on radiocephalic fistula patency. J Vasc Access. 2001;2(3):110–113. doi: 10.1177/112972980100200305. [DOI] [PubMed] [Google Scholar]
- 19.Johnson CP. Peroperative and intraoperative predictors of vascular access outcome. Vascular Access for Hemodialysis. 2002;VIII:143–156. [Google Scholar]
- 20.Kawecka A, Debska-Slizien A, Prajs J, Krol E, Zdrojewski Z, Przekwas M, et al. Remarks on surgical strategy in creating vascular access for hemodialysis: 18 years of one center’s experience. Ann Vasc Surg. 2005;19(4):590–598. doi: 10.1007/s10016-005-5020-z. [DOI] [PubMed] [Google Scholar]
- 21.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67(6):2462–2469. doi: 10.1111/j.1523-1755.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 22.Swindlehurst N, Swindlehurst A, Lumgair H, Rebollo MI, Mamode N, Cacciola R, et al. Vascular access for hemodialysis in the elderly. J Vasc Surg. 2011;53(4):1039–1043. doi: 10.1016/j.jvs.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Staramos DN, Lazarides MK, Tzilalis VD, Ekonomou CS, Simopoulos CE, Dayantas JN. Patency of autologous and prosthetic arteriovenous fistulas in elderly patients. Eur J Surg. 2000;166(10):777–781. doi: 10.1080/110241500447407. [DOI] [PubMed] [Google Scholar]
- 24.Richardson AI, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, et al. Should fistulas really be first in the elderly patient? J Vasc Access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
- 25.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45(2):420–426. doi: 10.1016/j.jvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Bonforte G, Rossi E, Auricchio S, Pogliani D, Mangano S, Mandolfo S, et al. The middle-arm fistula as a valuable surgical approach in patients with end-stage renal disease. J Vasc Surg. 2010;52(6):1551–1556. doi: 10.1016/j.jvs.2010.06.165. [DOI] [PubMed] [Google Scholar]
- 27.Smith GE, Gohil R, Chetter IC. Factors affecting the patency of arteriovenous fistulas for dialysis access. J Vasc Surg. 2012;55(3):849–855. doi: 10.1016/j.jvs.2011.07.095. [DOI] [PubMed] [Google Scholar]
- 28.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol. 2008;3(2):437–441. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allon M, Lok CE. Dialysis fistula or graft: the role for randomized clinical trials. Clin J Am Soc Nephrol. 2010;5(12):2348–2354. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 30.Berardinelli L, Vegeto A. Lessons from 494 permanent accesses in 348 haemodialysis patients older than 65 years of age: 29 years of experience. Nephrol Dial Transplant. 1998;13 (Suppl 7):73–77. doi: 10.1093/ndt/13.suppl_7.73. [DOI] [PubMed] [Google Scholar]
- 31.Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs.arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9(4):231–235. [PubMed] [Google Scholar]
- 32.Chan MR, Sanchez RJ, Young HN, Yevzlin AS. Vascular access outcomes in the elderly hemodialysis population: A USRDS study. Semin Dial. 2007;20(6):606–610. doi: 10.1111/j.1525-139X.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee T, Barker J, Allon M. Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol. 2007;18(6):1936–1941. doi: 10.1681/ASN.2006101119. [DOI] [PubMed] [Google Scholar]
- 34.Rocha A, Silva F, Queiros J, Malheiro J, Cabrita A. Predictors of steal syndrome in hemodialysis patients. Hemodial Int. 2012 doi: 10.1111/j.1542-4758.2012.00684.x. [DOI] [PubMed] [Google Scholar]
- 35.Davidson D, Louridas G, Guzman R, Tanner J, Weighell W, Spelay J, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46(6):408–412. [PMC free article] [PubMed] [Google Scholar]
- 36.Zamani P, Kaufman J, Kinlay S. Ischemic steal syndrome following arm arteriovenous fistula for hemodialysis. Vasc Med. 2009;14(4):371–376. doi: 10.1177/1358863X09102293. [DOI] [PubMed] [Google Scholar]
- 37.Ocak G, Halbesma N, le CS, Hoogeveen EK, van DS, Kooman J, et al. Haemodialysis catheters increase mortality as compared to arteriovenous accesses especially in elderly patients. Nephrol Dial Transplant. 2011;26(8):2611–2617. doi: 10.1093/ndt/gfq775. [DOI] [PubMed] [Google Scholar]
- 38.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42(5):1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo V, Martn M, Rufino M, Hernandez D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis. 2004;43(6):999–1007. doi: 10.1053/j.ajkd.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 40.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, et al. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int. 2007;71(6):555–561. doi: 10.1038/sj.ki.5002078. [DOI] [PubMed] [Google Scholar]
- 41.Tamura MK, Tan JC, O’Hare AM. Optimizing renal replacement therapy in older adults: a framework for making individualized decisions. Kidney Int. 2011 doi: 10.1038/ki.2011.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowling CB, O’Hare AM. Managing older adults with CKD: individualized versus disease-based approaches. Am J Kidney Dis. 2012;59(2):293–302. doi: 10.1053/j.ajkd.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 44.K-DOQI Clinical practice guidelines for vascular access. American Journal of Kidney Diseases. 2006;48(Suppl 1):S248–S273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 45.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, et al. CHAPTER 4: Vascular Access. J Am Soc Nephrol. 2006;17 (Suppl 1):S16–S23. doi: 10.1681/ASN.2005121372. [DOI] [PubMed] [Google Scholar]
- 46.Mendelssohn D, Beaulieu M, Kiaii M, Jindal K, macRae J, Kappel J, et al. Report of the Canadian Society of Nephrology Vascular Access Working Group. Seminars in Dialysis. 2012;25(1):22–25. doi: 10.1111/j.1525-139X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 47.Vachharajani TJ, Moossavi S, Jordan JR, Vachharajani V, Freedman BI, Burkart JM. Re-evaluating the Fistula First Initiative in Octogenarians on Hemodialysis. Clin J Am Soc Nephrol. 2011;6(7):1663–1667. doi: 10.2215/CJN.05830710. [DOI] [PubMed] [Google Scholar]
- 48.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 49.Couchoud C. Dialysis: Can we predict death in patients on dialysis? Nat Rev Nephrol. 2010;6(7):388–389. doi: 10.1038/nrneph.2010.42. [DOI] [PubMed] [Google Scholar]
- 50.Shingarev R, Maya ID, Barker-Finkel J, Allon M. Arteriovenous graft placement in predialysis patients: a potential catheter-sparing strategy. Am J Kidney Dis. 2011;58(2):243–247. doi: 10.1053/j.ajkd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia Cortes MJ, Viedma G, Sanchez Perales MC, Borrego FJ, Borrego J, Perez del BP, et al. Fistulae or catheter for elderly who start hemodialysis without permanent vascular access? Nefrologia. 2005;25(3):307–314. [PubMed] [Google Scholar]
- 52.Murray MA, Brunier G, Chung JO, Craig LA, Mills C, Thomas A, et al. A systematic review of factors influencing decision-making in adults living with chronic kidney disease. Patient Educ Couns. 2009;76(2):149–158. doi: 10.1016/j.pec.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. doi: 10.1136/bmj.c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bay WH, Van CS, Owens M. The hemodialysis access: preferences and concerns of patients, dialysis nurses and technicians, and physicians. Am J Nephrol. 1998;18(5):379–383. doi: 10.1159/000013380. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhry M, Bhola C, Joarder M, Zimmerman D, Quinan P, Mendelssohn D, et al. Seeing eye to eye: the key to reducing catheter use. J Vasc Access. 2011;12(2):120–126. doi: 10.5301/jva.2011.6390. [DOI] [PubMed] [Google Scholar]
- 56.Quinn RR, Lamping DL, Lok CE, Meyer RA, Hiller JA, Lee J, et al. The Vascular Access Questionnaire: assessing patient-reported views of vascular access. J Vasc Access. 2008;9(2):122–128. [PubMed] [Google Scholar]
- 57.Xi W, Harwood L, Diamant MJ, Brown JB, Gallo K, Sontrop JM, et al. Patient attitudes towards the arteriovenous fistula: a qualitative study on vascular access decision making. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr055. [DOI] [PubMed] [Google Scholar]
- 58.Quinn RR, Lamping DL, Lok CE, Meyer RA, Hiller JA, Lee J, et al. The Vascular Access Questionnaire: assessing patient-reported views of vascular access. J Vasc Access. 2008;9(2):122–128. [PubMed] [Google Scholar]
- 59.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7(4):523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 61.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 62.Lemmon GW, Murphy MP. Dialysis access steal syndromes. Perspect Vasc Surg Endovasc Ther. 2009;21(1):36–39. doi: 10.1177/1531003509333230. [DOI] [PubMed] [Google Scholar]
- 63.Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010;55(1):21–30. doi: 10.1053/j.ajkd.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4(12):1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garland JS, Holden RM, Groome PA, Lam M, Nolan RL, Morton AR, et al. Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis. 2008;52(5):849–858. doi: 10.1053/j.ajkd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Hassan NA, D’Orsi ET, D’Orsi CJ, O’Neill WC. The risk for medial arterial calcification in CKD. Clin J Am Soc Nephrol. 2012;7(2):275–279. doi: 10.2215/CJN.06490711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newman AB, Naydeck BL, Sutton-Tyrrell K, Feldman A, Edmundowicz D, Kuller LH. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104(22):2679–2684. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 68.Leon C, Asif A. Arteriovenous access and hand pain: the distal hypoperfusion ischemic syndrome. Clin J Am Soc Nephrol. 2007;2(1):175–183. doi: 10.2215/CJN.02230606. [DOI] [PubMed] [Google Scholar]
- 69.Salman L, Asif A, Beathard GA. Retrograde angiography and the risk of arteriovenous fistula perforation. Semin Dial. 2009;22(6):698–701. doi: 10.1111/j.1525-139X.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- 70.Khan FA, Vesely TM. Arterial problems associated with dysfunctional hemodialysis grafts: evaluation of patients at high risk for arterial disease. J Vasc Interv Radiol. 2002;13(11):1109–1114. doi: 10.1016/s1051-0443(07)61952-6. [DOI] [PubMed] [Google Scholar]
- 71.Duijm LE, Liem YS, van der Rijt RH, Nobrega FJ, van den Bosch HC, Douwes-Draaijer P, et al. Inflow stenoses in dysfunctional hemodialysis access fistulae and grafts. Am J Kidney Dis. 2006;48(1):98–105. doi: 10.1053/j.ajkd.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 72.Lee T, Safdar N, Mistry MJ, Wang Y, Chauhan V, Campos B, et al. Preexisting Venous Calcification Prior to Dialysis Vascular Access Surgery. Semin Dial. 2012 doi: 10.1111/j.1525-139X.2012.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu T, Willett WC, Rifai N, Rimm EB. Plasma fluorescent oxidation products as potential markers of oxidative stress for epidemiologic studies. Am J Epidemiol. 2007;166(5):552–560. doi: 10.1093/aje/kwm119. [DOI] [PubMed] [Google Scholar]
- 74.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 75.Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, et al. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25(3):224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- 76.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51(25):1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 77.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 78.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 2004;65(6):2371–2379. doi: 10.1111/j.1523-1755.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 79.Skaug EA, Aspenes ST, Oldervoll L, Morkedal B, Vatten L, Wisloff U, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol. 2012 doi: 10.1177/2047487312444234. [DOI] [PubMed] [Google Scholar]
- 80.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26(7):2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wasse H, Rivera AA, Huang R, Martinson DE, Long Q, McKinnon W, et al. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Semin Dial. 2011;24(6):688–693. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YO, Choi YJ, Kim JI, Kim YS, Kim BS, Park CW, et al. The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci. 2006;21(2):284–289. doi: 10.3346/jkms.2006.21.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YO, Song HC, Yoon SA, Yang CW, Kim NI, Choi YJ, et al. Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis. 2003;41(2):422–428. doi: 10.1053/ajkd.2003.50051. [DOI] [PubMed] [Google Scholar]
- 84.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]