Abstract
Purpose
Low birth weight (LBW) is one of the major public health problems in India. Hence, there is a need to identify risk factors that, when modified, will reduce the burden of unhealthy children on the healthcare system. The objective of this study was to determine whether periodontitis among mothers in the rural population of India is a risk factor for LBW babies.
Methods
A hospital-based case control study was conducted among 340 postpartum mothers. The cases consisted of 170 women who had given birth to babies weighing <2,500 g, while the control group consisted of 170 women who had given birth to babies weighing ≥2,500 g. Details of the mothers were taken from the hospital records and through a personal interview, and a full-mouth periodontal examination was performed postpartum, which included probing depth, clinical attachment level, and bleeding on probing on six sites per tooth.
Results
LBW cases had a significantly worse periodontal status than the controls, having an odds ratio (OR) of 2.94 (P=0.01). The multivariate logistic regression model demonstrated that periodontal disease is a significant independent risk factor with an adjusted odds ratio (aOR) of 2.85 for the LBW group (95% confidence interval [CI], 1.62-5.5). Other factors showing significant associations with LBW were pre-eclampsia (aOR, 4.49; 95% CI, 1.4-14.7), preterm labor (aOR, 5.5; 95% CI, 3.2-9.9), and vaginal type of delivery (aOR, 2.74; 95% CI, 1.4-5.2).
Conclusions
Periodontitis represents a strong, independent, and clinically significant risk factor for LBW. Periodontal therapy should form a part of the antenatal preventive care among rural women in India.
Graphical Abstract
Keywords: Case-control studies, Low birth weight infant, Periodontitis, Pregnancy outcome, Risk factors
INTRODUCTION
Over three-fifths of the 2.3 million child deaths in India in 2005 were caused by seven conditions: pneumonia, diarrheal diseases, neonatal infections, birth asphyxia, birth trauma, prematurity, and low birth weight (LBW) [1]. LBW remains the leading cause of morbidity and mortality among newborns despite the advances in obstetrical prevention, diagnostics, and therapy [2]. The World Health Organization (WHO 2005) defined LBW as a birth weight of less than 2,500 g [3]. LBW leads to long-term neurological complications, respiratory problems, congenital anomalies, and behavioral problems [4].
Multiple factors have been associated with LBW. Some of these known risk factors in mothers for LBW include young and old maternal age, low prepregnancy weight, obesity, multiple gestations, anemia, gestational diabetes, genitourinary tract infections, arterial hypertension, illicit drug use, cigarette smoking, low socioeconomic status (SES), inadequate prenatal care, short stature, excessive alcohol consumption, and previous preterm delivery [5]. Yet, very few of these factors have underlying biological or social mechanisms explaining the link. Young mothers have a less mature reproductive system, while older women have an anatomically and biologically aged organ system. History of preterm delivery is a strong predictive marker for future preterm labor leading to LBW due to inadequate growth of the gestational structures. Infections and diabetes lead to a state of increased systemic inflammation. However, a significant proportion of LBW is of unknown etiology [6]. It has been postulated that distant infections like periodontal diseases may be associated with LBW through similar mechanisms as other maternal infections [7]. Periodontal disease shares many common risk factors with LBW, such as age, smoking, low socioeconomic level, and systemic health status [8]. Periodontitis is an infectious disease caused by predominantly gram-negative, anaerobic, and microaerophilic bacteria that colonize in the subgingival area and cause local and systemic elevations of proinflammatory prostaglandins and cytokines [6]. Maternal periodontal infection has been proposed to influence LBW delivery through mechanisms involving inflammatory mediators or direct bacterial assault on the amnion leading to preterm labor and premature rupture of membranes [6,7,8].
Researchers have been interested in the potential causal association between the presence or progression of maternal periodontal disease and several pregnancy outcomes for almost 20 years [9]. This hypothesis is consistent with other medical literature suggesting that the inflammatory process in the fetal/placental unit and/or an elevated systemic inflammation may impact pregnancy outcomes [9]. A meta-analysis of case control studies showed a significant association between LBW and periodontal disease having an OR of 1.82 [10]. Recently, a case control study among the Iranian population found periodontal disease to be a risk factor for LBW [11]. Several authors found significant associations with LBW babies and periodontitis having an OR ranging from 1.67 to 3.48 [12,13,14]. This finding over the years has been confirmed in several studies conducted in various populations [15,16]. However, a few studies failed to show such an association [17,18,19]. Despite the efforts of many publications, case control studies, cohort studies, cross-sectional studies, and systematic reviews, no definite conclusion has emerged on the existence or relevance of such an association [20]. This has been mainly due to the variation in the population assessed, the presence of a range of potential confounding factors, and the variation in the definition of periodontitis across studies [9,20]. Further use of the composite outcome preterm low birth weight (PTLBW) is not encouraged, preterm birth (PTB) and LBW are independent adverse pregnancy outcomes and should be studied separately [20].
India is home to nearly 40% of all LBW babies in the developing world. Estimates based on the data available from studies based on institutional deliveries and small communities suggest that nearly one-third of all Indian infants weigh less than 2.5 kg at birth [21]. In spite of ongoing efforts to reduce LBW, the incidence remained roughly constant between 1984 and 2007 [21]. Rajnandgaon, an agrarian district of Chhattisgarh in central India, recorded one of the highest infant mortality rates (IMRs). The United Nations Development Program reported that Rajnandgaon district has a high IMR of 112/1,000/year compared to the national average of 61/1,000/year [22]. Prevention of LBW is a critical healthcare priority in this population for medical, social, and economic reasons.
Infant mortality is high in the rural parts of India and could be attributed to the lack of facilities to handle preterm and low weight newborns. The knowledge about the relevant risk factors will help increase awareness among the mothers as well as the doctors and health workers attending to them. The link between maternal periodontitis and LBW is a gray area, and whether LBW has a causal relationship with periodontitis or is a surrogate for another maternal factor remains unclear. If an association between periodontitis and LBW is present, this will be an important finding as periodontitis is a modifiable risk factor and can effectively be prevented and controlled at a population level. Therefore the objective of this study was to determine whether periodontitis among 18- to 35-year-old mothers is a risk factor for LBW babies in the rural population of Rajnandgaon.
MATERIALS AND METHODS
This was a retrospective, hospital-based case control (with a 1:1 ratio) study that involved postpartum mothers. Postpartum mothers were selected from Department of Obstetrics and Gynecology, Government District Hospital, Rajnandgaon, Chhattisgarh, from March 2011 to July 2012. This hospital offers free tertiary healthcare for a primarily rural population of more than 200,000 individuals. This study was conducted only after getting approval from the Institutional Ethics Committee (CDEC/22/2010/12/LW). A pilot study was conducted in the month of February 2010 to check the feasibility of the study and to pretest the study questionnaire. Informed consent was obtained from all the mothers.
Study population
Three hundred and forty postpartum primiparous mothers were selected for this study. Cases and controls consisted of 170 mothers in each group. Cases were defined according to the WHO definition of LBW and consisted of women who had given birth to babies weighing <2,500 g, and the controls were mothers who had given birth to normal birth weight (NBW) babies, that is, babies weighing ≥2,500 g.
The inclusion criteria for the mothers were that they belong to the age group of 18-35 years, be primiparous mothers, have a singleton pregnancy, belong to the rural population of the Rajnandgaon district, and have at least 20 teeth. The exclusion criteria were as follows: multiple pregnancies; human immunodeficiency virus seropositive mothers; mothers with a systemic disease such as cardiovascular disease, hepatic insufficiency, glomerulonephritis, hyperthyroidism, or epilepsy; placental or uterine abnormalities; stillborn babies; and mothers who received systemic antibiotics during pregnancy and had undergone periodontal therapy during pregnancy.
Sample size calculation
The sample size was calculated on the basis of the following assumption that the prevalence of periodontitis in 18- to 35-year-old females in the rural population of India is 6% [23], having an expected OR of 3 [12], a power value of 80%, and a type-1 error rate of 5%. The sample size was 170 subjects each for the case and the control groups (EpiInfo ver. 6, Centers for Disease Control and Prevention, Atlanta, GA, USA). Therefore, a total of 340 postpartum women were selected after delivery.
Data collection
The data were collected 2 days per week on a regular basis at the Rajnandgaon District Hospital. The cases were selected as the mother of the first LBW baby delivered on the day of the examination, and the next consecutive subject who delivered a NBW baby became the control. All the data were collected within 48 hours of delivery, and the same number of cases and controls were examined in each visit. The data from the mothers were collected by an interview schedule and a clinical examination form. All mothers who did not fulfill the inclusion criteria or did not give consent were excluded from this study.
The interview was conducted by a single person (P.S.J.) by using a structured questionnaire and other information was obtained from the medical records of the parturient. The mothers were interviewed in their hospital beds. The questionnaire form included information regarding personal details, household information, demographic data, behavioral habits, medical history, and obstetric history. Personal details included variables such as age, place of residence, and religion/caste. In the part on household information, details regarding marriage duration, consanguinity, type of housing, water supply, and SES were recorded. Demographic data included data on maternal schooling, maternal employment status over the last 9 months, and family income over the last month (calculated by summing the incomes of all the members of the household). The poverty threshold was measured for each mother's family by calculating the scores ranging from 0 to 4 with 13 parameters including landholding, type of house, clothing, food security, sanitation, consumer durables, literacy status, labor force, means of livelihood, status of children, type of indebtedness, and reasons for migration. Families with 17 points or less out of a maximum of 52 were classified to be below the poverty line (BPL) as defined by the Government of India. The other details that were recorded were dietary habits, brushing aids, duration of brushing, vigorous or strenuous activity during the last 3 months of pregnancy, alcohol consumption, and illegal drugs and tobacco use during pregnancy. In the section on obstetric history, the details recorded were the number of ante-natal consultations (ANC), last menses period, maternal general health during pregnancy, and any morbidity during pregnancy like hypertension, anemia, diabetes, infections during pregnancy (including genitourinary tract infections), pre-eclampsia, incompetent cervix, abruption placentae, intrauterine growth retardation, vaginal bleeding, clotting disorder, Rh factor isoimmunity, and their treatment.
PTB was also recorded and defined as spontaneous delivery in less than 37 completed weeks of gestation, followed by spontaneous labor or spontaneous rupture of the membranes. The gestational age was estimated on the basis of the last menstrual period, ultrasound scan (if present), and postnatal examination of a child. Gestation age was further categorized into very preterm delivery (22-31 weeks), moderate preterm delivery (32-33 weeks), and mild preterm (34-36 weeks); a mother delivering after 37 weeks was considered full term [24].
The following variables were recorded from the medical records: height, weight, body mass index (kg/m2; calculated using the height and weight values), blood pressure, hemoglobin, and blood group. If a patient had a systolic blood pressure of more than 140 mmHg, she was classified as having arterial hypertension. Anemia was marked as present for patients who had a hemoglobin level of ≤9 g. The data regarding the baby's weight and date of birth and type of delivery (vaginal or caesarean) were also taken from the hospital records. Birth weight was further categorized into LBW (≤2,499 g), very LBW (≤1,500 g), and extremely LBW (≤1,000 g) [3].
Periodontal examination
An intraoral examination was carried out in the maternity ward with the subject lying flat on her bed, head to the foot end of the bed in order to facilitate a reproducible examination position for the clinician. The clinical periodontal recordings in each woman included bleeding on probing (BOP), measurement of probing depth (PD), and clinical attachment level (CAL). PD was measured from the free gingival margin to the bottom of the gingival sulcus/periodontal pocket using a UNC 15 periodontal probe (Hu Friedy, Chicago, IL, USA). CAL was measured from the cement enamel junction to the base of the gingival sulcus/pocket. Distance was rounded down to the nearest whole millimeter for both the measures. A subject was identified as having periodontitis according to the WHO definition of periodontitis: pocket PD of ≥4 mm in at least one site. For analytical purposes, periodontitis severity and extent was coded as follows [25]: mild: presenting in at least three sites, in different teeth, with an attachment loss of ≥3 mm, but not three or more sites with an attachment loss of ≥5 mm; moderate: presenting in at least three sites, in different teeth, with an attachment loss of ≥5 mm, but not three sites with an attachment loss of ≥7 mm; severe: presenting in at least three sites, in different teeth, with an attachment loss of ≥7 mm. BOP was also recorded as present or absent and was determined positive if it occurred within 15 seconds of probing.
Examiner
The interview was conducted by a trained interviewer (P.S.J.) who collected the data from the mothers and babies, using a structured interview and medical registers. Two trained periodontists (S.N. and R.P.P.) performed the clinical examination and were blinded to the obstetric data and medical history. Calibration sessions to measure the agreement between the examiners were conducted, and intra- and interexaminer reliability was recorded until a satisfactory agreement was reached. The reproducibility expressed as a proportion of the agreement between clinical scores was 91% and 88% for the PD and the attachment level, respectively.
Statistical analysis
The statistical analysis was performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). The distribution of maternal socio-demographic characteristics and medical and reproductive histories according to LBW and NBW was examined by means of a chi-square test. In cells with low expected frequency, a Fisher exact test was used. An unpaired t-test with mean and standard deviation was conducted for the weight of the babies from the cases and the control. The risk factors included in the analyses were consanguinity (yes or no), use of smokeless tobacco (yes or no), number of antenatal checkups, intake of calcium and iron supplement, pre-eclampsia (present or absent), gestational diabetes (present or absent), anemia (present or absent), hypertension (present or absent), SES, gestational age, type of delivery (vaginal or caesarean), presence of complications during parturition, and periodontitis. In the first statistical analysis, a 2×2 table was set up for each independent variable in order to find out if there was any statistical association between periodontitis and LBW births. In the second analysis, a multiple logistic regression analysis was used to measure the effect of periodontitis on LBW, controlling the effects of the abovementioned risk factors. The unadjusted odds ratio (OR) and the adjusted odds ratio (aOR) were calculated with 95% confidence intervals. Statistical significance was considered at a 5% level.
RESULTS
The following are the results based on 340 inpatient postpartum mothers from the Government District Hospital, Rajnandgaon. None of the participants had been exposed to oral health education or periodontal therapy before the study.
Demographic and behavioral characteristics
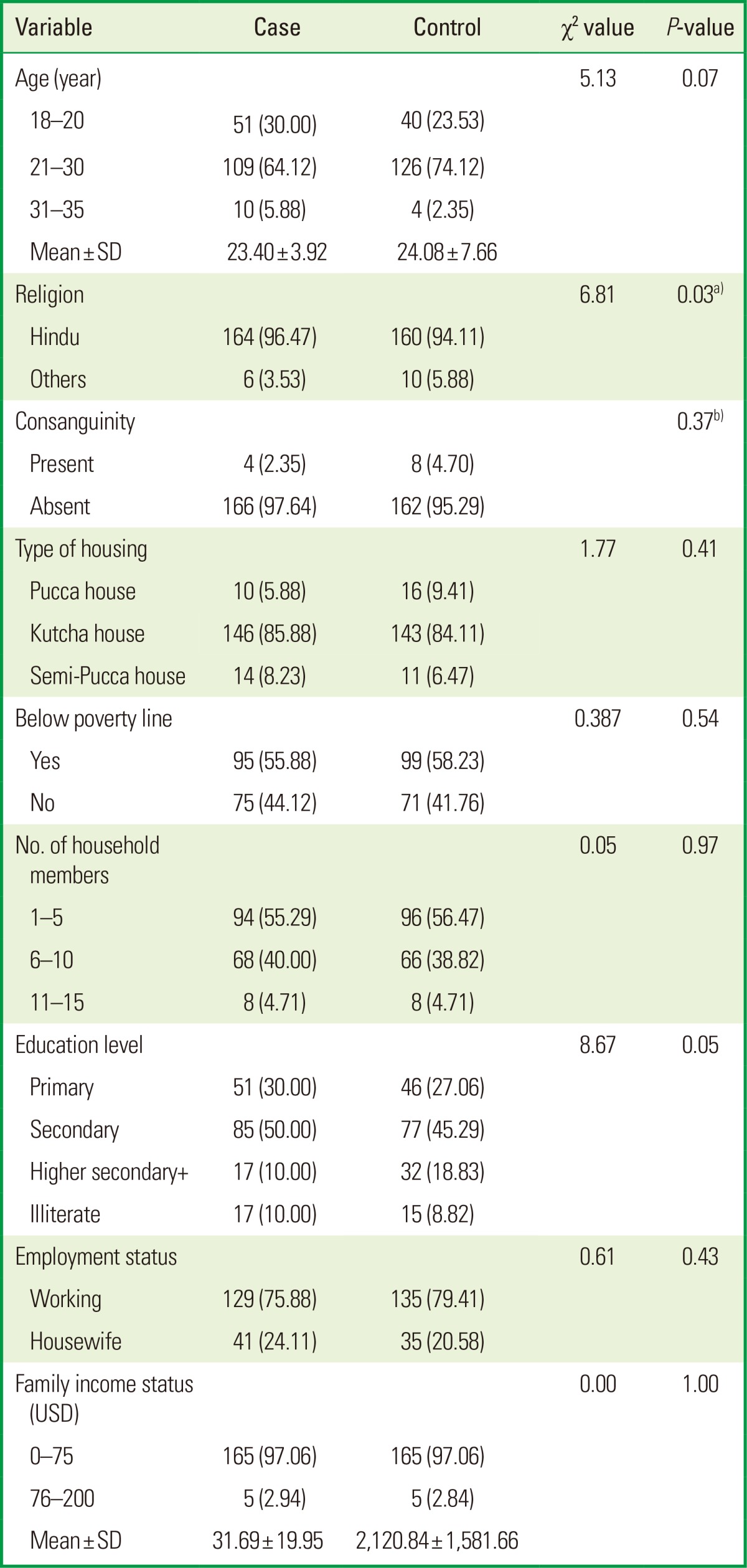
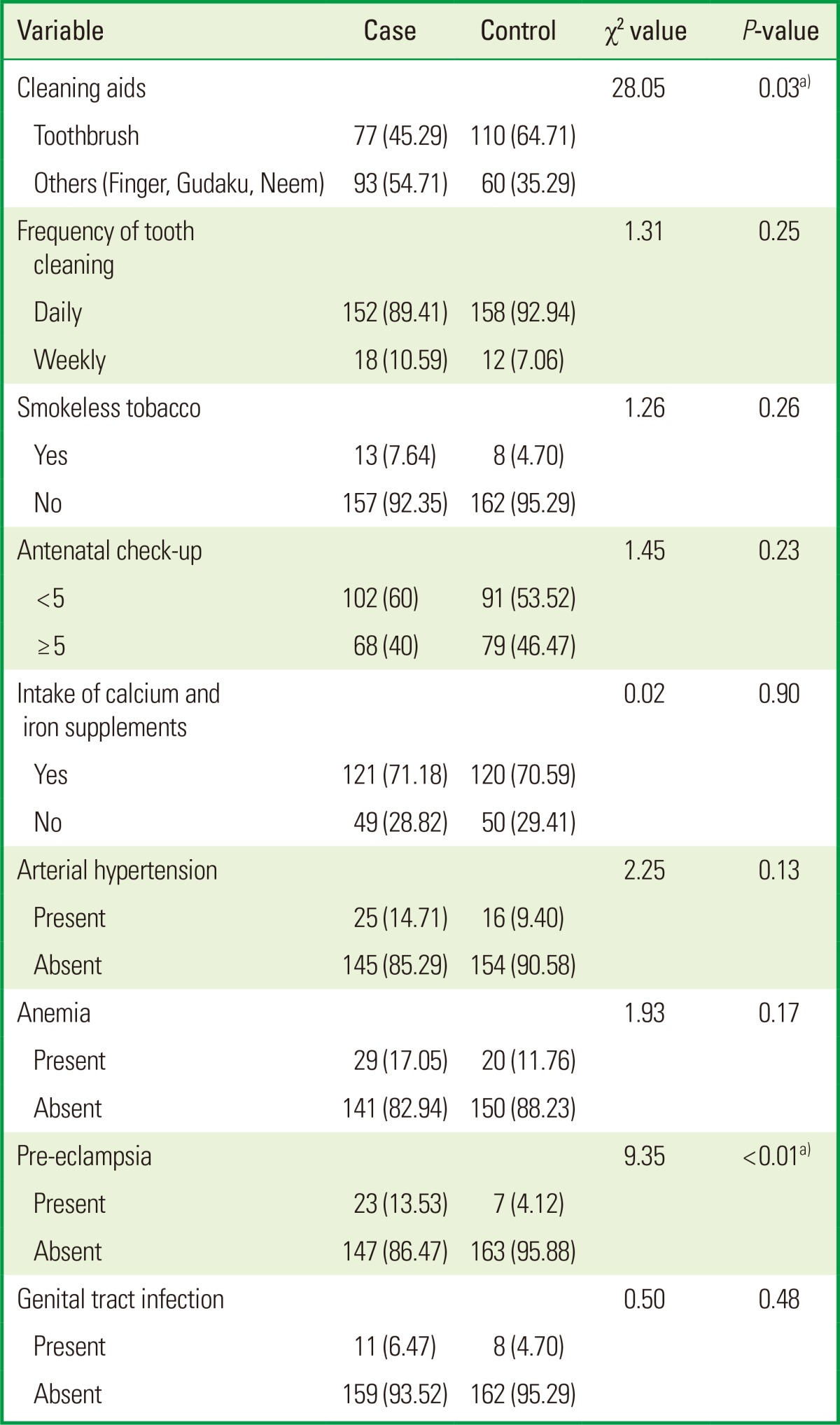
To ensure the homogeneity in the population, the cases and the controls were compared for all the associated factors. While the cases and the controls had a similar age range, significant differences were seen in religion (P<0.05), aids used for brushing (P<0.05), and pre-eclampsia (P<0.05). There was no patient in either group who was a smoker, consumed alcohol, and used abusive drugs or exercised strenuously; therefore, none of these variables were included for the analysis (Tables 1 and 2).
Table 1.
Sociodemographic characteristics of the cases and controls in a study on the association between maternal periodontal disease and low birth weight, in Rajnandgaon (n=340).
Values are presented as number (%) unless otherwise indicated.
SD: standard deviation, USD: United States dollar.
a)P<0.05 significant. b)Fisher exact test.
Table 2.
Behavioral characteristics of the cases and controls in a study on the association between maternal periodontal disease and low birth weight, in Rajnandgaon (n=340).
Values are presented as number (%).
a)P<0.05 significant.
Delivery characteristics and birth weight distribution
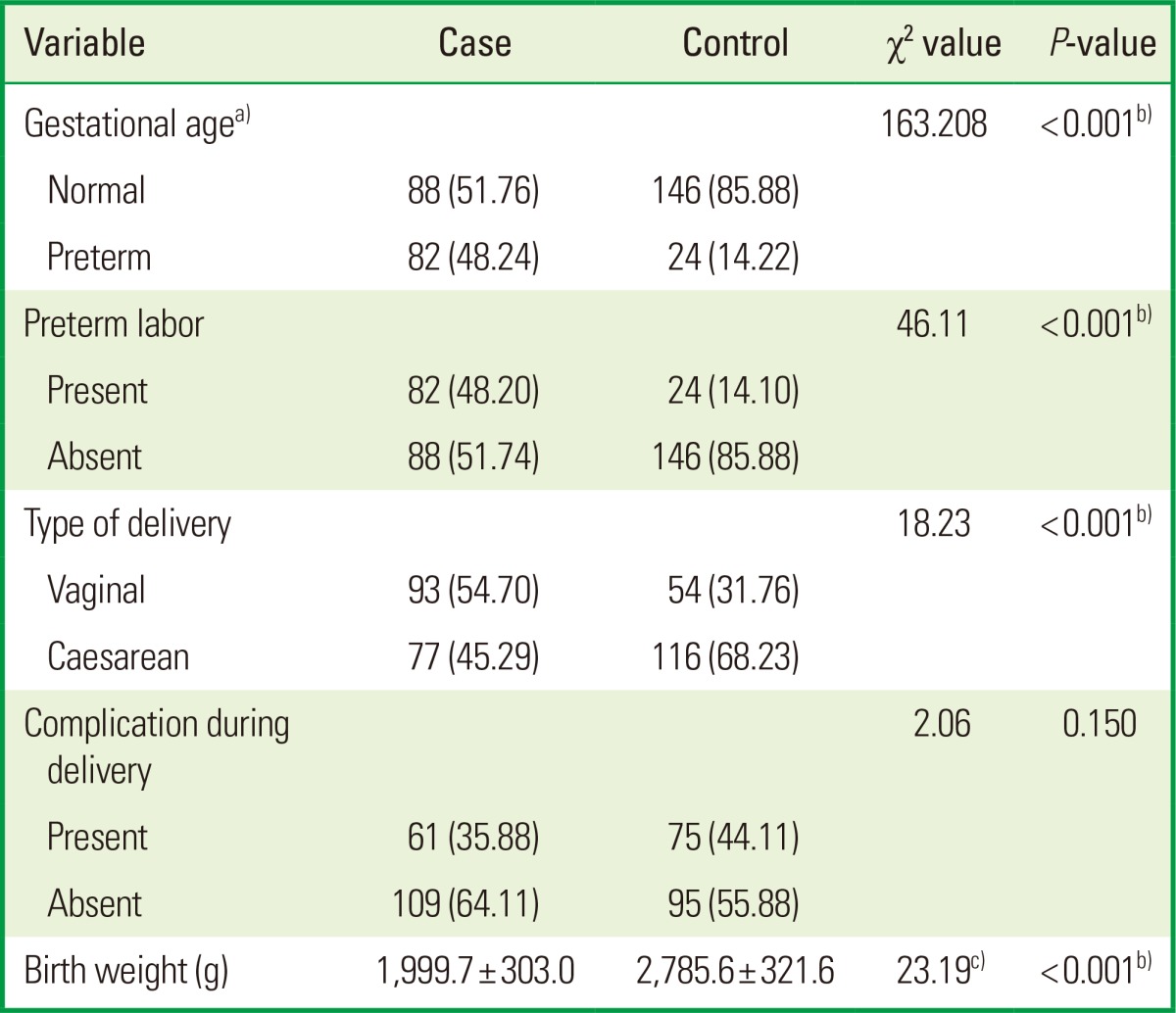
The case group had more mothers having PTBs than the control group, and the difference was significant (P<0.0001). Preterm labor was present in 48.2% of the cases as compared to 14.1% of the controls, and the difference was highly significant (P<0.001). 54.7% mothers in the case group and 31.8% of the mothers in the control group had normal parturition, while other mothers delivered through a caesarean section. The difference was highly significant (P<0.0001). 35% and 44.1% of the mothers in the case and the control groups, respectively, experienced complications during delivery. The mean weight at birth was 1,999 (±303) g in the case group and 2,785 (±412) g in the control group (Table 3).
Table 3.
Delivery characteristics among cases and controls.
Values are presented as number (%) or mean±standard deviation.
a)Due to less number of persons, preterm was considered without division. b)P<0.001 highly significant. c)Unpaired t-test.
Distribution of periodontitis
Among the cases, the frequency of periodontal disease was 52.9%, whereas among the controls, it was 27.6%; the difference was statistically significant (P<0.001). 5.3% patients had severe periodontitis in cases, whereas none in controls. 8.8% patients in cases and 4.1% patients in controls had moderate periodontitis, and mild periodontitis was seen in 38.8% in cases and 23.5% in controls (Table 4).
Table 4.
Periodontitis characteristics among cases and controls.
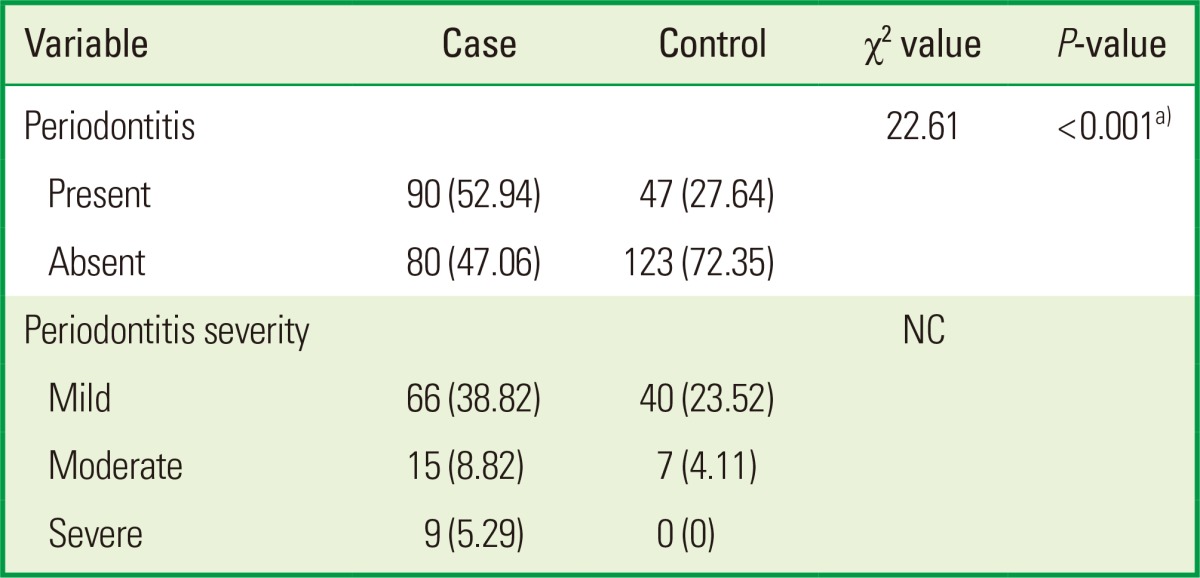
Values are presented as number (%).
NC: not calculated.
a)P<0.001 highly significant.
Multivariate logistic regression model-adjusting for the known risk factors
Factors that were statistically significant or near significant in the univariate analysis-namely, consanguinity, use of smokeless tobacco, number of ANCs, calcium and iron supplement intake, pre-eclampsia, gestational age, type of delivery, complications, hypertension, anemia, SES, and periodontitis-were included in the model. Among these, factors that were likely to pose an increased risk for LBW were pre-eclampsia (OR, 3.64; 95% CI, 1.5-8.7; aOR, 4.49; 95% CI, 1.4-14.7; P=0.01), type of delivery (OR, 2.60; 95% CI, 1.7-4.0; aOR, 2.74; 95% CI, 1.4-5.2; P=0.002), and periodontitis (OR, 2.94; 95% CI, 1.9-4.6; aOR, 2.80; P=0.001). Consanguinity, use of smokeless tobacco, number of ANCs, calcium and iron intake, anemia, SES, and arterial hypertension were not significant risk factors for this sample. In the crude association analysis, the chance that the child might present LBW was approximately three times as large among mothers with periodontitis (crude OR, 2.94; 95% CI, 1.9-4.6); periodontitis remained a significant risk factor with an adjusted OR of 2.80 (Table 5).
Table 5.
Multiple logistic regression analysis having low birth weight as outcome variable.
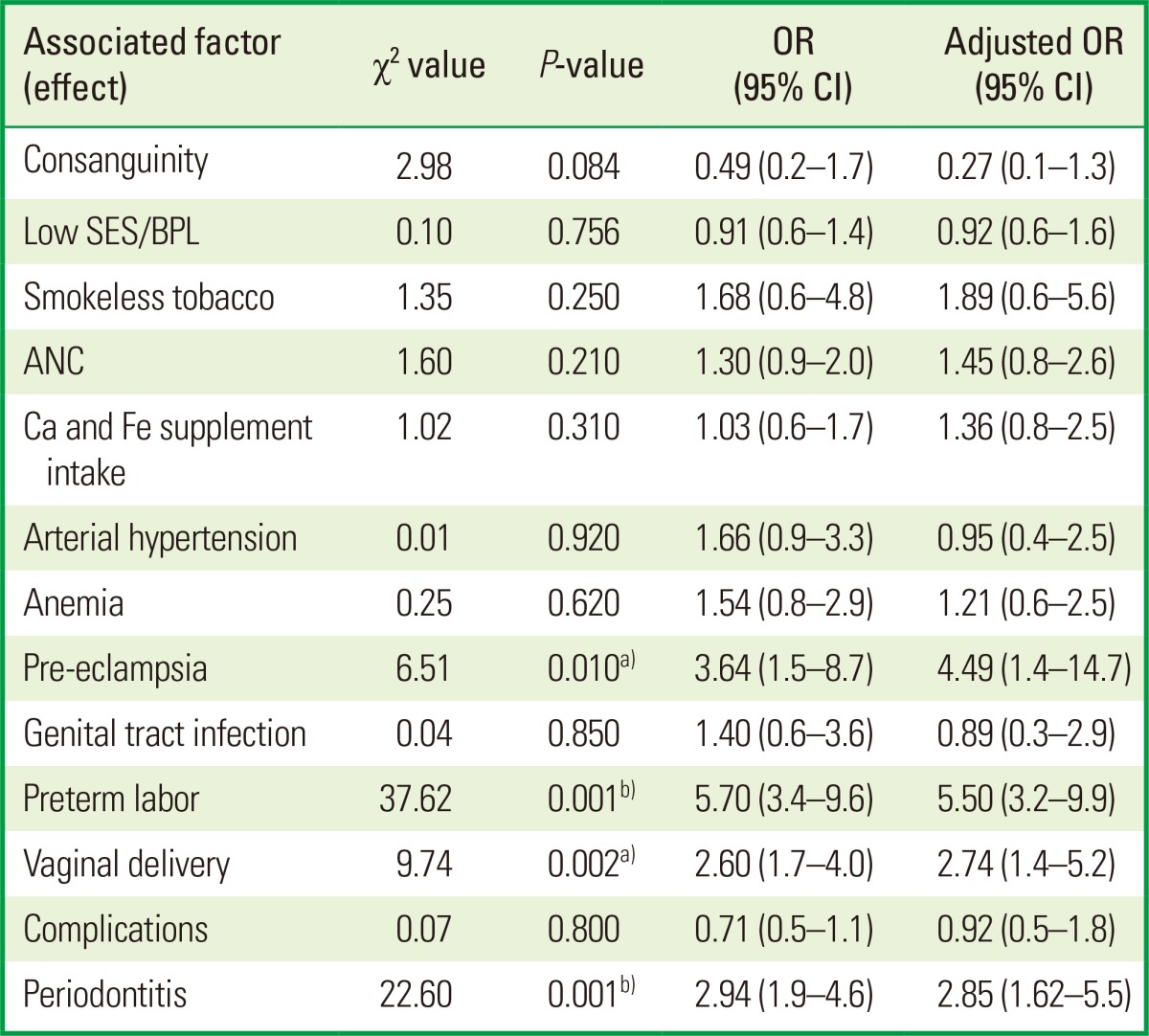
OR: odds ratio, CI: confidence interval, SES: socio-economic status, BPL: below poverty line, ANC: ante-natal consultation.
a)P<0.05 significant. b)P<0.001 highly significant.
DISCUSSION
In this study, we tried to determine whether periodontitis was a risk factor for LBW babies while distinguishing between the other main causes of LBW. The relation between periodontal disease and pregnancy has been under investigation for over a decade, but the quest is still inconclusive [9,10,20]. The population selected was unique as it represented the most underserved population with a very high IMR. Previous studies have considered a variety of outcomes, such as PTB, LBW (<2,500 g), or preterm LBW as a composite outcome in their quest for finding the relationship with periodontitis. In this study, the LBW of infants was assessed instead of using a composite outcome.
In our study, postpartum indigenous mothers with a low socio-economic status were selected from the Government District Hospital, Rajnandgaon (Chhattisgarh). The women were enrolled immediately after delivery, and none of them had visited a dentist during their pregnancy. A homogenous indigenous population, living in a rural area with an almost equal accessibility to health services, renders this study reliable when it comes to sample recruitment. All the potential confounding factors, which were the characteristics of the population under study, were taken into account in the questionnaire. 18- to 35-year-old mothers were selected, as young and high maternal ages are known risk factors. Both the case and the control groups were similar in terms of age, education level, employment status, family income, and demographic and SES. Similar populations were considered in studies by Dasanayake et al. [26] and Lopez et al. [27] where the samples were mostly of Afro-American or Hispanic origins, often of low socio-economic status, and having poor oral hygiene and periodontal conditions. Our sample included a sufficient number of women to allow an analysis with adequate statistical power and calculate the OR for each of the risk factors.
In this study, more mothers in the case group had periodontitis than in the control group. This association was originally suggested by Offenbacher et al. [28] and confirmed in further studies carried out in Chile [29], and Thailand [30]. Offenbacher et al. [28,30] suggested that maternal periodontal infection was significantly associated with a higher prevalence rate of PTLBW and could lead to a seven-fold risk. Recent studies [11,13,15,16] also found evidence for an association between periodontitis of the pregnant woman and LBW. The Croatian cohort [31] shows by far the worst periodontal status (OR, 8.13; CI, 2.73-45.9) reported in studies investigating the correlation between periodontal disease and adverse pregnancy outcomes. In contrast, in studies dealing with an association of periodontitis and LBW, some authors found no such association [17,18,19]. Moore et al. [32] in England, Lunardelli and Peres [33] in Brazil, Noack et al. [34] in Germany, and Mitchell-Lewis et al. [35] in America found that periodontitis was not a detectable risk factor for preterm LBW.
In most studies, the population having a relatively low OR had either an American or a European origin, in which the occurrence of both periodontitis and LBW was very low. We selected a rural population having a high IMR in which periodontitis and LBW was a common finding among mothers. The difference in these results may also be explained by different definitions of periodontitis. In the abovementioned studies, there is a lack of clarity in the criterion for the clinical diagnosis of the exposure measurement [9] and the possible inclusion of false positive diagnoses of periodontal disease, given the shakiness of the definition of individuals with periodontitis [20].
We considered periodontitis to be present in mothers having at least 1 site of PD≥4 mm (WHO criterion). As the population of interest was young, it was prudent to select a definition that identified less severe destruction. Offenbacher et al. [28] and Marakoglu et al. [36] similarly considered a PD of ≥4 mm as periodontitis. In this study, six sites in all the teeth in the mouth were analyzed. A full-mouth recording presents a possibility of obtaining a clearer image of the prevalence of periodontitis in the studied sample. As in almost all the recently published articles [25,33,34], we performed a full periodontal examination; hence, we can deduce that the periodontal status has been analyzed correctly. Nonetheless, some authors still use partial-mouth recording despite reports that such a practice results in an underestimation of the level of disease [32,37]. Although the examination time should be kept at a minimum, full-periodontal recording is not a time-consuming procedure and cannot be considered tiresome or painful in general, and the possible risk is minimal.
The pathological mechanisms by which chronic periodontitis may cause, or trigger, an inflammatory response resulting in the premature termination of a pregnancy remain unclear. Offenbacher et al. [28] observed that the progression of periodontal destruction was associated with an increase in the prostaglandin E2 levels in the gingival fluid. This would further lead to the local production of cytokines in the periodontal pocket and in the amniotic fluid. These proinflammatory cytokines and chemokines (transforming growth factor-α, interleukin [IL] 1, IL-6, and IL-8), either of a local or a more distant origin, are involved in pre-term labor [6,7].
Another reason for having more mothers with periodontitis in the case group could be attributed to the inefficiency of their oral hygiene practices, which could activate periodontal disease and make this disease even more harmful to the maternal-fetal equilibrium. There were statistically significant differences in the use of oral hygiene aids, and a similar finding was reported by Cruz et al. [13]. Many patients used a Neem stick for brushing instead of a toothbrush, which is a twig from the Neem tree. Some others used gudaku, a paste form of tobacco rubbed in the gingiva and teeth, commonly used by Chhattisgarhi women.
There are various risk factors that are related to LBW, and to examine the association between periodontitis and LBW, a regression model was used, adjusting for the known risk factors (consanguinity, low SES, anemia, hypertension, and genital tract infections). In the population analyzed, the greatest risk indicators were related to maternal factors; these were pre-eclampsia, preterm labor, and vaginal type of delivery. Similarly to our study, Bassani et al. [25] and Nabet et al. [37] found pre-eclampsia to have a high OR of 3.07 (2.03-4.65). In contrast, Vettore et al. [18] did not find any significant findings. The type of delivery was also seen as a risk factor by Agueda et al. [38]; they found caesarian delivery to be a risk factor. Further, similar to our study, several studies [39,40] have shown preterm labor to be a risk factor. Genitourinary infection is a widely accepted risk for prematurity and LBW [7,8,9], but in our study, genitourinary infection was not observed to be a risk factor. Hypertension and anemia did not reach any statistical significance as a risk factor. Similar results were seen by Vettore et al. [18]. In this Chhattisgarh population, the incidences of smoking and alcohol consumption, the traditional risk factors for LBW, were very low. None of the mothers considered in this study were smokers or consumed alcohol. However, tobacco is used by Chhattisgarhi women in the form of gudaku, and some of these also chew tobacco (pan). However, the difference between the cases and the controls was not significant 1.21 (0.74-1.98).
The limitations of this study are as follows: (1) In a case control study, there may be a memory recall bias among the participants. (2) For defining periodontitis, only the PD was considered, which may be an overestimation of the actual amount of destruction because during pregnancy, there may be an enlargement of the gingiva. (3) A hospital-based population is generally not an accurate representative of a population that has a significant number of noninstitutional deliveries.
It must also be stressed that despite the limitations mentioned above, this was one of the few Indian investigations performed in a population that has a high IMR. This case-control study was designed with a satisfactory study power for evaluating the complex question of the abovementioned association. Further, the cofactors associated with LBW were measured to observe the effect of periodontitis on LBW without the effects of any confounding factors. Despite the observational and retrospective nature of the study design, the methodology of the present study was justifiable, given that the topic can still be classified as controversial and that the consistency of the hypothesis needs to be investigated. Therefore, it is believed that the findings from this study may contribute, along with other investigations conducted with designs of greater rigor, towards understanding the abovementioned problem that still presents many knowledge gaps. Conducting a population-based case control study in this population will be challenging but nonetheless will identify the true risk factors as many mothers who have non-institutional deliveries will be included. However, until such a study is conducted, it is reasonable to conclude that maintaining good periodontal health in women planning babies will go a long way in significantly reducing the IMR of Rajnandgaon.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. R. M. Zade and Dr. Ramesh A. for their help and support during the conduct of the study.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Million Death Study Collaborators. Bassani DG, Kumar R, Awasthi S, Morris SK, Paul VK, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010;376:1853–1860. doi: 10.1016/S0140-6736(10)61461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152:425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The incidence of low birth weight: an update. Wkly Epidemiol Rec. 1984;59:205–211. [Google Scholar]
- 4.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 5.da Silva TR. Nonbiological maternal risk factor for low birth weight on Latin America: a systematic review of literature with meta-analysis. Einstein (Sao Paulo) 2012;10:380–385. doi: 10.1590/s1679-45082012000300023. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann Periodontol. 2001;6:153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26(3 Pt 2):230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams CE, Davenport ES, Sterne JA, Sivapathasundaram V, Fearne JM, Curtis MA. Mechanisms of risk in preterm low-birth-weight infants. Periodontol 2000. 2000;23:142–150. doi: 10.1034/j.1600-0757.2000.2230115.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanz M, Kornman K Working group 3 of joint EFP/AAP workshop. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S164–S169. doi: 10.1111/jcpe.12083. [DOI] [PubMed] [Google Scholar]
- 10.Corbella S, Taschieri S, Francetti L, De Siena F, Del Fabbro M. Periodontal disease as a risk factor for adverse pregnancy outcomes: a systematic review and meta-analysis of case-control studies. Odontology. 2012;100:232–240. doi: 10.1007/s10266-011-0036-z. [DOI] [PubMed] [Google Scholar]
- 11.Haerian-Ardakani A, Eslami Z, Rashidi-Meibodi F, Haerian A, Dallalnejad P, Shekari M, et al. Relationship between maternal periodontal disease and low birth weight babies. Iran J Reprod Med. 2013;11:625–630. [PMC free article] [PubMed] [Google Scholar]
- 12.Moliterno LF, Monteiro B, Figueredo CM, Fischer RG. Association between periodontitis and low birth weight: a case-control study. J Clin Periodontol. 2005;32:886–890. doi: 10.1111/j.1600-051X.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 13.Cruz SS, Costa Mda C, Gomes-Filho IS, Rezende EJ, Barreto ML, Dos Santos CA, et al. Contribution of periodontal disease in pregnant women as a risk factor for low birth weight. Community Dent Oral Epidemiol. 2009;37:527–533. doi: 10.1111/j.1600-0528.2009.00492.x. [DOI] [PubMed] [Google Scholar]
- 14.Siqueira FM, Cota LO, Costa JE, Haddad JP, Lana AM, Costa FO. Intrauterine growth restriction, low birth weight, and preterm birth: adverse pregnancy outcomes and their association with maternal periodontitis. J Periodontol. 2007;78:2266–2276. doi: 10.1902/jop.2007.070196. [DOI] [PubMed] [Google Scholar]
- 15.Shirmohammadi A, Abdollahifard S, Chitsazi MT, Behlooli S. Relationship between maternal periodontal disease and Apgar score of newborns. J Periodontal Implant Sci. 2012;42:212–216. doi: 10.5051/jpis.2012.42.6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YL, Liou JD, Pan WL. Association between maternal periodontal disease and preterm delivery and low birth weight. Taiwan J Obstet Gynecol. 2013;52:71–76. doi: 10.1016/j.tjog.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Ali TB, Abidin KZ. Relationship of periodontal disease to pre-term low birth weight infants in a selected population: a prospective study. Community Dent Health. 2012;29:100–105. [PubMed] [Google Scholar]
- 18.Vettore MV, Leal Md, Leao AT, da Silva AM, Lamarca GA, Sheiham A. The relationship between periodontitis and preterm low birth-weight. J Dent Res. 2008;87:73–78. doi: 10.1177/154405910808700113. [DOI] [PubMed] [Google Scholar]
- 19.Gomes-Filho IS, da Cruz SS, Rezende EJ, da Silveira BB, Trindade SC, Passos JS, et al. Periodontal status as predictor of prematurity and low birth weight. J Public Health Dent. 2006;66:295–298. doi: 10.1111/j.1752-7325.2006.tb04088.x. [DOI] [PubMed] [Google Scholar]
- 20.Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes--systematic review. J Clin Periodontol. 2013;40(Suppl 14):S181–S194. doi: 10.1111/jcpe.12063. [DOI] [PubMed] [Google Scholar]
- 21.United Nations Children's Fund; World Health Organization. Low birthweight: country, regional and global estimates [Internet] New York: UNICEF; 2004. [cited 2011 Nov 22]. Available from: http://www.childinfo.org/files/low_birthweight_from_EY.pdf. [Google Scholar]
- 22.Government of Chhattisgarh. Human development report Chhattisgarh [Internet] Raipur: Government of Chhattisgarh; 2005. [cited 2011 Nov 22]. Available from: http://www.undp.org/content/dam/india/docs/human_develop_report_2005_chhattisgarh_full_report.pdf. [Google Scholar]
- 23.Corbet EF, Zee KY, Lo EC. Periodontal diseases in Asia and Oceania. Periodontol 2000. 2002;29:122–152. doi: 10.1034/j.1600-0757.2002.290107.x. [DOI] [PubMed] [Google Scholar]
- 24.Ip M, Peyman E, Lohsoonthorn V, Williams MA. A case-control study of preterm delivery risk factors according to clinical subtypes and severity. J Obstet Gynaecol Res. 2010;36:34–44. doi: 10.1111/j.1447-0756.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassani DG, Olinto MT, Kreiger N. Periodontal disease and perinatal outcomes: a case-control study. J Clin Periodontol. 2007;34:31–39. doi: 10.1111/j.1600-051X.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 26.Dasanayake AP, Boyd D, Madianos PN, Offenbacher S, Hills E. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J Periodontol. 2001;72:1491–1497. doi: 10.1902/jop.2001.72.11.1491. [DOI] [PubMed] [Google Scholar]
- 27.Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. J Periodontol. 2005;76(11 Suppl):2144–2153. doi: 10.1902/jop.2005.76.11-S.2144. [DOI] [PubMed] [Google Scholar]
- 28.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67(10 Suppl):1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 29.Dasanayake AP. Poor periodontal health of the pregnant woman as a risk factor for low birth weight. Ann Periodontol. 1998;3:206–212. doi: 10.1902/annals.1998.3.1.206. [DOI] [PubMed] [Google Scholar]
- 30.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6:164–174. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 31.Bosnjak A, Relja T, Vucicevic-Boras V, Plasaj H, Plancak D. Pre-term delivery and periodontal disease: a case-control study from Croatia. J Clin Periodontol. 2006;33:710–716. doi: 10.1111/j.1600-051X.2006.00977.x. [DOI] [PubMed] [Google Scholar]
- 32.Moore S, Ide M, Coward PY, Randhawa M, Borkowska E, Baylis R, et al. A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. Br Dent J. 2004;197:251–258. doi: 10.1038/sj.bdj.4811620. [DOI] [PubMed] [Google Scholar]
- 33.Lunardelli AN, Peres MA. Is there an association between periodontal disease, prematurity and low birth weight? A population-based study. J Clin Periodontol. 2005;32:938–946. doi: 10.1111/j.1600-051X.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 34.Noack B, Klingenberg J, Weigelt J, Hoffmann T. Periodontal status and preterm low birth weight: a case control study. J Periodontal Res. 2005;40:339–345. doi: 10.1111/j.1600-0765.2005.00808.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell-Lewis D, Engebretson SP, Chen J, Lamster IB, Papapanou PN. Periodontal infections and pre-term birth: early findings from a cohort of young minority women in New York. Eur J Oral Sci. 2001;109:34–39. doi: 10.1034/j.1600-0722.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 36.Marakoglu I, Gursoy UK, Marakoglu K, Cakmak H, Ataoglu T. Periodontitis as a risk factor for preterm low birth weight. Yonsei Med J. 2008;49:200–203. doi: 10.3349/ymj.2008.49.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabet C, Lelong N, Colombier ML, Sixou M, Musset AM, Goffinet F, et al. Maternal periodontitis and the causes of preterm birth: the case-control Epipap study. J Clin Periodontol. 2010;37:37–45. doi: 10.1111/j.1600-051X.2009.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agueda A, Ramon JM, Manau C, Guerrero A, Echeverría JJ. Periodontal disease as a risk factor for adverse pregnancy outcomes: a prospective cohort study. J Clin Periodontol. 2008;35:16–22. doi: 10.1111/j.1600-051X.2007.01166.x. [DOI] [PubMed] [Google Scholar]
- 39.Kurdi AM, Mesleh RA, Al-Hakeem MM, Khashoggi TY, Khalifa HM. Multiple pregnancy and preterm labor. Saudi Med J. 2004;25:632–637. [PubMed] [Google Scholar]
- 40.Hasegawa K, Furuichi Y, Shimotsu A, Nakamura M, Yoshinaga M, Kamitomo M, et al. Associations between systemic status, periodontal status, serum cytokine levels, and delivery outcomes in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2003;74:1764–1770. doi: 10.1902/jop.2003.74.12.1764. [DOI] [PubMed] [Google Scholar]


