Abstract
Objective. The aim of this study was to determine the clinical correlates and predictors of rheumatoid vasculitis (RV).
Methods. A retrospective cohort of patients with RV evaluated at a tertiary referral centre between 1 January 2000 and 1 January 2010 was identified. RV cases were compared in a 1:2 ratio to controls (RA without vasculitis) to identify risk factors for developing RV.
Results. Eighty-six RV cases (58% women, 88% white) were identified. Histopathological confirmation was available for 58% of patients. Cutaneous vasculitis was the most common presentation, followed by vasculitic neuropathy. The median age at presentation was 63 years and the median duration of RA was 10.8 years. One third were current smokers. The majority were seropositive and had elevated inflammatory markers. Treatment was with a range of immunomodulating agents. At 6 months, 38% of patients achieved complete remission, 52% had partial improvement and 10% noted no clinical improvement. Thirty-six per cent relapsed by 5 years and 26% died. After adjusting for age and disease duration, current smoking at RA diagnosis [odds ratio (OR) 1.98], coexistent peripheral vascular disease (OR 3.98), cerebrovascular disease (OR 6.48), severe RA (OR 2.02) (characterized by radiographic erosions, nodulosis on clinical examination or requirement of joint surgery) and the use of biologics (OR 2.80) were found to increase the odds for developing RV; the use of HCQ (OR 0.54, CI 0.31, 0.94) and low-dose aspirin (OR 0.42, CI 0.21, 0.85) was associated with decreased odds for developing RV.
Conclusion. This largest single-centre series of patients with RV suggests that even in recent years, RV remains a serious complication of RA and is associated with significant mortality.
Keywords: rheumatoid arthritis, systemic vasculitis, rheumatoid vasculitis, biologics
Introduction
RA is a quintessential systemic disease. Extra-articular manifestations (EAMs) develop in 40% of patients and contribute to significant disease-related morbidity and mortality. Among these, systemic rheumatoid vasculitis (RV), characterized by inflammation of mid-size arteries and capillaries, is associated with a particularly dire outcome [1, 2], with up to 40% of patients dying within 5 years due to damage from vasculitis and/or consequences of immunosuppressive therapy [3, 4].
RV can be clinically heterogeneous and can simultaneously affect multiple vascular beds. Clinical manifestations may include deep cutaneous ulcers, peripheral gangrene, vasculitic neuropathy, inflammatory eye disease and visceral infarction, all associated with poor outcomes. Long-standing RA, male sex, smoking, rheumatoid nodules and, in particular, HLA class I and class II genotypes have been associated with an increased risk of RV [5–7].
Treat-to-target treatment paradigms and the use of biologic response modifiers (BRMs) have revolutionized the treatment of RA with better disease control, decreased incidence of systemic complications and overall improved outcomes. However, RV is still encountered in clinical practice and it is unclear whether the risk factors for this serious complication of RA have changed in recent years. The objectives of our study were to address gaps in our current knowledge of RV and determine risk factors for development of RV by conducting a case–control study.
Methods
Study design
This study was approved by the Mayo Clinic Institutional Review Board and was designed as a retrospective matched case–control study. All patients included gave written consent for use of their records for research. Cases and controls were identified retrospectively from a recent cohort of RA patients evaluated at the Mayo Clinic (Rochester, MN, USA) between 1 January 2000 and 1 January 2010. All patients met the 1987 ACR classification criteria for RA.
RV case identification was by electronic medical records diagnosis search followed by a chart review. Given the absence of an International Classification of Diseases, Ninth Revision (ICD-9)/Hospital Adaptation of the International Classification of Diseases A (HICDA) code for RV, we used a combination of several HICDA codes for RA and vasculitis to identify 505 cases. Ten patients who denied authorization for chart review for research purposes were excluded due to Minnesota law. Medical records of the remaining 495 patients were reviewed by a rheumatologist (A.M.) to confirm classification criteria for RA and identify patients with newly diagnosed (between 2000 and 2010) vasculitis. Patients with a prior history of RV (before 2000) were excluded. A patient with RA was defined as having RV based on histopathological confirmation of vasculitis on tissue biopsy and/or based on a strong clinical or radiological suspicion of vasculitis attributable to RA with no alternate explanation per the treating physician. A separate search for RV was run in the Mayo Clinical Notes Search tool of the Enterprise Data Trust Portal, which is designed to identify all patients with these terms anywhere in their medical records to validate retrieval and allow complete identification of cases.
For each RV case, two comparator subjects were identified from RA patients evaluated at the Mayo Clinic, Rochester, during the same time period. The controls were RA patients without RV, matched on age at index date, ascertained in an identical manner to the cases except for exclusion of those with vasculitis. The index date for control subjects was defined as the RV incidence date of the corresponding patient with RV. The absence of vasculitis was confirmed by a detailed review of available inpatient and outpatient medical records up to the corresponding index date and 1 year beyond it.
Data collection
Data abstracted from the medical records was collected on a standardized data collection form using the Research Electronic Data Capture (REDCap) tools hosted at the Mayo Clinic [8]. Demographics, smoking status, clinical features of RA (articular and EAM), laboratory studies, medications and co-morbidities (based on physician-based diagnoses listed in the medical record) were abstracted. For RV cases, the following variables were also collected: date and method of RV diagnosis, clinical presentation, smoking status at RV diagnosis, laboratory studies and treatment. Short-term (6 months) treatment outcomes (based on physician assessment) for RV were collected as complete remission, partial response or no improvement. Remission was defined as the absence of any clinical signs of active vasculitis with no new vasculitic manifestations. The 5-year relapse rate and mortality rate were also computed.
Statistical analysis
A sample of 50 cases and 100 controls provided us 79% power to detect an odds ratio (OR) of 3.0 for a risk factor with 50% prevalence and 20% correlation of exposure in matched sets of cases and controls using a two-sided test with a significance level of 0.05.
Descriptive statistics {mean [s.d.] or median [interquartile range (IQR)] for continuous variables and number [percentage] for categorical variables} were used to summarize the clinical features and treatment outcomes of patients with RV. Conditional logistic regression models were used to examine potential risk factors for RV. Rates of long-term outcome (i.e. relapse and mortality) were estimated using Kaplan–Meier methods. Cox models were used to examine risk factors for relapse. SAS version 9.2 (SAS, Cary, NC, USA) was used for statistical analysis.
Results
Study cohort and clinical characteristics
Between 2000 and 2010, 86 patients with RV were identified. All cases met the 1984 Scott and Bacon criteria [9] for systemic RV. Patients with isolated nailfold infarcts or rheumatoid nodules were not included as cases. Histopathology confirmation was available in 58% cases. Diagnosis was established by a Mayo Clinic physician in 89.5% cases while the rest were diagnosed at an outside institution.
Details of the clinical characteristics at RV presentation are listed in Table 1. The median age at RV diagnosis was 63 years (range 51–71) with a median duration of RA of 10.8 years (range 2.7–21). The most common presentation of vasculitis was cutaneous, followed by vasculitic neuropathy. One third of the cases were current smokers at presentation and one third had another EAM of RA. Radiographic erosions were noted in 54% of cases. The majority had anaemia and elevated inflammatory markers at presentation and were seropositive. For treatment of RA, 69% had been on CSs prior to developing RV. Fifty-eight cases had been on MTX previously, while 25 cases had been exposed to three or more DMARDs. Thirty-four cases had been treated with a BRM (all being anti-TNF agents).
Table 1.
Clinical characteristics of 86 rheumatoid vasculitis cases seen at the Mayo Clinic (Rochester, MN, USA) between 2000 and 2010
| Characteristic | n (%) or median (IQR) |
|---|---|
| Clinical presentation | |
| Cutaneous vasculitisa | 56 (65) |
| Vasculitic neuropathyb | 30 (35) |
| CNS vasculitis | 7 (8) |
| Mesenteric vasculitis | 2 (2) |
| Scleritis/episcleritis | 2 (2) |
| Pulmonary angiitis | 1 (1) |
| Necrotizing glomerulonephritis | 1 (1) |
| Demographic characteristics | |
| Age, years | 63 (51–71) |
| Sex, female | 50 (58) |
| Race, white | 76 (88) |
| BMI, kg/m2 | 26.4 (23.4–29.3) |
| Smoking status at RV diagnosis | |
| Current smokers | 25 (29) |
| Former smokers | 23 (26.7) |
| RA characteristics | |
| Duration of RA, years | 10.8 (2.7–21) |
| Smoking status at RA diagnosis | |
| Current smokers | 34 (40) |
| Former smokers | 13 (15.3) |
| Tender joint count (28 joints), mean (s.d.)c | 1.6 (4.1) |
| Swollen joint count (28 joints), mean (s.d.)c | 3.3 (5.4) |
| Rheumatoid nodules (skin only) | 38 (44) |
| Radiographic erosions | 46 (54) |
| Any joint replacement surgery (small, medium or large joints) | 30 (35) |
| ≥1 extra-articular manifestations | 25 (29) |
| Laboratory features | |
| Anaemia, haemoglobin < 12 g/dl | 43 (55) |
| Leucocytosis, >10.5 × 109 | 21/78 (27) |
| Thrombocytosis, >450 × 109] | 13/78 (17) |
| Elevated ESR | 50 (66) |
| Elevated CRP | 42 (69) |
| RF | 72 (84) |
| ACPA | 30/45 (67) |
| ANA positive | 24/82 (29) |
| ANCA positive | 29/75 (39) |
| pANCA | 28/75 (37) |
| MPO | 4/75 (5) |
| Hypocomplementaemiad | 10/71 (14) |
| Cryoglobulinaemia | 4/73 (6) |
| Treatment of RA prior to RV | |
| Corticosteroids | 59 (69) |
| MTX | 58 (69) |
| ≥3 DMARDs | 25 (29) |
| Any biologic | 34 (42) |
aCutaneous vasculitis included vasculitic purpura (with biopsy confirmation of leucocytoclastic vasculitis), upper or lower extremity ulcers (attributed to vasculitis, with other common aetiologies such as peripheral arterial disease or infection ruled out) and digital ischaemia/infarcts or gangrene. bVasculitic neuropathy included mononeuritis multiplex on EMG or pure motor/sensorimotor neuropathy on EMG study and confirmed on nerve biopsy. cBased on data available from 58 patients. dHypocomplementaemia was defined as low levels of serum total, C3 or C4 complements (normal total serum complement 30–75 U/ml, serum C3 75–175 mg/dl, serum C4 14–40 mg/dl). Only one patient had an isolated low C4 level.
We sought to evaluate whether RV occurring in patients with RA treated with BRMs differed from RV occurring in patients who were not on BRMs. Clinical correlates of RV cases by biologic use are summarized in Table 2. Among RV patients, those treated with a BRM for their RA were significantly younger (56 vs 65 years, P = 0.004) and had a lower incidence of vasculitic neuropathy (21% vs 47%, P = 0.015) than those treated without a BRM. There were no statistically significant differences in the occurrence of other clinical presentations of RV by biologic use other than vasculitic neuropathy.
Table 2.
Clinical correlates of rheumatoid vasculitis by biologic use for RA
| Characteristics at diagnosis of RV | Biologic use (n = 34) | No biologic (n = 47) | P-value |
|---|---|---|---|
| Duration of RA, median (IQR), years | 11.7 (5.5–22) | 7.9 (0.6–15.7) | 0.09 |
| Age, median (IQR), years | 56.4 (44.7–64.9) | 65.3 (53.8–73.4) | 0.004 |
| Ever smoker | 23 (67.6) | 23 (48.9) | 0.09 |
| ESR, mean (s.d.), mm/h | 43.8 (36.7) | 49 (35.1) | 0.38 |
| CRP, mean (s.d.), mg/l | 36.5 (42.7) | 52.1 (57.1) | 0.31 |
| Vasculitic neuropathy | 7 (20.6) | 22 (46.8) | 0.01 |
| ANCA positive | 11 (40.7) | 16 (37.2) | 0.77 |
| Cryoglobulinaemia | 1 (3.7) | 2 (4.8) | 0.83 |
| Hypocomplementaemia | 6 (21.4) | 3 (7.5) | 0.09 |
Values are n (%) unless indicated otherwise.
Risk factors for RV
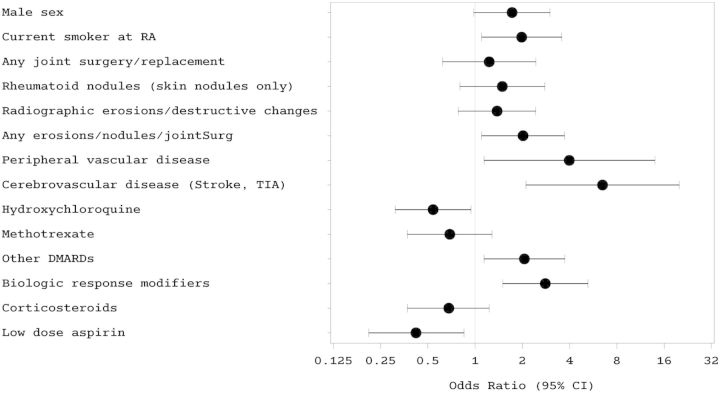
The 86 RV cases were compared with 172 controls to determine risk factors for RV (Fig. 1 and Table 3). Risk factors for RV were younger age at RA diagnosis, current smoking status at RA diagnosis, peripheral vascular disease, cerebrovascular disease, severe RA (characterized by erosions, nodulosis and/or joint surgery) and the use of other DMARDs (besides HCQ and MTX) and biologics for RA treatment. The use of HCQ and low-dose aspirin were found to lower the risk for RV. Other factors not found to be significant included RF, ACPA, ANA, rheumatoid lung disease, SS, cervical spine involvement, rheumatoid pericarditis, hypertension, hyperlipidaemia, diabetes mellitus, cardiovascular disease, atrial fibrillation, venous thromboembolism, the use of three or more DMARDs for RA and statin use.
Fig. 1.
Forest plot depicting risk factors for rheumatoid vasculitis in RA patients seen at the Mayo Clinic (Rochester, MN, USA) between 2000 and 2010
Values plotted are odds ratios and 95% CIs. (Other DMARDs in this forest plot indicate oral DMARDs besides HCQ and MTX.)
Table 3.
Risk factors for rheumatoid vasculitis among patients with RA seen at the Mayo Clinic (Rochester, MN, USA), 2000–10
| Cases (n = 86) | Controls (n = 172) | Odds ratio (95% CI) | |
|---|---|---|---|
| Sex, male | 36 (42) | 53 (31) | 1.72 (0.98, 3.00) |
| Age at RA diagnosis, mean (s.d.), years | 47.7 (14.5) | 52.4 (13.4) | 0.98 (0.97, 0.99) |
| BMI, mean (s.d.), kg/m2 | 27.1 (5.5) | 27.6 (5.2) | 1.00 (0.94, 1.06) |
| Current smoker at RA diagnosis | 34 (40) | 39 (23) | 1.98 (1.10, 3.56) |
| Rheumatoid nodules | 38 (44) | 45 (26) | 1.49 (0.80, 2.78) |
| Radiographic erosions | 46 (54) | 66 (39) | 1.38 (0.78, 2.43) |
| Any joint surgery | 30 (35) | 37 (22) | 1.23 (0.62, 2.44) |
| Any erosions, nodulosis and/or joint surgery | 62 (72) | 84 (49) | 2.02 (1.1, 3.71) |
| Rheumatoid lung disease | 9 (10) | 11 (6) | 1.59 (0.60, 4.21) |
| SS | 8 (9) | 18 (11) | 0.85 (0.34, 2.10) |
| C-spine disease | 10 (12) | 6 (4) | 2.03 (0.66, 6.24) |
| Rheumatoid pericarditis | 6 (7) | 4 (2) | 2.67 (0.72, 9.96) |
| ≥2 extra-articular manifestations | 7 (8) | 2 (1) | 5.05 (0.98, 26.06) |
| Positive RF | 72 (84) | 137 (82) | 0.80 (0.38, 1.67) |
| Positive ACPA | 30 (67) | 31 (51) | 1.88 (0.80, 4.40) |
| Positive ANA | 24 (29) | 24 (19) | 1.93 (0.96, 3.89) |
| Hypertension | 39 (45) | 62 (36) | 1.63 (0.91, 2.92) |
| Hyperlipidaemia | 25 (29) | 47 (28) | 1.29 (0.69, 2.40) |
| Diabetes mellitus | 12 (14) | 15 (9) | 1.79 (0.77, 4.12) |
| Cardiovascular disease | 12 (14) | 23 (14) | 0.90 (0.40, 2.04) |
| Peripheral vascular disease | 9 (10) | 4 (2) | 3.98 (1.14, 13.99) |
| Cerebrovascular disease | 14 (16) | 5 (3) | 6.48 (2.11, 19.97) |
| Atrial fibrillation | 3 (4) | 6 (4) | 0.83 (0.19, 3.65) |
| Venous thromboembolism | 8 (9) | 8 (5) | 2.23 (0.79, 6.32) |
| MTX for RA | 58 (69) | 121 (70) | 0.69 (0.37, 1.28) |
| HCQ for RA | 30 (37) | 85 (51) | 0.54 (0.31, 0.94) |
| ≥3 DMARDs for RA | 25 (29) | 27 (16) | 1.57 (0.81, 3.04) |
| Biologic use for RA | 34/81 (42) | 26/170 (15) | 2.80 (1.50,5.23) |
| Low-dose aspirin | 15 (17) | 50 (29) | 0.42 (0.21,0.85) |
| Statins | 19 (22) | 34 (20) | 1.36 (0.69,2.68) |
Values are n (%) unless indicated otherwise. Statistically significant variables are shown in bold.
Treatment and outcomes
Treatment strategies used for RV are summarized in Table 4. Nearly all patients received CSs in oral or i.v. form. The median starting dose of oral steroids was a prednisone equivalent of 40 mg (IQR 27.5–60). Twenty-nine per cent (24/83) of patients were treated with CYC (14 oral, 9 i.v. and 1 both). Systemic features (fever, weight loss, fatigue) (P = 0.02) and vasculitic neuropathy (P = 0.02) were statistically significantly higher among RV patients who were treated with CYC than those who were not. Forty-six patients received other DMARDs and 21 received a BRM for treatment.
Table 4.
Therapeutic agents used for the treatment of rheumatoid vasculitis and outcomes at the Mayo Clinic (Rochester, MN, USA), 2000–10
| Treatment | |
| Corticosteroids (oral and/or i.v.) | 83/86 (99) |
| Orala | 69/83 (83) |
| i.v. pulse therapy | 2/83 (2) |
| Oral and i.v. pulse | 12/83 (14) |
| CYC (oral or i.v.) | 24 (29) |
| Oral | 14 (16) |
| i.v. | 9 (10) |
| Oral and i.v. | 1 (1) |
| Other DMARDs | 46 (55) |
| HCQ 7 patients | |
| MTX 26 patients | |
| AZA 12 patients | |
| MMF 3 patients | |
| Minocycline 1 patient | |
| Biologic response modifiers | 21/74b (28) |
| Anti-TNF agents 12 patients | |
| Rituximab 6 patients | |
| Abatacept 1 patient | |
| Anakinra 2 patients | |
| Outcomes | |
| Follow-up, median (IQR), months | 16 (2.4–59) |
| Response to treatment (6 months after treatment initiation) | |
| Complete response | 33 (38) |
| Partial response | 45 (52) |
| No response | 8 (10) |
| Relapse at 5 years (similar or different clinical presentation) | 36% |
| Mortality rate (at 5 years) | 26% |
Values are n (%) unless indicated otherwise. aThe median starting dose of oral steroid was a prednisone equivalent of 40 mg (IQR 27.5–60). bData missing on 12 patients.
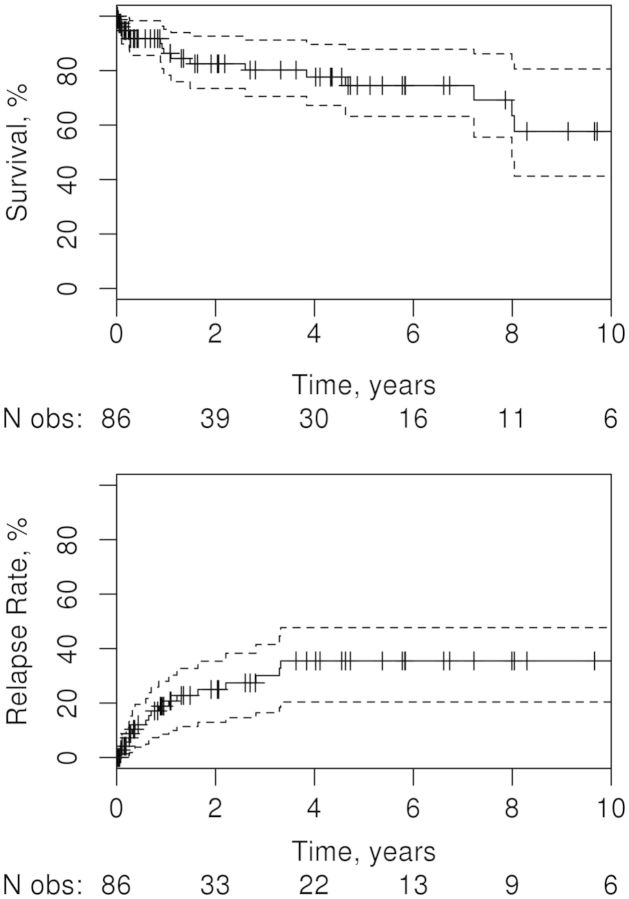
Six months after initiation of treatment, 38% of patients were noted to have achieved complete remission of RV symptoms, 52% had some improvement and 10% had no improvement. The median follow-up was 16 months (IQR 2.4–59). By 5 years, 36% of patients had a relapse of vasculitis (similar or different clinical presentation). Predictors of relapse are summarized in Table 5. Smoking at RV diagnosis (index date), lower ESR at presentation with RV and CYC use were found to significantly increase the risk of relapse. The 5-year mortality rate was high, at 26%. By 10 years there had been 18 deaths, 19 relapses and 6 patients were still under observation (Fig. 2).
Table 5.
Predictors of relapse among patients with rheumatoid vasculitis
| Characteristic at RV diagnosis | Hazard ratio (95% CI) |
|---|---|
| Age, years | 0.78a (0.54, 1.12) |
| Duration of RA, years | 1.09a (0.74, 1.62) |
| Current smoker | 3.12 (1.25, 7.79) |
| ESR, mm/h | 0.83a (0.69, 0.99) |
| CRP, mg/l | 1.02a (0.91, 1.15) |
| RF positive | 0.68 (0.24, 1.89) |
| ACPA positive | 1.76 (0.48, 6.41) |
| ANCA positive | 0.88 (0.32, 2.39) |
| Hypocomplementaemia | 0.90 (0.26, 3.11) |
| MTX use | 0.61 (0.20, 1.84) |
| Biologic use | 1.18 (0.44, 3.21) |
| CYC use | 2.77 (1.12, 6.84) |
Statistically significant variables are shown in bold. aHazard ratio reported per 10-unit increase.
Fig. 2.
Kaplan–Meier plots depicting survival rate (upper panel) and relapse rate (lower panel) in 86 patients with rheumatoid vasculitis
The solid line in each plot shows the estimated rate over time, with hashed lines showing where patients are censored; dotted lines are 95% CIs. The numbers beneath each plot show the number of patients still under observation at the corresponding time points.
Discussion
This study describes the largest single-centre series of patients with RV to date, highlighting the clinical presentation, therapy and outcomes of vasculitis occurring as a complication of RA.
Clinical presentation
The clinical presentation of RV continues to remain heterogeneous and similar to that reported in older case series [10–12]. The most common manifestation of RV in our study was cutaneous vasculitis, followed by neurologic, similar to prior studies [7, 13]. Nailfold infarcts may occur in isolation and have a prognosis similar to uncomplicated RA [14] and hence were not included as RV. As in prior reports, rare cases of rheumatoid pachymeningitis, ocular disease, pulmonary angiitis, mesenteric vasculitis and necrotizing glomerulonephritis continue to be observed and have not been eliminated completely despite improvements in RA management and a reported decrease in RA severity [15] in recent years. Coronary involvement is a rare, yet known presentation of RV [13]. There were no reported cases of coronary angiitis in our cohort.
RV is reported to occur in patients with long-standing RA (generally >10 years in duration). Recent data from the Norfolk registry reported an average disease duration of 15.6 years [16], while in our study the median duration was slightly lower, at 10.8 years. The disease activity of RA is often reported to be low (burnt out) when RV presents, and this is affirmed by the low mean tender and swollen joint counts in our patients. Clinically, patients with RV have been described to have severe RA with destructive joint disease and EAMs, in particular FS [5]. More than half of our cohort of RV patients had radiographic erosions or destructive changes in their joints and one third had another EAM, although none had FS. Nodulosis among our cohort was also lower (44% of patients compared with 86%) than reported previously [13]. These observations may be due to improved control of RA in recent years.
Laboratory data
RV onset is often heralded by a systemic inflammatory response [17]. The majority of our patients had elevated ESR, CRP and anaemia, although leucocytosis (27%) and thrombocytosis (16.7%) were not as frequent, similar to prior reports [13]. Deposition of immune complexes appears to contribute to vascular inflammation and end-organ damage in RV. High levels of immune complexes and circulating autoantibodies, particularly RF, are often present in patients with RV [18] and were noted in >80% of our patients. High serum levels of RF are often detected at RV onset [19]. Given the retrospective collection of data, we did not have RF titres available on all cases. Moreover, in those where the data were available, the methodology of testing and normal laboratory ranges were not uniform and hence titres were not directly comparable.
Anti-CCP antibodies are associated with progressive joint damage and severe EAMs in RA. Laskari et al. [20] reported 93% of 25 RV patients were positive for anti-CCP compared with 70% of RA patients without vasculitis. In our cohort, anti-CCP positivity was lower than in other studies. However, CCP data were available for fewer than half of the RV cohort. The frequency of hypocomplementaemia [13, 21] and cryoglobulinaemia [22] was much lower in our cohort of RV than in prior studies. ANCAs were detected in 29 of 86 patients, 28 of who had a pANCA pattern on immunofluorescence. Of these 28, only 4 had a positive MPO antibody while 11 had a positive ANA, which could have accounted for the pANCA pattern as a fixation artefact.
Treatment of RV
Given the rarity, severity and heterogeneous clinical presentation of RV, management is largely empirical using sustained aggressive immunosuppression. The results of two small, open-label studies [9, 23] and past experience favours the use of high-dose CSs and CYC, especially in those with severe disease (neuropathy and necrotizing arteritis on biopsy) [9]. Almost all patients in our cohort received CSs, and one third received CYC. Patients treated with CYC had a higher prevalence of vasculitic neuropathy and systemic features of vasculitis. CYC treatment was found to be a significant predictor of relapse among our RV patients, likely due to confounding by indication. Heurkens et al. [24] randomized 19 patients with systemic rheumatoid vasculitis (SRV) with predominantly cutaneous features to AZA 2 mg/kg and prednisone 60 mg vs conventional anti-rheumatic therapy and demonstrated greater clinical efficacy and a lower incidence of side effects, relapse and mortality in the AZA group. Only 12 of 86 patients in our cohort were treated with AZA, and a few patients received other DMARDs including MTX and MMF. D-penicillamine [25] and chlorambucil [26], demonstrated to be effective agents for RV in the 1960s, were not used in any of our patients. There are few reports of initial efficacy of plasma exchange in SRV, often followed by relapse [25, 27]. Only 1 of our 86 patients with vasculitic neuropathy received it, without any benefit.
The role of anti-TNF therapy in RV continues to remain uncertain, with reports of benefit in some patients [28], whereas there are >200 cases of anti-TNF-induced vasculitis in the literature [29–34]. Distinguishing RV due to active RA vs drug-induced vasculitis can be challenging. In our cohort, 34 patients were treated with an anti-TNF agent prior to the development of RV. Only one of these was suspected by the treating clinician to have a drug-induced vasculitis. In all others, RV was attributed to RA disease severity and/or inadequate immunosuppression.
Various biologic agents were used for the treatment of RV, including anti-TNF agents, rituximab, abatacept and anakinra. Due to the small sample size, disease heterogeneity, variable clinical response and retrospective nature of our analysis, conclusions regarding the efficacy of individual biologic agents for treatment of RV could not be drawn.
Mortality in RV
Several studies have reported high morbidity and mortality rates (33–43%) with RV [1, 4, 21, 35]. Data from Olmsted County [36] and the US Veterans Administration [37] demonstrate the declining incidence of RV, likely attributable to better treatment of RA, including the increasing use of DMARDs and biologics. One would expect this success to translate into better survival for patients with RV. Unfortunately, recent data from the Norfolk registry [16] demonstrate that although the average incidence of RV has decreased from 9.1 cases/million in 1998–2000 to 3.9 cases/million in 2001–10, the overall mortality rates remain similar and high, with 1-year mortality rates of 14% and 12%, respectively, and 5-year mortality rates of 51% and 60%, respectively, in the two time periods. With an observed mortality rate of 26% over 5 years, our data support their conclusion and highlight the grave outcomes for RV patients.
Risk factors for RV
After adjusting for age and disease duration at the index date, the risk of developing RV was associated with male sex, as observed previously [7, 13, 38], although it did not reach statistical significance. Watts et al. [7] estimated the lifetime risk of developing RV at 1 in 9 among male RA patients compared with 1 in 38 female RA patients. The reasons for male predominance remain unclear, but have been attributed to smoking and coincidental cardiovascular disease. Current smoking at RA diagnosis was found to significantly increase the odds of developing RV in our cohort. This has been shown in prior studies [39] and attributed to the immunomodulatory effects of smoking via interaction with HLA-DRB1 alleles [40] and non-specific vascular damage leading to systemic inflammation [39, 41].
Rheumatoid nodules have been long considered to predict the occurrence of systemic vasculitis [18, 42] and are regarded as a sign of microvascular extra-articular inflammation. In our study, nodulosis alone did not predict higher odds for RV, but the odds doubled when combined with other attributes of severity in RA such as radiographic erosions and joint surgery.
Although individual extra-articular features such as rheumatoid lung disease, SS, pericarditis and C-spine disease were not associated with an increased risk of RV independently, the risk was increased with two or more of these together, signifying a higher overall disease burden in these patients developing RV. Unlike a prior case–control study [18] that studied RV patients from 1980 to 1993 and found ever positive RF to predict higher odds of RV, in our study, having any positive serologies (RF, anti-CCP or ANA) did not predict a higher risk of RV.
Co-morbid peripheral vascular and cerebrovascular disease substantially increased the odds of RV in our cohort by four to six times. This may represent the extra burden of vascular pathology in multiple arterial beds that these patients are predisposed to developing, probably from a genetic predisposition compared with their counterpart RA controls. In particular, interactions between HLA-C*03 and killer-immunoglobulin-like receptors on CD28null T cells [41, 43] have been implicated in the pathogenesis of vasculitis and other EAMs in RA.
The use of a BRM for RA treatment was associated with higher odds of RV. This is probably attributable to a channelling bias, as patients treated with a BRM were also more likely to have severe disease and develop RV by virtue of that. As discussed above, the dilemma of a drug-induced vasculitis persists, but overall the risk seems to be low. The use of three or more DMARDs signifies RA that is increasingly refractory to conventional combination DMARD therapy and the increased need to introduce biologic treatments. In our study it did show a positive association, but it did not reach statistical significance, probably due to the small sample.
To our knowledge, no protective factors have been identified for RV in any prior reports.
In our study, use of HCQ (OR 0.54, P = 0.03) was associated with lower odds of developing RV. The exact reason for this is unclear but may be attributed to its multimodal anti-inflammatory and immunomodulatory actions [44] or may reflect a milder form of RA. Low-dose aspirin use also decreased the odds of developing RV (OR 0.42, P = 0.02). This may be attributed to an antithrombotic effect that results from the inhibition of platelet thromboxane A2 and its resultant effect on vascular homeostasis in RA.
Study strengths
This is the largest single-centre series describing RV in the biologic era in a US cohort. It augments previous reports by providing a detailed descriptive analysis of clinical correlates, treatments and short-term outcomes among patients diagnosed with RV in the more recent decade, addressing an important knowledge gap. We also ascertained risk factors for RV in a systematic case–control design and determined protective factors that have not been described in the literature before, both modifiable to some extent. Whether patients having a combination of severe RA with nodulosis and joint interventions will benefit from low-dose aspirin or HCQ is debatable.
Study limitations
This study is limited by its retrospective nature. In the absence of diagnostic codes for RV, we used a combination of codes for vasculitis and RA to identify patients with RV. Using this approach we may have missed a few cases of RV, although to minimize the loss of cases, we validated the retrieval by a different method to allow complete identification of cases.
In the absence of updated criteria for RV, several factors, including drug use and mimickers, make it challenging to correctly diagnose vasculitis attributable to RA. While not all patients had histopathologically confirmed RV, the majority had histopathological or radiological confirmation of RV. Finally, there is a referral bias inherent in our institution’s status as a tertiary care academic medical centre. As a result, our cohort may not be representative of a general US-based medical practice. However, RV is a rare and severe manifestation of RA and can best be studied in a referral-based cohort. The generalizability of our findings may be limited primarily to the US white population, reflecting the racial composition of our current clinical practice.
Conclusion
RV is among the most serious complications of RA. Although epidemiological data suggest that the overall incidence of RV is declining, our data suggest that RV remains a difficult to treat EAM of RA with high risk of relapse and short-term mortality. Even today, conventional treatment with high-dose CSs and CYC remains the treatment of choice for RV for most clinicians. Despite better treatment options, clinical presentation has remained the same as >30 years ago and mortality rates remain high.
Rheumatology key messages.
Cutaneous vasculitis and vasculitic neuropathy are the most common clinical manifestations of rheumatoid vasculitis (RV).
Smoking, vascular disease and RA severity are risk factors for RV.
Even today, RV remains a serious complication of RA and is associated with significant mortality.
Funding: This work was funded by the Mayo Clinic Margaret Harvey Schering Clinician Career Development Award Fund for Arthritis Research and supported by grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The sponsors of these grants did not have any involvement with the study design, data collection, analysis and interpretation of data, writing of the manuscript or the decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Erhardt CC, Mumford PA, Venables PJ, et al. Factors predicting a poor life prognosis in rheumatoid arthritis: an eight year prospective study. Ann Rheum Dis. 1989;48:7–13. doi: 10.1136/ard.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62–7. [PubMed] [Google Scholar]
- 3.Puechal X, Said G, Hilliquin P, et al. Peripheral neuropathy with necrotizing vasculitis in rheumatoid arthritis. A clinicopathologic and prognostic study of thirty-two patients. Arthritis Rheum. 1995;38:1618–29. doi: 10.1002/art.1780381114. [DOI] [PubMed] [Google Scholar]
- 4.Voskuyl AE, Zwinderman AH, Westedt ML, et al. The mortality of rheumatoid vasculitis compared with rheumatoid arthritis. Arthritis Rheum. 1996;39:266–71. doi: 10.1002/art.1780390213. [DOI] [PubMed] [Google Scholar]
- 5.Turesson C, Matteson EL. Vasculitis in rheumatoid arthritis. Curr Opin Rheumatol. 2009;21:35–40. doi: 10.1097/BOR.0b013e32831c5303. [DOI] [PubMed] [Google Scholar]
- 6.Turesson C, O’Fallon WM, Crowson CS, et al. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts RA, Carruthers DM, Symmons DP, et al. The incidence of rheumatoid vasculitis in the Norwich Health Authority. Br J Rheumatol. 1994;33:832–3. doi: 10.1093/rheumatology/33.9.832. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott DG, Bacon PA. Intravenous cyclophosphamide plus methylprednisolone in treatment of systemic rheumatoid vasculitis. Am J Med. 1984;76:377–84. doi: 10.1016/0002-9343(84)90654-5. [DOI] [PubMed] [Google Scholar]
- 10.Bywaters EG, Scott JT. The natural history of vascular lesions in rheumatoid arthritis. J Chronic Dis. 1963;16:905–14. doi: 10.1016/0021-9681(63)90139-5. [DOI] [PubMed] [Google Scholar]
- 11.Schmid FR, Cooper NS, Ziff M, et al. Arteritis in rheumatoid arthritis. Am J Med. 1961;30:56–83. doi: 10.1016/0002-9343(61)90064-x. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson M, Torrance WN. Clinical background of rheumatoid vascular disease. Ann Rheum Dis. 1967;26:475–80. doi: 10.1136/ard.26.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott DG, Bacon PA, Tribe CR. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine. 1981;60:288–97. [PubMed] [Google Scholar]
- 14.Watts RA, Carruthers DM, Scott DG. Isolated nail fold vasculitis in rheumatoid arthritis. Ann Rheum Dis. 1995;54:927–9. doi: 10.1136/ard.54.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silman A, Davies P, Currey HL, et al. Is rheumatoid arthritis becoming less severe? J Chronic Dis. 1983;36:891–7. doi: 10.1016/0021-9681(83)90011-5. [DOI] [PubMed] [Google Scholar]
- 16.Ntatsaki EM, Mooney J, Scott DGI, et al. Systemic rheumatoid vasculitis in the biologic era. Br Soc Rheumatol. 2012;51(Suppl 3) Abstract O38. [Google Scholar]
- 17.Turesson CM. Extraarticular features of rheumatoid arthritis and systemic involvement. In: Hochberg MC, et al., editors. Rheumatology. 4th edn. Philadelphia: Mosby; 2008. pp. 773–83. [Google Scholar]
- 18.Voskuyl AE, Zwinderman AH, Westedt ML, et al. Factors associated with the development of vasculitis in rheumatoid arthritis: results of a case-control study. Ann Rheum Dis. 1996;55:190–2. doi: 10.1136/ard.55.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turesson C, McClelland RL, Christianson TJ, et al. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:70–5. doi: 10.1136/ard.2006.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskari K, Ahmadi-Simab K, Lamken M. Are anticyclic citrullinated peptide autoantibodies (anti-CCP) a sural marker for rheumatoid vasculitis in a cohort of patients with systemic vasculitis? Ann Rheum Dis. 2008;67(Suppl II):355. doi: 10.1136/ard.2009.110411. [DOI] [PubMed] [Google Scholar]
- 21.Geirsson AJ, Sturfelt G, Truedsson L. Clinical and serological features of severe vasculitis in rheumatoid arthritis: prognostic implications. Ann Rheum Dis. 1987;46:727–33. doi: 10.1136/ard.46.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisman M, Zvaifler N. Cryoimmunoglobulinemia in rheumatoid arthritis. Significance in serum of patients with rheumatoid vasculitis. J Clin Invest. 1975;56:725–39. doi: 10.1172/JCI108144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis. Effects of systemic immunosuppression. Ophthalmology. 1984;91:1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 24.Heurkens AH, Westedt ML, Breedveld FC. Prednisone plus azathioprine treatment in patients with rheumatoid arthritis complicated by vasculitis. Arch Intern Med. 1991;151:2249–54. [PubMed] [Google Scholar]
- 25.Jaffe IA. The treatment of rheumatoid arthritis and necrotizing vasculitis with penicillamine. Arthritis Rheum. 1970;13:436–43. doi: 10.1002/art.1780130412. [DOI] [PubMed] [Google Scholar]
- 26.Kahn MF, Bedoiseau M, de Seze S. Immunosuppressive drugs in the management of malignant and severe rheumatoid arthritis. Proc R Soc Med. 1967;60:130–3. [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman JA, Casey HL, McIlwain H, et al. Limited plasmapheresis in rheumatoid arthritis with vasculitis. Arthritis Rheum. 1979;22:1146–50. doi: 10.1002/art.1780221019. [DOI] [PubMed] [Google Scholar]
- 28.Puechal X, Miceli-Richard C, Mejjad O, et al. Anti-tumour necrosis factor treatment in patients with refractory systemic vasculitis associated with rheumatoid arthritis. Ann Rheum Dis. 2008;67:880–4. doi: 10.1136/ard.2007.081679. [DOI] [PubMed] [Google Scholar]
- 29.Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955–8. [PubMed] [Google Scholar]
- 30.Ramos-Casals M, Brito-Zeron P, Munoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine. 2007;86:242–51. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 31.Ramos-Casals M, Brito-Zeron P, Soto MJ, et al. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol. 2008;22:847–61. doi: 10.1016/j.berh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Ramos-Casals M, Roberto Perez A, Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9:188–93. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Saint Marcoux B, De Bandt M. Vasculitides induced by TNFalpha antagonists: a study in 39 patients in France. Joint Bone Spine. 2006;73:710–3. doi: 10.1016/j.jbspin.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Sokumbi O, Wetter DA, Makol A, et al. Vasculitis associated with tumor necrosis factor-alpha inhibitors. Mayo Clin Proc. 2012;87:739–45. doi: 10.1016/j.mayocp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vollertsen RS, Conn DL. Vasculitis associated with rheumatoid arthritis. Rheum Dis Clin North Am. 1990;16:445–61. [PubMed] [Google Scholar]
- 36.Myasoedova E, Crowson CS, Turesson C, et al. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995–2007 versus 1985–1994: a population-based study. J Rheumatol. 2011;38:983–9. doi: 10.3899/jrheum.101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartels C, Bell C, Rosenthal A, et al. Decline in rheumatoid vasculitis prevalence among US veterans: a retrospective cross-sectional study. Arthritis Rheum. 2009;60:2553–7. doi: 10.1002/art.24775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vollertsen RS, Conn DL, Ballard DJ, et al. Rheumatoid vasculitis: survival and associated risk factors. Medicine. 1986;65:365–75. [PubMed] [Google Scholar]
- 39.Struthers GR, Scott DL, Delamere JP, et al. Smoking and rheumatoid vasculitis. Rheumatol Int. 1981;1:145–6. doi: 10.1007/BF00541260. [DOI] [PubMed] [Google Scholar]
- 40.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 41.Turesson C, Schaid DJ, Weyand CM, et al. Association of HLA-C3 and smoking with vasculitis in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2776–83. doi: 10.1002/art.22057. [DOI] [PubMed] [Google Scholar]
- 42.Turesson C, McClelland RL, Christianson T, et al. Clustering of extraarticular manifestations in patients with rheumatoid arthritis. J Rheumatol. 2008;35:179–80. [PubMed] [Google Scholar]
- 43.Martens PB, Goronzy JJ, Schaid D, et al. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–14. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 44.Wallace DJ, Gudsoorkar VS, Weisman MH, et al. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol. 2012;8:522–33. doi: 10.1038/nrrheum.2012.106. [DOI] [PubMed] [Google Scholar]