Abstract
The exaggerated sexual swellings exhibited by females of some primate species have been of interest to evolutionary biologists since the time of Darwin. We summarize existing hypotheses for their function and evolution and categorize these hypotheses within the context of 3 types of variation in sexual swelling size: 1) variation within a single sexual cycle, 2) variation between the sexual cycles of a single female, and 3) differences between females. We then propose the Paternal Care Hypothesis for the function of sexual swellings, which posits that exaggerated sexual swellings function to elicit the right quantity and quality of male care for a female's infant. As others have noted, swellings may allow females to engender paternity confusion, or they may allow females to confer relative paternal certainty on one male. Key to our hypothesis is that both of these scenarios create an incentive for one or more males to provide care. This hypothesis builds on previous hypotheses but differs from them by highlighting the elicitation of paternal care as a key function of swellings. Our hypothesis predicts that true paternal care (in which males accurately differentiate and provide assistance to their own offspring) will be most common in species in which exaggerated swellings accurately signal the probability of conception, and males can monopolize females during the window of highest conception probability. Our hypothesis also predicts that females will experience selection to behave in ways that either augment paternity confusion or enhance paternal certainty depending on their social and demographic contexts.
Keywords: infanticide, parental care, primates, sexual swellings
INTRODUCTION
Females of many primate species exhibit exaggerated sexual swellings—large, conspicuous, and sometimes brightly colored swellings of the anogenital area (and sometimes other correlated areas) that occur during the periovulatory phase of the sexual cycle. Exaggerated sexual swellings are thought to have evolved multiple times (Dixson 1983; Nunn 1999). They occur in some Old World Monkeys and some apes but have not been reported in New World Monkeys or Strepsirrhines.
Several hypotheses have been proposed to explain the function and evolution of exaggerated sexual swellings. Each of these hypotheses builds, either explicitly or implicitly, on observations about 1 of the 3 possible ways in which this trait may vary. Specifically, variation in the size of sexual swellings may be measured within one cycle of one individual female, within one individual female across multiple cycles, or across individuals (Figure 1). By extension, sexual swellings may signal 3 different types of information to the receiver. They may signal the timing of ovulation within a cycle, they may signal the probability of conception between cycles, and they may signal differences in quality between individuals (Zinner et al. 2002). We categorize each of the hypotheses that have been proposed according the type of variation that it addresses. We refer to these categories as addressing questions about “Signaling Within a Cycle, Signaling Between Cycles, and Signaling Between Individuals.” Hypotheses in all of these categories have strengths, but empirical support for them varies markedly. Notably, they are usually not mutually exclusive; hypotheses in some or all of the categories may accurately describe the function(s) of exaggerated sexual swellings.
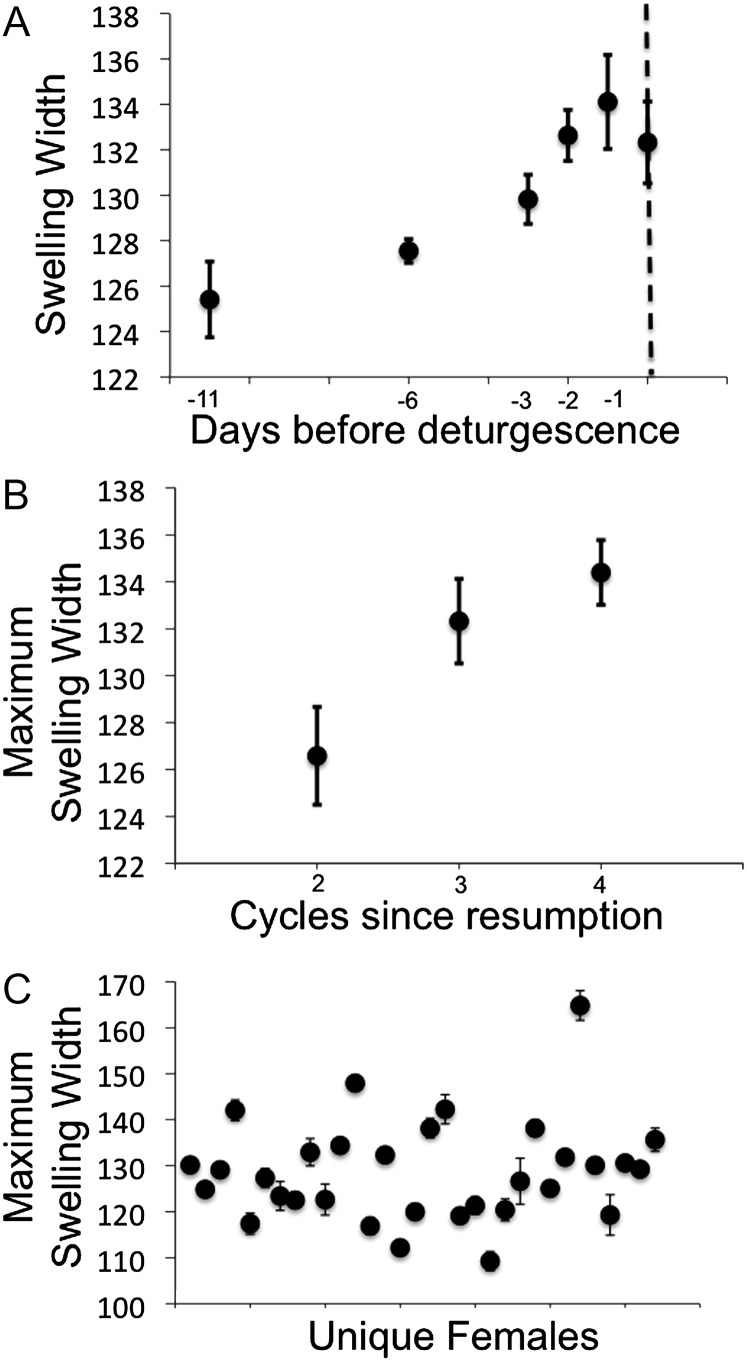
Figure 1.
Examples of how sexual swelling size varies in the Amboseli baboon population in southern Kenya (A) within one cycle of an individual, (B) between cycles of one individual, and (C) across individuals. (A) Changes in swelling size over the course of one nonconceptive cycle for one female. Each point represents the mean width (±standard deviation [SD]) of the swelling measured from multiple images on a single day. The vertical dashed line represents the first day of deturgescence. (B) Changes in maximum swelling size for 3 consecutive estrous cycles within one female; cycles are numbered consecutively from when the female resumed cycling (after the birth of the previous offspring). Each point represents the mean width (±SD) of the swelling measured from multiple images on the day prior to deturgescence in each cycle. The female's fourth cycle after resumption was a conceptive cycle. (C) Differences in maximum swelling size across females; each point represents the mean maximum swelling size (±SD) for one conceptive cycle of one female.
Here, we propose the Paternal Care Hypothesis for the function of exaggerated sexual swellings in primates. In particular, we propose that a key function of exaggerated sexual swellings is to elicit the optimum quantity and quality of male investment in a female's infant. This optimum will vary with demographic and behavioral contexts and will be subject to ecological and phylogenetic constraints. In some contexts, the most valuable role for male investment will be to help prevent infanticide during the period of infant vulnerability; in these contexts, the best option for females might be to confuse paternity, thereby increasing the number of males that are motivated to defend an infant. In other contexts, greater fitness benefits may accrue to offspring through more enduring, longer term investment, though that investment may be less intense at any one time than protection against infanticide; in these contexts, the best option might be to confer paternal certainty on a single male who then concentrates his paternal effort in the offspring he has fathered. We posit that variation in the information content of sexual swellings tracks this variation in the optimal quantity and quality of male investment (excluded from this prediction are swellings that occur when females are pregnant, a phenomenon known in some species, which may have a different function).
FUNCTIONS OF EXAGGERATED SEXUAL SWELLINGS IN PRIMATES
Below, we briefly describe signaling within a cycle, signaling between cycles, and signaling between individuals as categories of hypotheses for the function of sexual swellings (as noted above, excluding cases where swellings occur during pregnancy). We summarize the empirical support for these hypotheses so far, and then we describe the relevance of sexual swellings for understanding paternal care. Finally, we outline our hypothesis and its predictions.
Category 1: signaling within a cycle
Most hypotheses that have been proposed to explain either the function or the evolution of exaggerated sexual swellings focus on the variation that sexual swellings display within any given estrous cycle. These hypotheses include the Best Male Hypothesis, the Obvious Ovulation Hypothesis, the Many Males Hypothesis, the Male Services Hypothesis, and the Graded Signal Hypothesis (summarized in Nunn 1999; see also Clutton-Brock and Harvey 1976; Hrdy 1981; Hamilton 1984; van Noordwijk 1985; Hrdy and Whitten 1987). The Graded Signal Hypothesis (Nunn 1999) is currently the prevailing and most comprehensive one; it posits that exaggerated swellings function simultaneously to confuse paternity and concentrate the probability of paternity in the highest quality male. Specifically, swellings can confuse paternity by extending the period of mating and hence promoting multiple mating, and they can concentrate paternity in the “best” male by inciting the most intense competition when the probability of ovulation is highest (Nunn 1999). All of these within-cycle hypotheses build on the notion that the timing of ovulation is correlated with the period of maximal swelling within each estrous cycle, but each posits that a different set of benefits have driven the evolution of exaggerated swellings (for a thorough review, see Nunn 1999).
Ample empirical evidence supports the idea that swelling size can signal the probability of conception within a cycle. Specifically, in most species that have been examined, the increase in swelling size within each estrous cycle signals the approach of ovulation. For example, ovulation in baboons typically occurs within the 5-day window prior to deturgescence (going down) of the swelling, and the period of maximal turgescence encompasses ovulation in both captive (Wildt et al. 1977; Daspre et al. 2009) and wild populations (Higham et al. 2008a). A similar correspondence between maximal turgescence and ovulation has been reported in some macaque species (Mohle et al. 2005), in sooty mangabeys (Whitten and Russell 1996), and in chimpanzees (Dahl et al. 1991; Emery and Whitten 2003). Furthermore, male mate-guarding attention increases dramatically as swelling size increases within a cycle in several species, and even subtle swelling size changes within the period of maximal turgescence may play a critical role in signaling ovulation (e.g., Emery and Whitten 2003; Deschner et al. 2004 in chimpanzees; Higham et al. 2008b in baboons). Indeed, as predicted by the Graded Signal Hypothesis (Nunn 1999), the fact that the swelling has a long duration promotes multiple mating by females, potentially confusing paternity; at the same time, the generally good relationship between ovulation and maximal swelling in a range of species can serve to concentrate paternity. However, the precision with which maximal swelling predicts ovulation varies across species (e.g., see Reichert et al. 2002; Engelhardt et al. 2005), suggesting that the balance between concentrating and confusing paternity may be evolutionarily labile and that lineages may differ in this balance.
Category 2: differentiating between cycles
Recent empirical studies support the idea that exaggerated swellings also allow males to differentiate between the cycles of a given female. After giving birth, most female primates experience a period of postpartum amenorrhea, followed by the resumption of sexual cycles. Once cycles resume, female primates usually cycle repeatedly without conceiving, even when mating activity is plentiful (Drea 2005). This hypothesis predicts that the maximum size of a sexual swelling on a given cycle signals the probability of conception on that cycle relative to other cycles of that female. That is, it predicts that successive swellings of a female within a single reproductive interval are progressively larger and that the conceptive cycle is the largest swelling in that reproductive interval. It also predicts that males will expend more effort on cycles that exhibit a relatively large swelling for that female. For males to successfully differentiate between cycles of a given female, males must be able to track individual females over the course of several cycles and differentiate swelling size between cycles of the same female.
Recent data from several primate species support the “Differentiating Between Cycles Hypothesis.” Olive baboons (Papio anubis; Higham et al. 2008b) and chimpanzees (Pan troglodytes; Emery and Whitten 2003; Deschner et al. 2004) showed increases in absolute swelling size with increasing cycle number (i.e., with each successive cycle in a reproductive interval, the female displayed larger swellings). In yellow baboons (Papio cynocephalus), conceptive cycles were associated with larger swellings than nonconceptive cycles, and high-ranking males monopolized a larger fraction of the female receptive period for conceptive cycles than they did for nonconceptive cycles (Gesquiere et al. 2007; see also Alberts et al. 2006). In chacma baboons as in yellow baboons, high-ranking males biased their mate-guarding effort toward conceptive over nonconceptive cycles (Bulger 1993; Weingrill et al. 2003; see Daspre et al. 2009 for data that are qualitatively similar for olive baboons). Interestingly, the Weingrill et al. (2003) study also showed that recently immigrated high-ranking males did not bias their mate-guarding effort toward conceptive cycles until after an initial period of residence, suggesting that time with the females provided males with critical information for assessing the probability of conception for a given cycle. Taken together, these data strongly suggest that increases in swelling size across successive cycles, in at least some species, enable males to assess the probability of conception for that female and that males adjust their mating investment accordingly, as predicted by the Differentiating Between Cycles Hypothesis.
Category 3: signaling differences between individuals
Pagel (1994) posited that swelling size in female primates, like eyespots on the peacock's tail (Petrie et al. 1991; Petrie and Williams 1993; Petrie 1994), is an honest indicator of the individual's quality. For exaggerated swellings to function as a reliable indicator analogous to the spots on a peacock's tail, swelling size differences between females must be consistent indicators not of transient female quality (such as fecundability that changes within an individual because of temporal changes in her physiological state) but of a relatively enduring female superiority over other females (including those in the same physiological state). That is, they must signal not simply differences in the quality of the mating opportunity (one female is ready to conceive because she has been cycling for several months, and a second female is less likely to conceive because she has been cycling for a shorter time), but differences in the quality of 2 females even given that they are both likely to conceive on that cycle. Such differences may result from good genes or from consistently better health that arises, for example, from a maternal effect. Such differences in female quality could be manifested in the survival or fertility of their offspring.
Several studies have attempted to test the Signaling Differences Between Individuals Hypothesis, but to date, none have provided robust evidence in its favor. Zinner et al. (2002) outlined the 3 types of evidence that are necessary to support the hypothesis that exaggerated swellings are reliable indicators of quality. First, differences between females in their maximal swelling size must be consistent across multiple cycles and so reflect relatively enduring differences between females in swelling size. Second, a female's swelling size relative to other females must indicate her permanent, rather than transitory, quality as a mate. That is, it must signal not simply “this is the right time to mate with me because I am ready to conceive,” but “among all females that are ready to conceive, I am the superior choice” (for instance, because her infants will survive with higher probability). Third, males must bias their mating effort toward females with larger swellings.
Recent studies have found support for the first type of evidence (consistent differences in swelling size between females) in wild chimpanzees (Deschner et al. 2004) and wild chacma baboons (Huchard et al. 2009); in these species, some females have consistently larger swellings than other females. This is not true in semicaptive mandrills, however (Setchell and Wickings 2004).
Reports of the second type of evidence (swelling size indicates quality as a mate) have been mixed. A correlation between swelling size and number of surviving offspring was found in wild chacma baboons (Huchard et al. 2009) but not in semicaptive mandrills (Setchell and Wickings 2004). One study reported a correlation between swelling size and reproductive success in wild olive baboons (Domb and Pagel 2001), but a reanalysis of their data showed that the effect was explained by differences between social groups: In some social groups, females had larger body size, larger swellings, and higher infant survival, and male–male competition was more intense, all possibly as a result of enhanced nutrition through partial access to human food sources (Zinner et al. 2002).
Reports of the third type of evidence have come from 2 studies in wild baboons, that is, they have reported that males bias their mating effort toward females with larger maximal swelling sizes (Domb and Pagel 2001; Huchard et al. 2009). However, neither study controlled for the variation between cycles that individual females of several species, including baboons, are known to exhibit (Deschner et al. 2003; Gesquiere et al. 2007; Higham et al. 2008b). Consequently, these 2 studies were unable to differentiate between a situation in which the female with the larger swelling was simply the female who had experienced more cycles in her current reproductive interval, and so was closer to conception (supporting the Signaling Between Cycles Hypothesis), versus a situation in which the female with the larger swelling was the higher quality female (supporting the Differentiating Between Individuals Hypothesis). In fact, one study in wild chimpanzees found direct evidence that, when presented with the choice, males preferred females who were periovulatory over females with larger swellings (Deschner et al. 2004). Like Deschner et al. (2004), Emery and Whitten (2003) concluded that their data on captive chimpanzees did not support the Differentiating Between Individuals hypothesis and instead cite their study as evidence for the Signaling Between Cycles Hypothesis. Taken together, the prevailing evidence does not support the Differentiating Between Individuals hypothesis.
The fact that evidence for this hypothesis is mixed may reflect the fact that sexual swellings do not signal differences in quality between individuals, or it may simply reflect variation across species in the functional significance of sexual swellings—in some species, swellings may reflect individual differences, and in others, they may not. The evolutionary lability of this trait may mean that the selective forces that currently shape its expression differ among species. In this context, however, it is useful to note that the Signaling Within A Cycle hypothesis has received consistent support in a wide range of species: In virtually, all species examined to date, an increase in swelling size within each estrous cycle signals the approach of ovulation (although, as noted, species vary in how precisely maximal swelling size predicts ovulation). The Differentiating Between Cycles hypothesis has not been explicitly examined until recently, but when it has, this hypothesis, too, has received consistent support. The more extensive support for these 2 hypotheses justifies a close focus on explanations for swelling size variation within and between cycles, which is the aim of this paper.
PATERNAL CARE IN PRIMATES
Traditional models for the evolution of paternal care require that males experience a high degree of paternal certainty before they provide care, so that, in species with internal fertilization, paternal care is usually associated with monogamy (Kleiman and Malcolm 1981; Snowden and Suomi 1982). In general, this prediction is well supported in mammals. However, 2 lines of evidence—1 theoretical and 1 empirical—support the notion that male primates in a range of social systems—including polygynandrous species—experience selection to provide paternal care. First, the average wild male primate will experience just a handful of successful conceptions over the course of his life because primates have slow life histories and low reproductive rates. Infant mortality is relatively high in most wild primates, as it is in other wild mammals (reviewed in Dunbar 1987), leading to the strong inference that selection may be as strong on behaviors that increase infant survival as it is on behaviors that increase fertility. Indeed, evolutionary demographic analyses tell us this is so. In long-lived species like primates, lifetime fitness will generally be much more sensitive to survival than to fertility (Brault and Caswell 1993; McDonald 1993; Wisdom et al. 2000; Caswell 2001; Le Gouar et al. 2011; Sergio et al. 2011; Sim et al. 2011). In fact, changes in survival—including survival during infancy and the juvenile period—may account for ∼90% of the total sensitivity of fitness in long-lived species (McDonald 1993). Studies of behavioral ecology often focus on variation in fertility as a key source of variation in fitness (and indeed it can be), but the generally greater importance of survival to fitness in long-lived species is well known among evolutionary demographers. Furthermore, primates, as long-lived species, are archetypal representatives of this pattern, and there should be strong selection on male primates, as on females, to contribute to offspring survival.
Second, males of many primate species provide care to immatures. Some primate societies are monogamous or polyandrous, and male care is most extensive and conspicuous in these lineages (Fernandez-Duque et al. 2009). However, even in polygynous and polygynandrous species, male care of offspring has been documented repeatedly (e.g., Brandt et al. 1970; Altmann 1980; Kleiman and Malcolm 1981; Snowden and Suomi 1982; Smuts 1985; Whitten 1987; Palombit et al. 1997; Borries et al. 1999; Buchan et al. 2003; Zhao and Pan 2006; Nguyen et al. 2009; Xiang et al. 2009; Moscovice et al. 2010; Rosenbaum et al. 2011).
If selection on infant survival is strong and male primates are known to invest in immatures, why is it so commonly believed that male primates do not provide paternal care? A key reason has been the difficulty of linking male care to genetically determined paternity. However, only recently have genetic paternity tests been carried out in wild primates; the very few that have examined male-immature relationships in the light of paternity results have found mixed but compelling evidence (see “Related evidence” below and Ménard et al. 1992; Kuester and Paul 1996; Buchan et al. 2003; Lehmann et al. 2006; Charpentier et al. 2008; Moscovice et al. 2009; Huchard et al. 2010; Wroblewski 2010). The paucity of genetic paternity data in most natural primate populations has led some to propose that male care is more likely to represent mating investment than parental care—that is, males care for offspring as a mating incentive to mothers (van Schaik and Paul 1996). However, even this argument explicitly acknowledges that males in multimale species invest in immatures. A second possible reason that paternal care has been overlooked in polygynandrous primates is that the most conspicuous forms of parental care—provisioning and carrying—are uncommon behaviors among male primates. However, other forms of parental care, including grooming, defending, playing, huddling, and providing a safe zone for feeding and resting, may be quite important for infant welfare (Kleiman and Malcolm 1981) and are commonly performed by male primates (e.g., Brandt et al. 1970; Altmann 1980; Hamilton 1984; Smuts 1985; Whitten 1987; Strier 1996; van Schaik and Paul 1996; Palombit et al. 1997; Warren and Williamson 2001; Buchan et al. 2003; Lehmann et al. 2006; Zhao and Pan 2006; Moscovice et al. 2009, 2010; Nguyen et al. 2009; Xiang et al. 2009; Wroblewski 2010).
These 2 lines of evidence—the importance of immature survival for fitness in long-lived species and the numerous reports in multimale primates of males providing care to immatures—call for a reconsideration of the long-held view that male primates, except in monogamous species, provide little or no care to immatures. In addition, evidence is accumulating that male care matters for immatures even in multimale species: Males of several species protect immatures from harassment or the threat of infanticide (Palombit et al. 1997; Borries et al. 1999; Moscovice et al. 2009; Nguyen et al. 2009), and father–offspring coresidence enhances a component of offspring fitness in baboons (Charpentier et al. 2008), one of the few species to date in which sufficient data exist for addressing this question. These results lead us to argue for a broad reassessment of the notion that male care in multimale primate species is of little consequence to immatures.
SEXUAL SWELLINGS AND PATERNAL CARE
One potential consequence of the evolution of sexual swellings is that if these signals are accurate indicators of the probability of ovulation and conception, and males evolve sensitive detection systems in response to them, sexual swellings represent a mechanism for estimating the probability of paternity within a multimale mating system. That is, even in the absence of monogamy, the probabilistic nature of the signal associated with sexual swellings allows males to estimate their probability of paternity for any given conception, potentially with reasonable accuracy, if they are capable of sensitively tracking probabilities over time and closely monitoring the mating behavior of the female during the conceptive period. Unexpectedly, then, exaggerated sexual swellings create a situation in which paternal certainty may sometimes be quite high among primate species that exhibit multiple mating by females, an idea first presented by Hamilton (1984). This in turn creates a context in which males will benefit from providing paternal care.
Paternity confusion and paternal certainty
Females can benefit from paternity confusion in multimale multifemale societies when their infants are vulnerable to infanticide by generating a set of males who are potential caretakers of their infants. Paternity confusion is unlikely to lower the risk of an infanticidal attack because infanticidal males are typically new immigrant males that have recently attained a position of high reproductive activity in a group (reviewed in van Schaik 2000, see also Boggess 1984; Collins et al. 1984; Crockett and Sekulic 1984; Fossey 1984; Leland et al. 1984; Vogel and Loch 1984; Borries and Koenig 2000; Crockett and Janson 2000; Palombit et al. 2000; Steenbeek 2000). In other words, infanticidal males are not typically among the “confused.” However, those males that are among the confused—male group members who mated with the female during her conceptive cycle—will be both available and motivated to deter infanticidal males because they have a potential stake in the infant. Thus, although paternity confusion is unlikely to reduce infantide risk, it can reduce the probability that an infanticidal attack will be successful.
The Graded Signal Hypothesis for exaggerated sexual swellings, and other hypotheses that invoke paternity confusion (reviewed in Nunn 1999), implicitly assume that males use the information available in sexual swellings to estimate their probability of paternity and use this information to adjust the level of care and protection they direct toward infants. These benefits of confusing paternity raise the puzzling question of why females should ever confer paternal certainty; why not always confuse paternity? The Graded Signal Hypothesis (Nunn 1999) resolves this puzzle by positing that females in multimale multifemale societies are faced with 2 problems that exaggerated sexual swellings can solve, of which potential infanticide is only one. Nunn proposes that the second problem is pressure to produce a high quality offspring, a problem that exaggerated sexual swellings solve, he posits, by biasing insemination—that is, by “concentrating paternity” in the best quality male.
Our argument is, instead, that the 2 parallel functions of exaggerated sexual swellings, namely confusing paternity and concentrating paternity, represent alternative solutions to the same problem: obtaining the right quantity and quality of male investment in a female's infant. In this view, exaggerated swellings still function as a graded signal, the difference being that our hypothesis posits that the benefits of focusing versus diffusing the signal revolve around obtaining appropriate male care.
In contexts where infanticide risk is high, a female may benefit most from confusing paternity: Multiple potential fathers may then have a high enough probability of paternity to protect an offspring from infanticidal males, even if they do not have high enough paternal certainty to engage in sustained or intensive care once infants are past the most vulnerable stage. Indeed, studies in both baboons and Hanuman langurs have found that, in populations where infanticide risk is high, both actual fathers and possible fathers (males that were present when an infant was conceived and copulated with the mother around the time of conception) were protective of a given infant (Palombit et al. 1997; Borries et al. 1999; Moscovice et al. 2009, 2010). In contrast, when infanticide risk is low, a female may benefit most from conferring paternal certainty (e.g., exhibiting strong preferences for and cooperating most with) on a male who can then provide care in multiple forms for an extended period. The paternal care documented among the Amboseli baboons (Buchan et al. 2003), in which sexually selected infanticide is quite rare, may be an example of this type of extended care. Amboseli males intervened in conflicts on behalf of their own offspring throughout the juvenile period (Buchan et al. 2003), and, as noted earlier, offspring whose biological fathers were present for a longer time during their juvenile period reached maturity earlier than offspring whose fathers resided with them for a shorter time (Charpentier et al. 2008). These data indicate protracted investment by fathers in their offspring, resulting in a long-term fitness consequence of paternal care even in the absence of infanticidal risk.
The paternal care hypothesis
At the heart of the Paternal Care Hypothesis is the idea that sexual swellings motivate males to adjust their behavior to provide the optimal quantity and quality of paternal care. We hypothesize that exaggerated sexual swellings allow males to estimate the probability that they are the father of a given offspring by signaling within a conceptive cycle when ovulation is most likely to occur and by differentiating between cycles to provide information about when conception is most likely to occur. That is, our hypothesis bridges those hypotheses that fall within the Signaling Within a Cycle and the Differentiating Between Cycles categories because variation both within and between cycles of a female probably signals information to males about paternity. By concentrating their mating efforts during the periovulatory period of conceptive cycles, males can increase their likelihood of paternity. Furthermore, they can potentially assess the probability that they have achieved paternity by monitoring the mating behavior of the female during this period (see also Hamilton 1984; Nunn 1999; Daspre et al. 2009). This assessment of paternity on the part of male primates means that they may use the information content of sexual swellings to dial up or down the intensity, frequency, and duration of care and protection they provide to immatures, according to the probability that they are the immatures' father. This hypothesis generates several predictions.
Prediction 1
Among multimale multifemale primate species, true paternal care (care that is disproportionately directed to a male's own offspring) will be more common in species that exhibit exaggerated sexual swellings than in species that do not. Furthermore, among species with exaggerated sexual swellings, true paternal care will occur only when swelling size accurately signals the probability of conception across cycles and the probability of ovulation within a cycle. That is, we expect true paternal care to evolve more commonly in those species in which exaggerated sexual swellings accurately predict ovulation, and we expect true paternal care to be uncommon or absent in those species in which sexual swellings are less accurate signals of ovulation. Testing these predictions will also require data from multiple species, using phylogenetic comparative methods.
Prediction 2
Among species with sexual swellings, we expect true paternal care to be more common in instances in which males can closely monitor the behavior of estrous females and can effectively monopolize mating with her during that period (for instance, in smaller groups or more open habitats). Note that this prediction allows not only for variation among species but also for variation within species and even within populations in the extent of paternal care provided to each offspring. Testing this prediction will require detailed behavioral data on male and female behavior during conceptive sexual cycles and on paternal behavior toward neonates or older juveniles.
Prediction 3
Whether or not female primates display exaggerated sexual swellings, they may experience selection to behave in ways that either augment paternity confusion or enhance paternal certainty depending on their social and demographic context. That is, if a female can predict that her offspring will receive greater benefit from confused paternity rather than from enhanced paternal certainty, she should attempt to bias her mating behavior to favor paternal confusion, even at the expense of intense or long-lasting paternal care (as described in Clarke et al. 2009). In contrast, a female living in a context in which extended paternal care is more valuable than short-term investment in anti-infanticidal defense should behave in a way to confer paternal certainty, thereby potentially increasing the intensity and duration of paternal care from a single male.
Related evidence
Very few studies have both identified paternity and done careful analyses of father–offspring interactions, in species for which the concordance between swelling size and ovulation is known. Some recent data support the general prediction that a good concordance between swelling size and ovulation will promote paternal care. For instance, males bias their caring behavior toward their own offspring in baboons (Palombit et al. 1997; Buchan et al. 2003; Moscovice et al. 2010), a species in which swelling size within a cycle is a good predictor of ovulation (Wildt et al. 1977; Higham et al. 2008a; Daspre et al. 2009). Two studies in chimpanzees have also found that males discriminate their offspring from unrelated immatures (Lehmann et al. 2006; Wroblewski 2010), although the extent or significance of paternal care in this species is not yet known.
In contrast, we expect paternal care to be less intense and protracted in species in which swelling size is a poor predictor of ovulation, such as long-tailed macaques (Engelhardt et al. 2005) or bonobos (Reichert et al. 2002). We would also predict that paternal care would be low in species, such as Barbary macaques, in which sexual swelling size is a good indicator of ovulation (Brauch et al. 2007), but in which a male is rarely able to monopolize access to a female at any point during the estrous cycle (Taub 1980; Small 1990; Kuester and Paul 1992). In fact, evidence from 2 populations of Barbary macaques suggests that true paternal care (as opposed to extensive male–infant interactions of other sorts) is uncommon or absent in this species (Ménard et al. 1992; Kuester and Paul 1996).
Future research
We hope to stimulate research that will 1) describe the variation in caring behaviors that males provide to immatures and quantify how males distribute these behaviors among immatures in a range of species, 2) carefully examine the relationship between variation in swelling size and ovulation in species that exhibit exaggerated swellings, and 3) describe and quantify variation in the extent to which males can monitor the behavior of estrous females in these species (this may vary between species, within species, and between individuals in a population). With respect to measuring male care, a key component will be examining the multiple ways in which males invest in offspring, including behaviors that are subtle or indirect but may be of enormous significance, such as maintaining proximity to infants in a manner that reduces their vulnerability to harassment or increases their feeding efficiency (Altmann 1980; Bales 1980; Smuts 1985; Palombit et al. 1997; Moscovice et al. 2009; Nguyen et al. 2009). The current paucity of data pertaining to these questions presents a rich set of opportunities for future research.
Mechanisms other than exaggerated sexual swellings may be available to concentrate or confuse paternity. Our main focus here was on sexual swellings, but others may function simultaneously and independently (for instance, see our prediction 3 about female behavior). Importantly, our hypothesis does not propose to explain the initial evolution of exaggerated swellings. Rather, we propose that whatever the selective forces were that originally shaped them, exaggerated sexual swellings now represent a system that can sometimes confer a high degree of paternity certainty in multimale multifemale social systems. At the same time, it is flexible enough to also allow females to confuse paternity when circumstances favor it. Male primates, as members of a lineage with slow life histories—that is, long lives and low fertility—should experience strong selection to influence the health and well being of the relatively few offspring that they will produce in their lifetimes. We predict that the era of research on paternal care in primates, including those living in multimale multifemale societies, is just beginning.
FUNDING
National Science Foundation (DEB 0919200 and NSF DEB 0846286); National Institute of Health (R01AG034513-01 and NIA P01-AG031719 to S.C.A.); and National Science Foundation Graduate Research Fellowship, Fulbright Fellowship, and grant from the L.S.B. Leakey Foundation (to C.L.F.).
Acknowledgments
We thank Tim Clutton-Brock and John Fleagle for discussions that inspired various portions of this paper. We thank Jeanne Altmann, Beth Archie, Charlie Nunn and Patrick Chiyo for helpful comments on the manuscript. The manuscript was significantly enhanced during the review process by the suggestions of reviewers.
References
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- Altmann J. Baboon mothers and infants. Cambridge (MA): Harvard University Press; 1980. [Google Scholar]
- Bales KB. Cumulative scaling of paternalistic behavior in primates. Am Nat. 1980;116:454–461. [Google Scholar]
- Boggess J. Infant killing and male reproductive strategies in langurs (Presbytis entellus) In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 283–310. [Google Scholar]
- Borries C, Koenig A. Infanticide in hanuman langurs: social organization, male migration, and weaning age. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge (UK): Cambridge University Press; 2000. pp. 99–122. [Google Scholar]
- Borries C, Launhardt K, Epplen C, Epplen JT, Winkler P. Males as infant protectors in hanuman langurs (Presbytis entellus) living in multimale groups—defence pattern, paternity and sexual behaviour. Behav Ecol Sociobiol. 1999;46:350–356. [Google Scholar]
- Brandt EM, Irons R, Mitchell G. Paternalistic behavior in 4 species of macaques. Brain Behav Evol. 1970;3:415–420. doi: 10.1159/000125484. [DOI] [PubMed] [Google Scholar]
- Brauch K, Pfefferle D, Hodges K, Mohle U, Fischer J, Heistermann M. Female sexual behavior and sexual swelling size as potential cues for males to discern the female fertile phase in free-ranging Barbary macaques (Macaca sylvanus) of Gibraltar. Horm Behav. 2007;52:375–383. doi: 10.1016/j.yhbeh.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Brault S, Caswell H. Pod-specific demography of killer whales (Orcinus orca) Ecology. 1993;74:1444–1454. [Google Scholar]
- Buchan JC, Alberts SC, Silk JB, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- Bulger JB. Dominance rank and access to estrous females in male savanna baboons. Behaviour. 1993;127:67–103. [Google Scholar]
- Caswell H. Matrix population models: construction, analysis and interpretation. Sunderland (MA): Sinauer Associates; 2001. [Google Scholar]
- Charpentier MJE, Van Horn RC, Altmann J, Alberts SC. Paternal effects on offspring fitness in a multimale primate society. Proc Natl Acad Sci U S A. 2008;105:1988–1992. doi: 10.1073/pnas.0711219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PMR, Henzi SP, Barrett L. Sexual conflict in chacma baboons, Papio hamadryas ursinus: absent males select for proactive females. Anim Behav. 2009;77:1217–1225. [Google Scholar]
- Clutton-Brock TH, Harvey PH. Evolutionary rules and primate societies. In: Bateson PPG, Hinde RA, editors. Growing points in ethology. Cambridge (UK): Cambridge University Press; 1976. pp. 195–237. [Google Scholar]
- Collins DA, Busse CD, Goodall J. Infanticide in two populations of savanna baboons. In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 193–216. [Google Scholar]
- Crockett CM, Janson CH. Infanticide in red howlers: female group size, male membership, and a possible link to folivory. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge (UK): Cambridge University Press; 2000. pp. 75–98. [Google Scholar]
- Crockett CM, Sekulic R. Infanticide in red howler monkeys (Alouatta seniculus) In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 173–192. [Google Scholar]
- Dahl JF, Nadler RD, Collins DC. Monitoring the ovarian cycles of Pan troglodytes and P. paniscus—a comparative approach. Am J Primatol. 1991;24:195–209. doi: 10.1002/ajp.1350240306. [DOI] [PubMed] [Google Scholar]
- Daspre A, Heistermann M, Hodges JK, Lee PC, Rosetta L. Signals of female reproductive quality and fertility in colony-living baboons (Papio h. anubis) in relation to ensuring paternal investment. Am J Primatol. 2009;71:529–538. doi: 10.1002/ajp.20684. [DOI] [PubMed] [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus . Anim Behav. 2003;66:551–560. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Observations on the evolution and behavioral significance of sexual skin in female primates. Adv Study Behav. 1983;13:63–106. [Google Scholar]
- Domb LG, Pagel M. Sexual swellings advertise female quality in wild baboons. Nature. 2001;410:204–206. doi: 10.1038/35065597. [DOI] [PubMed] [Google Scholar]
- Drea CM. Bateman revisited: the reproductive tactics of female primates. Integr Comp Biol. 2005;45:915–923. doi: 10.1093/icb/45.5.915. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Demography and reproduction. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago (IL): University of Chicago Press; 1987. pp. 240–249. [Google Scholar]
- Emery MA, Whitten PL. Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes) Behav Ecol Sociobiol. 2003;54:340–351. [Google Scholar]
- Engelhardt A, Hodges JK, Niemitz C, Heistermann M. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis) Horm Behav. 2005;47:195–204. doi: 10.1016/j.yhbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mendoza SP. The biology of paternal care inhuman and nonhuman primates. Annu Rev Anthropol. 2009;38:115–130. [Google Scholar]
- Fossey D. Infanticide in mountain gorillas (Gorilla gorilla beringei) with comparative notes on chimpanzees. In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 217–236. [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ., III . Significance of paternal investment by primates to the evolution of adult male-female associations. In: Taub DM, editor. Primate paternalism. New York: Van Nostrand Reinhold; 1984. pp. 309–335. [Google Scholar]
- Higham JP, Heistermann M, Ross C, Semple S, MacLarnon A. The timing of ovulation with respect to sexual swelling detumescence in wild olive baboons. Primates. 2008;49:295–299. doi: 10.1007/s10329-008-0099-9. [DOI] [PubMed] [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: information content of size and color. Horm Behav. 2008;53:452–462. doi: 10.1016/j.yhbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. The woman that never evolved. Cambridge (MA): Harvard University Press; 1981. [Google Scholar]
- Hrdy SB, Whitten PL. Patterning of sexual activity. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago (IL): University of Chicago Press; 1987. pp. 370–384. [Google Scholar]
- Huchard E, Alvergne A, Fejan D, Knapp LA, Cowlishaw G, Raymond M. More than friends? Behavioural and genetic aspects of heterosexual associations in wild chacma baboons. Behav Ecol Sociobiol. 2010;64:769–781. [Google Scholar]
- Huchard E, Courtiol A, Benavides JA, Knapp LA, Raymond M, Cowlishaw G. Can fertility signals lead to quality signals? Insights from the evolution of primate sexual swellings. Proc R Soc B Biol Sci. 2009;276:1889–1897. doi: 10.1098/rspb.2008.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG, Malcolm JR. The evolution of male parental investment in mammals. In: Gubernik DJ, Klopfer PH, editors. Parental care in mammals. New York: Plenum Publishing Corporation; 1981. pp. 347–387. [Google Scholar]
- Kuester J, Paul A. Influence of male competition and female mate choice on male mating success in Barbary macaques (Macaca sylvanus) Behaviour. 1992;120:192–217. [Google Scholar]
- Kuester J, Paul A. Female-female competition and male mate choice in Barbary macaques (Macaca sylvanus) Behaviour. 1996;133:763–790. [Google Scholar]
- Le Gouar PJ, Schekkerman H, van der Jeugd HP, Boele A, van Harxen R, Fuchs P, Stroeken P, van Noordwijk AJ. Long-term trends in survival of a declining population: the case of the little owl (Athene noctua) in the Netherlands. Oecologia. 2011;166:369–379. doi: 10.1007/s00442-010-1868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Fickenscher G, Boesch C. Kin biased investment in wild chimpanzees. Behaviour. 2006;143:931–955. [Google Scholar]
- Leland L, Struhsaker TT, Butynski TM. Infanticide by adult males in three primate species of Kibale Forest, Uganda: a test of hypotheses. In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 151–172. [Google Scholar]
- McDonald DB. Demographic consequences of sexual selection in the long-tailed manakin. Behav Ecol. 1993;4:297–309. [Google Scholar]
- Ménard N, Scheffrahn W, Vallet D, Vidane C, Reber C. Application of blood protein electrophoresis and DNA fingerprinting to the analysis of paternity and social characteristics of wild Barbary macaques. In: Martin RD, Dixson AF, Wickings EJ, editors. Paternity in primates: genetic tests and theories. Basel (Switzerland): Karger; 1992. pp. 155–174. [Google Scholar]
- Mohle U, Heistermann M, Dittami J, Reinberg V, Wallner B, Hodges JK. Patterns of anogenital swelling size and their endocrine correlates during ovulatory cycles and early pregnancy in free-ranging Barbary macaques (Macaca sylvanus) of Gibraltar. Am J Primatol. 2005;67:279. doi: 10.1002/ajp.20161. [DOI] [PubMed] [Google Scholar]
- Moscovice LR, Di Fiore A, Crockford C, Kitchen DM, Wittig R, Seyfarth RM, Cheney DL. Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Anim Behav. 2010;79:1007–1015. [Google Scholar]
- Moscovice LR, Heesen M, Di Fiore A, Seyfarth RM, Cheney DL. Paternity alone does not predict long-term investment in juveniles by male baboons. Behav Ecol Sociobiol. 2009;63:1471–1482. doi: 10.1007/s00265-009-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Van Horn RC, Alberts SC, Altmann J. “Friendships” between new mothers and adult males: adaptive benefits and determinants in wild baboons (Papio cynocephalus) Behav Ecol Sociobiol. 2009;63:1331–1344. doi: 10.1007/s00265-009-0786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordwijk M. Sexual behaviour of Sumatran long-tailed macaques. Ethology. 1985;70:277–296. [Google Scholar]
- Nunn CL. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav. 1999;58:229–246. doi: 10.1006/anbe.1999.1159. [DOI] [PubMed] [Google Scholar]
- Pagel M. Evolution of conspicuous advertisement in old world monkeys. Anim Behav. 1994;47:1333–1341. [Google Scholar]
- Palombit RA, Cheney DL, Fischer J, Johnson S, Rendall D, Seyfarth RM, Silk JB. Male infanticide and defense of infants in chacma baboons. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge (UK): Cambridge University Press; 2000. pp. 123–152. [Google Scholar]
- Palombit RA, Seyfarth RM, Cheney DL. The adaptive value of ‘friendships’ to female baboons: experimental and observational evidence. Anim Behav. 1997;54:599–614. doi: 10.1006/anbe.1996.0457. [DOI] [PubMed] [Google Scholar]
- Petrie M. Improved growth and survival of offspring of peacocks with more elaborate trains. Nature. 1994;371:598–599. [Google Scholar]
- Petrie M, Halliday T, Sanders C. Peahens prefer peacocks with more elaborate trains. Anim Behav. 1991;41:323–331. [Google Scholar]
- Petrie M, Williams A. Peahens lay more eggs for peacocks with larger trains. Proc R Soc Lond Ser B Biol Sci. 1993;251:127–131. [Google Scholar]
- Reichert KE, Heistermann M, Hodges JK, Boesch C, Hohmann G. What females tell males about their reproductive status: are morphological and behavioural cues reliable signals of ovulation in bonobos (Pan paniscus)? Ethology. 2002;108:583–600. [Google Scholar]
- Rosenbaum S, Silk JB, Stoinski TS. Male-immature relationships in multi-male groups of mountain gorillas (Gorilla beringei beringei) Am J Primatol. 2011;73:356–365. doi: 10.1002/ajp.20905. [DOI] [PubMed] [Google Scholar]
- van Schaik CP. Infanticide by male primates: the sexual selection hypothesis revisited. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge (UK): Cambridge University Press; 2000. pp. 27–60. [Google Scholar]
- van Schaik CP, Paul A. Male care in primates: does it ever reflect paternity? Evol Anthropol. 1996;5:152–156. [Google Scholar]
- Sergio F, Tavecchia G, Blas J, Lopez L, Tanferna A, Hiraldo F. Variation in age-structured vital rates of a long-lived raptor: implications for population growth. Basic Appl Ecol. 2011;12:107–115. [Google Scholar]
- Setchell JM, Wickings EJ. Sexual swelling in mandrills (Mandrillus sphinx): a test of the reliable indicator hypothesis. Behav Ecol. 2004;15:438–445. [Google Scholar]
- Sim IMW, Rebecca GW, Ludwig SC, Grant MC, Reid JM. Characterizing demographic variation and contributions to population growth rate in a declining population. J Anim Ecol. 2011;80:159–170. doi: 10.1111/j.1365-2656.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- Small MF. Promiscuity in Barbary macaques (Macaca sylvanus) Am J Primatol. 1990;20:267–282. doi: 10.1002/ajp.1350200403. [DOI] [PubMed] [Google Scholar]
- Smuts BB. Sex and friendship in baboons. Hawthorne (NY): Aldine; 1985. [Google Scholar]
- Snowden CT, Suomi SJ. Paternal behavior in primates. In: Fitzgerald HE, Mullins JA, Gage P, editors. Child nurturance, vol 3: studies of development in nonhuman primates. New York: Plenum Press; 1982. pp. 63–108. [Google Scholar]
- Steenbeek R. Infanticide by males and female choice in wild Thomas's langurs. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge (UK): Cambridge University Press; 2000. pp. 153–177. [Google Scholar]
- Strier KB. Male reproductive strategies in new world primates. Hum Nat. 1996;7:105–123. doi: 10.1007/BF02692107. [DOI] [PubMed] [Google Scholar]
- Taub DM. Female choice and mating strategies among wild Barbary macaques (Macaca sylvanus L.) In: Lindburg DG, editor. The macaques: studies in ecology, behavior and evolution. New York: Van Nostrand Reinhold; 1980. pp. 287–344. [Google Scholar]
- Vogel C, Loch H. Reproductive parameters, adult male replacements, and infanticide among free-ranging langurs (Presbytis entellus) at Jodhpur (Rajasthan), India. In: Hausfater G, Hrdy SB, editors. Infanticide: comparative and evolutionary perspectives. New York: Aldine Publishing Company; 1984. pp. 237–256. [Google Scholar]
- Warren Y, Williamson EA. Carriage of infants by a silverback mountain gorilla. Folia Primatol. 2001;72:245–247. doi: 10.1159/000049944. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Lycett JE, Barrett L, Hill RA, Henzi SP. Male consortship behaviour in chacma baboons: the role of demographic factors and female conceptive probabilities. Behaviour. 2003;140:405–427. [Google Scholar]
- Whitten PL. Infants and adult males. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago (IL): University of Chicago Press; 1987. pp. 343–357. [Google Scholar]
- Whitten PL, Russell E. Information content of sexual swellings and fecal steroids in sooty mangabeys (Cercocebus torquatus atys) Am J Primatol. 1996;40:67–82. doi: 10.1002/(SICI)1098-2345(1996)40:1<67::AID-AJP5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Wildt DE, Doyle LL, Stone SC, Harrison RM. Correlation of perineal swelling with serum ovarian hormone levels, vaginal cytology, and ovarian follicular development during the baboon reproductive cycle. Primates. 1977;18:261–270. [Google Scholar]
- Wisdom MJ, Mills LS, Doak DF. Life stage simulation analysis: estimating vital-rate effects on population growth for conservation. Ecology. 2000;81:628–641. [Google Scholar]
- Wroblewski EE. Paternity and father-offspring relationships in wild chimpanzees, Pan troglodytes schweinfurthii [PhD Dissertation] [Minneapolis (MN)]: University of Minnesota; 2010. [Google Scholar]
- Xiang ZF, Sayers K, Grueter CC. Direct paternal care in black-and-white snub-nosed monkeys. J Zool. 2009;278:157–162. [Google Scholar]
- Zhao Q, Pan WS. Male-immature interactions seem to depend on group composition in white-headed langur (Trachypithecus leucocephalus) Acta Ethol. 2006;9:91–94. [Google Scholar]
- Zinner D, Alberts SC, Nunn CL, Altmann J. Significance of primate sexual swelling. Nature. 2002;420:142–143. doi: 10.1038/420142a. [DOI] [PubMed] [Google Scholar]