Abstract
NRAS mutations are common in human melanoma. To produce a mouse model of NRAS-driven melanoma, we expressed oncogenic NRAS (NRASG12D) in mouse melanocytes. When NRASG12D was expressed in the melanocytes of developing embryos, it induced melanocyte proliferation and congenital melanocytic lesions reminiscent of human blue nevi, but did not induce cutaneous melanoma. Unexpectedly however, it did induce early onset primary melanoma of the central nervous system (CNS). The tumors were rapidly proliferating and caused neurological symptoms, rapid health deterioration and death. NRAS is not a common driver oncogene of primary melanoma of the CNS in adults, but we report two cases of primary melanoma of the CNS in children, both of which carried oncogenic mutations in NRAS. We conclude that acquisition of somatic mutations in NRAS in CNS melanocytes is a predisposing risk factor to primary melanoma of the CNS in children and present a mouse model of this disease.
Keywords: childhood cancer, CNS melanoma, oncogenic NRAS, congenital naevi
INTRODUCTION
Malignant melanoma is a potentially fatal form of cancer that develops from specialized pigment cells called melanocytes. In humans, melanocytes are common in the epidermis (cutaneous melanocytes), but they also inhabit the dermis, eyes, ears, heart, central nervous system (CNS), and mucosal surfaces of the mouth and genital areas (1, 2). Thus, although the most common form of melanoma (~90% of cases) occur on hair-bearing skin (cutaneous melanoma), primary melanomas also develop in other sites of the body. Melanoma of the non hair-bearing skin (acral and mucosal melanomas) accounts for ~5% (3), and 1% (4) of cases respectively, while uveal melanoma accounts for ~3% (5). In general the rare forms have poorer prognosis, probably because they are diagnosed at a late stage.
Genetic analyses suggest that melanomas from different anatomical sites represent genetically distinct diseases. BRAF and NRAS are mutated in ~45% and ~20% respectively of cutaneous melanomas. By contrast, in acral melanoma, BRAF mutations occur in only ~16% of cases, NRAS mutations are absent or very rare, and KIT mutations occur in ~20% of patients (6, 7). Furthermore, in uveal melanomas BRAF, NRAS and KIT mutations appear to be extremely rare and instead this disease is driven by mutations in GNAQ, GNA11 and BAP1 (8-10). Primary melanoma of the central nervous system (CNS) is another rare melanoma and is thought to arise from the melanocytes of the leptomeninges. Melanocytic lesions of the CNS range from benign (leptomeningeal melanocytosis and melanocytoma) to malignant (leptomeningeal melanomatosis and melanoma) tumors (11-13). In children, these neoplasms often (but not always) occur in the context of neurocutaneous melanosis (NCM), a rare non-hereditary neurocutaneous syndrome presenting with giant (>20cm) and/or multiple congenital melanocytic nevi (CMN) (14-16). Until recently, little was known about the genetic drivers of CNS melanoma, particularly in children. However, it was recently reported that the adult disease is associated with mutations in GNAQ and GNA11 (17-19), and in one case, NRAS (19). Notably, ~80% of human CMN harbor somatic mutations in NRAS (20, 21) and giant CMN are associated with increased risk of cutaneous and leptomeningeal melanoma (22).
To study melanoma biology, mouse models of melanoma driven by oncogenic BRAF or RAS have been developed (23-29), and we have used conditionally-inducible alleles based on Cre-recombinase/LoxP technology to express oncogenes in mouse melanocytes (24, 25, 30, 31). In adult mice, BRAFV600E induced skin darkening at 2 months, blue nevus-like lesions at 4 months, and melanoma in ~80% of the animals within 2 years (24). In contrast, when BRAFV600E was expressed in the melanocytes of developing embryos (congenital expression), it induced developmental abnormalities and embryonic lethality (31).
Here we investigated if oncogenic NRAS could induce melanoma when it was expressed at physiological levels using the endogenous Nras gene. We found that expression of NRASG12D in the melanocytes of adult mice induced skin darkening and blue nevus-like lesions, but not cutaneous melanoma. Expression of NRASG12D in the melanocytes of embryonic mice also induced skin darkening and congenital blue nevus-like lesions, but again, it did not induce cutaneous melanoma. However, when it was expressed in congenital nevi, NRASG12D induced melanoma of the CNS and critically, the course of the disease in these mice closely resembled the course of disease in two children who developed melanoma of the CNS driven by oncogenic NRAS. We conclude that acquired somatic mutation in NRAS in the melanocytes of the leptomeninges is a predisposing risk factor to childhood melanoma of the CNS and we have developed a mouse model of this disease.
RESULTS
To develop NRAS-driven melanoma models, we expressed NRASG12D at physiological levels in mouse melanocytes. To achieve this we used mice in which NRASG12D is expressed from the endogenous Nras gene under the control of a LoxP-STOP-LoxP (LSL) cassette (NrasLSL-G12D)(32), the removal of which by Cre-recombinase released NRASG12D expression in a conditional-inducible manner (Supplementary Fig. 1A). We crossed the NrasLSL-G12D mice onto mice in which tamoxifen-activated Cre-recombinase (CreERT2) was expressed in melanocytes using a tyrosinase enhancer/promoter fragment (Tyr::CreERT2 mice; see Supplementary Fig. 1A)(32, 33). Although CreERT2 was expressed in the melanocytes of these mice from approximately embryonic day 10.5 (E10.5), it was only activated when the mice are treated with tamoxifen.
We painted tamoxifen onto the shaven skin on the backs of the mice at approximately two months of age to induce NRASG12D expression. Within 4-8 months, we observed visible darkening of the skin (Supplementary Fig. 1B), in the homozygous (NrasLSL-G12D/LSL-G12D;Tyr::CreERT2) mice and weak darkening of the skin in 50% of the heterozygous (Nras+/LSL-G12D;Tyr::CreERT2) mice. The darkening was more apparent in the tamoxifen-treated areas, although systemic effects also occurred, with darkening of the tails of the homozygous mice (Supplementary Fig. 1B). Microscopic examination of the skin revealed small paucicellular nevi in the deep dermal and periadnexal regions of the skin of the heterozygous mice and larger multicellular nevi in the homozygous mice (Supplementary Fig. 1C). However, despite this clear evidence that NRASG12D drives melanocyte proliferation, none of the mice developed tumors even after 24-months of expression (Supplementary Fig. 1D).
Next we crossed the NrasLSL-G12D mice onto mice expressing unmodified Cre-recombinase from the tyrosinase promoter/enhancer (Tyr::CreA; see Fig. 1A) to induce NRASG12D expression in the developing melanocytes of embryonic mice. We previously reported that expression of BRAFV600E in embryonic melanocytes caused hydrocephaly, disruption to the development of the eyes and hearts, and embryonic lethality (31). We were therefore intrigued that live-born Nras+/LSL-G12D;Tyr::CreA/° and NrasLSL-G12D/LSL-G12D;Tyr::CreA/° offspring were obtained at the expected ratio and did not present any signs of hydrocephaly (Fig 1B), or developmental abnormalities of the eyes or hearts (Supplementary Fig. 2). Notably, all mice presented with darkening of the skin, tails, paws and snouts that was evident from day 1, persisted throughout life and was more pronounced in the homozygous than heterozygous mice (Fig. 1B).
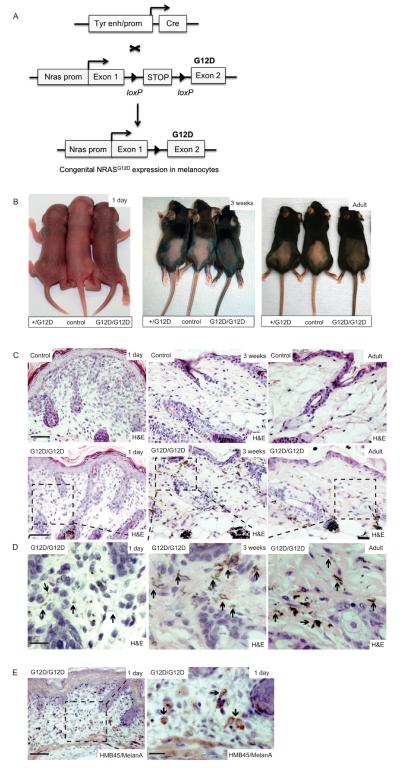
Figure 1. NRASG12D induces skin pigmentation and congenital nevi.
(A) Schematic representation of the conditional-inducible approach used to express NRASG12D in embryonic mouse melanocytes. A tyrosinase gene enhancer/promoter construct (Tyr enh/prom) was used to express Cre-recombinase (Cre) in melanocytes from embryonic day ~10.5 (38). NRASG12D was expressed from the endogenous mouse Nras gene using a conditional-inducible targeted allele in which exon 2 is mutated to introduce the G12D mutation (32). The loxP-STOP-loxP cassette blocks NRASG12D expression, but its removal by Cre-recombinase releases the block on expression.
(B) Photographs showing skin pigmentation in control, Nras+/LSL-G12D;Tyr::CreA/° (+/G12D), and NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) mice, at 1 day, 3 weeks, and in adulthood.
(C) Upper panels: photomicrographs of H&E stained skin in 1 day old, 3 week old and adult mouse skin. Scalebar = 200μm. Lower panels: low power photomicrographs of H&E stained skin in 1 day old, 3 week old and adult NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) mice. Hyperpigmented dendritic melanocytes are visible at low magnification in the 3 week old and adult mice. Scalebar = 200μm. n=6 mice / experimental group.
(D) High power photomicrographs of H&E stained skin from boxed areas in the lower panel from (C) in 1 day old, 3 week old and adult NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) mice. Hyperpigmented dendritic melanocytes in the papillary and reticular dermis, and along the hair follicles and adnexal glands are indicated (black arrows) and are sparse in the skin of day 1 old mice, but are prominent in 3 week old and adult mice. Scalebar = 20μm.
(E) Photomicrographs of HMB45/MelanA stained skin from a 1 day old NrasLSL-G12D/LSL- G12D;Tyr::CreA/° (G12D/G12D) mouse, demonstrating the presence of melanocytes (black arrows). The area boxed in the left hand panel is enlarged in the right hand panel. Scale bars = 200μm (left panel) and 20μm (right panel).
These mice also developed benign paucicellular dermal melanocytic lesions reminiscent of human blue nevi (Fig. 1C). Hyperpigmented dendritic melanocytes were visible in 3 week old and adult mice, particularly between the collagen bundles of the reticular dermis and along the adnexae of the deep dermal layers (Fig. 1C). Notably, these hyperpigmented dendritic melanocytic lesions stained positive for HMB45/MelanA and were present in 1 day old mice (Fig. 1D, 1E) confirming that expression of NRASG12D in embryonic melanocytes induced congenital nevi.
Despite clearly driving melanocyte proliferation in the congenital setting, NRASG12D did not induce cutaneous melanoma. However, at a median of 4 months for the homozygous animals, and 12.5 months for the heterozygous animals (Fig. 2A), the mice developed neurological symptoms that presented as hyperreactivity to normal stimuli and motor dysfunction, including incoordination and tremor with impaired gait. Most animals developed increasing cranial perimeter or cranial deformity and the symptoms progressed rapidly to akathisia and marked general malaise that necessitated sacrifice.
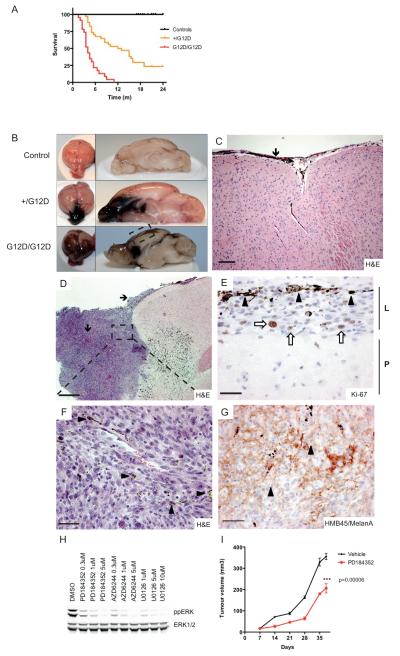
Figure 2. NrasG12D induces CNS tumors in mice.
(A) Kaplan-Meier plot showing survival in months (m) of study mice. The experimental groups consisted of Nras+/LSL-G12D;Tyr::CreA/° (+/G12D; n=33) and NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D; n=23) mice. The control groups consisted of Tyr::CreA/° (n=22), Nras+/LSL-G12D (n=13) and NrasLSL-G12D /LSL-G12D (n=15) mice.
(B) Photographs showing representative whole brain and sagital sections of the brains of control, Nras+/LSL-G12D;Tyr::CreA/° (+/G12D) and NrasLSL-G12D/LSL- G12D;Tyr::CreA/° (G12D/G12D) mice.
(C) Photomicrograph of an H&E stained brain section from a NrasLSL-G12D/LSL-G12D;Tyr::CreA/° mouse (boxed area in the left image), displaying proliferation of pigmented melanocytes in the leptomeninges along the external surface of the brain parenchyma (black arrow). Scalebar = 100μm.
(D) Photomicrograph of an H&E stained frontal lobe melanoma from an NrasLSL-G12D/LSL-G12D;Tyr::CreA/° mouse, showing leptomeningeal spread of melanoma cells (black arrows). Scalebar = 1mm.
(E) Photomicrograph showing nuclear Ki67 staining (white arrows) of a representative melanoma from a NrasLSL-G12D/LSL-G12D;Tyr::CreA/° mouse (boxed area in upper left image). Note that some cells are melanin-laden (black arrowheads). Scalebar = 20μm.
(F) High power photomicrograph of boxed area in upper left image of an H&E stained melanoma with atypical, epithelioid cells. Tumor cells frequently presented intracytoplasmic melanin (black arrowheads). Scalebar = 25μm.
(G) Photomicrograph showing HMB45/MelanA staining in a representative melanoma from a NrasLSL-G12D/LSL-G12D;Tyr::CreA/° mouse. Note the predominant membranous staining and presence of intracytoplasmic deposits of melanin in the melanoma cells (black arrowheads). Scalebar = 50μm.
(H) Western blot analysis of ppERK and total ERK levels of Nras mutant melanoma cells following MEK inhibition for 3h using PD184352, U0126 and AZD6244.
(I) In vivo allograft experiment, using Nras mutant cells from a mouse brain melanoma showing the effect of the MEK inhibitor PD184352 on intradermal tumor growth in C57Bl/6 mice. n = 6 PD184352-treated mice and 9 vehicle-treated mice.
The brains of the affected animals revealed marked darkening of the leptomeninges that followed the sulci and fissures, and was more prominent in the frontoparietal region (Fig. 2B). We observed an increased number of pigmented melanocytes that followed the gyri and sulci and enveloped the ventricles (Fig. 2C). The darkly pigmented areas were composed of large melanocytic lesions comprising pleomorphic cells that invaded the brain parenchyma (Fig. 2D). The tumor cells were rapidly dividing (mitotic index >10 per mm2) and stained positive for Ki67 (Fig. 2E), ppERK and Cyclin D1 (Supplementary Fig. 3A, B). They were melanin-laden (Fig. 2F) and stained positive for HMB45/MelanA (Fig. 2G, Supplementary Fig. 3A, B) and S100 (Supplementary Fig. 3C), but negative for the glial cell marker GFAP (Supplementary Fig. 3D).
To provide evidence that oncogenic NRAS was expressed, we reverse transcribed RNA from the tumors and PCR amplified an Nras fragment across the exon 2/exon 3 boundary. Sequencing of this fragment revealed that NrasG12D was expressed in the tumors, but not the normal brains of the Tyr::CreA/° littermate controls (Supplementary Fig. 4). Macroscopic and microscopic examination of the skin did not present evidence of cutaneous melanoma (Fig. 1B-D) and we did not observe primary tumors in the uvea, hearts (Supplementary Fig. 2) or oral and genital mucosa (data not shown), the other tissues in which melanocytes reside. We therefore diagnosed primary melanoma of the CNS and cells derived from these tumors displayed constitutive ERK activity that was sensitive to the MEK inhibitors PD184352, U0126 and AZD6244 (Fig. 2H). Importantly, PD184352 also delayed the growth of tumor allografts formed by these cells in syngeneic immuno-competent mice (Fig. 2I).
We observed hyperpigmentation of the leptomeninges in the brains of asymptomatic mice. The pigmented cells followed the gyri and sulci and covered the parietal lobe in an arboriform pattern (Fig. 3A). Even in the immediate post-natal period (1 day old mice), the leptomeninges were thickened and hyperpigmented and presented HMB45/MelanA positive cells (Fig. 3B, 3C). Thus, although NRASG12D induced hyperproliferation of melanocytes in both the skin and the CNS, those in the skin developed into congenital cutaneous nevi, whereas those in the leptomeninges progressed to become aggressive and invasive primary CNS melanoma.
Figure 3. NRASG12D induces melanocytosis in embryonic mice.
(A) Photograph showing a representative whole brain from an NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) and control mouse at 3 weeks of age. Note the hyperpigmentation of leptomeninges following the gyri (black arrow) and sulci (black arrowhead) and the arboriform pattern over the parietal lobe (white arrow).
(B) Upper panels: photomicrographs of H&E stained mouse brains of control mice at 1 day and 3 weeks of age showing a single array of non-pigmented leptomeninges lining the cerebral parenchyma (black arrows). Scalebar = 50μm. Lower panel: photomicrographs of H&E stained mouse brains of NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) mice at 1 day and 3 weeks of age showing hyperpigmentation and thickening of the leptomeninges (black arrows). Scalebar = 50μm. n = 6 mice / experimental group.
(C) Photomicrographs of an HMB45/MelanA stained mouse brain (arrows indicate individual cells) from an NrasLSL-G12D/LSL-G12D;Tyr::CreA/° (G12D/G12D) mouse at 1 day of age. Scalebar = 50μm. The boxed area in the left panel is shown in higher magnification in the right panel.
We were struck by the similarities in the disease in our mice and those we observed in two cases of primary melanoma of the CNS that occurred in young children. The first case involved a 4-year old Caucasian boy who presented with somnolence and motor dysfunction. Initial clonic seizures of the right leg led to secondary generalized tonic-clonic seizures that were followed by paralysis of the right arm and leg that slowly recovered over 24 hours. Magnetic resonance imaging (MRI) revealed a hyperintense, contrast-enhancing lesion in the left parieto-occipital region, following the gyri and sulci. Cerebral spinal fluid (CSF) analysis with cytology and CT scans of the thorax and abdomen were normal and no pigmented lesions were found on the skin.
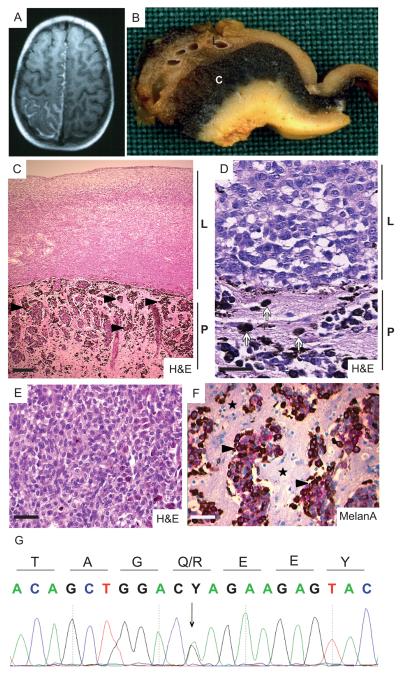
Despite treatment with valproic acid, carbamazepine and phenytoin, the seizures recurred and subsequent MRI revealed extension of the lesion to the right parietal cortex (Fig. 4A). After six months, the child developed permanent right hemiparalysis and aphasia. The lesion was resected and despite radiotherapy, the child passed away. Macroscopic examination of the cerebral tissue showed marked thickening and brownish discoloration of the leptomeninges and a zone of intense black discoloration underlying the cerebral cortex (Fig. 4B). Microscopically the lesions revealed a proliferation of atypical cells in the leptomeninges and Virchow-Robin spaces, with invasion of the adjacent cerebral cortex (Fig. 4C). The tumor cells were highly mitotic (>10 per mm2) and adopted an epithelioid morphology with nuclear pleomorphism (Fig. 4D, 4E). Tumor cells stained positive for MelanA (Fig. 4F), HMB45 and S100 (Supplementary Fig. 5A, 5B) and the malignant cells in the Virchow-Robin spaces and cerebral cortex contained copious amounts of melanin, whereas cells in the leptomeninges were sparsely pigmented (Fig. 4C, 4D). These features are consistent with a diagnosis of leptomeningeal melanomatosis and DNA sequencing did not uncover mutations in BRAF, HRAS, GNAQ, GNA11, CDKN2A or TP53 (data not shown), but did reveal a c.182A>G, p.(Q61R) mutation in NRAS (Fig. 4G).
Figure 4. Diagnosis of leptomeningal melanomatosis carrying an oncogenic mutation in NRAS in patient 1.
(A) Axial T1-weighted MRI revealing a hyperintense, contrast-enhancing lesion in the left parieto-occipital region following the gyri and sulci.
(B) Photograph showing macroscopic appearance of the cerebral tissue from patient 1. Brownish discoloration of the thickened leptomeninges (L) and black discoloration of the underlying cerebral cortex (C) are evident.
(C) Photomicrograph showing H&E staining of the melanoma revealing the proliferation of hypopigmented cells in the leptomeninges (L) and invasion of hyperpigmented cells (black arrowheads) into the CNS parenchyma (P). Scalebar = 100μm.
(D) Photomicrograph showing H&E staining of the melanoma. Note the epithelioid morphology of the non-pigmented atypical cells in the leptomeningeal compartment (L) and pigmented tumor cells (white arrows) invading the CNS parenchyma (P). Scalebar = 25μm.
(E) Photomicrograph showing detail of epithelioid morphology of pleomorphic melanoma cells in the leptomeningeal compartment. Scalebar = 50μm.
(F) Photomicrograph showing MelanA staining of the pigmented melanoma cells (black arrowheads) in the CNS parenchyma (black stars). Scalebar = 50μm.
(G) Forward sequence of DNA from the primary CNS melanoma from patient 1 showing the presence of an NRAS c.182A>G, p.(Q61R) mutation (arrow).
The second case involved a boy with a giant cutaneous congenital melanocytic nevus over the lumbrosacral region, with multiple satellite congenital nevi over the trunk, face, arms and upper legs. Biopsies at 2 and 5 years revealed a congenital melanocytic nevus with diffuse infiltration of nevomelanocytes into the dermis and subcutaneous tissue (Fig. 5A-C). Single cells in the dermis splayed the collagen bundles and extended around and within the periadnexal structures (Fig. 5A, 5C). At age 7 the boy presented with motor dysfunction and paraesthesia of the left hand, progressive weakness of the left leg and accompanying headache and vomiting. MRI revealed a large, heterogeneous, hyperintense tumor in the right frontotemporal area, causing midline shift and compression of the ventricles and mesencephalon (Fig. 5D). The patient quickly deteriorated and passed away despite tumor debulking and supportive treatment with mannitol and dexamethasone. The tumor was composed of highly mitotic (>10 per mm2) epithelioid cells with irregular nuclei, prominent nucleoli (Fig. 5E, 5F) and positive staining for MelanA (Fig. 5G), HMB45 and S100 (Supplementary Fig. 5C, 5D). Dermatological examination did not reveal any clinical changes to suggest primary cutaneous melanoma arising over CMN. In the absence of cutaneous melanoma, we diagnosed primary melanoma of the CNS. DNA sequencing did not reveal mutations in BRAF, HRAS, GNAQ, GNA11, CDKN2A or TP53 (data now shown), but did reveal a c.181C>A, p.(Q61K) mutation in NRAS in the tumor (Fig. 5H) and the congenital nevus (Supplementary Fig. 5E).
Figure 5. Diagnosis of NRAS mutated leptomeningal melanomatosis in patient 2.
(A) Photomicrograph showing H&E staining of full-thickness skin from the congenital melanocytic nevus (CMN). Scalebar = 0.5mm.
(B) Photomicrograph of the CMN showing detailed H&E staining of melanocytic nests (arrows) in the papillary dermis and single melanocytes in the reticular dermis (arrowhead). Scalebar = 300μm.
(C) Photomicrograph of the CMN showing detailed H&E staining of the deep dermal melanocytes in the collagen and along the hair follicle (arrows). Scalebar = 300μm.
(D) T1-weighted magnetic resonance image (MRI) with contrast, revealing a large tumor in the right frontotemporal region with meningeal attachment.
(E) H&E staining of the CNS melanoma showing epithelioid tumor cells with low pigment content. Scalebar = 50μm.
(F) H&E staining of the CNS melanoma. Note the atypical tumor cells with irregular nuclei and frequent nucleoli. Scalebar = 30μm.
(G) Photomicrograph showing cytoplasmic brown chromogen MelanA staining in melanoma cells (black arrowhead) invading brain parenchyma (black star) of patient 2. Scalebar = 50μm.
(H) Forward sequence of DNA from the primary CNS melanoma from patient 2 showing the presence of an NRAS c.181C>A, p.(Q61K) mutation (arrow).
DISCUSSION
Here we show that oncogenic NRAS induced dose-dependent hyperpigmentation of the skin when expressed in the mature melanocytes of adult mice, or the developing melanocytes of embryonic mice. This is consistent with previous data showing that NRASQ61K also increased skin pigmentation when expressed in embryonic mouse melanocytes using the tyrosinase promoter, and that oncogenic KRAS induced skin hyperpigmentation when expressed in mature melanocytes from the endogenous Kras gene (30) or from an Actb (β-Actin) promoter fragment (25). We also show that like KRASG12V, when NRASG12D was expressed in mature melanocytes, it induced paucicellular nevi in the deep dermal layers of the skin that resembled human blue nevi. We also show that when NRASG12D was expressed in developing melanocytes it induced congenital blue nevus-like lesions, complementing a recent report showing that NRASQ61K also induced congenital nevi when expressed using a tyrosinase promoter fragment (34).
We previously reported that when BRAFV600E was expressed in embryonic melanocytes, it disrupted heart and eye development, and caused embryonic lethality (31), but show here that NRASG12D did not induce these effects. The basis of this difference is unclear, but a possible explanation is that BRAFV600E transforms developing melanocytes more readily than NRASG12D, causing them to disrupt the development of the organs that they colonize. Alternatively, perhaps oncogenic NRAS induces melanocyte senescence or apoptosis, so that unlike the BRAFV600E melanocytes, the NRASG12D melanocytes are unable to disrupt the development of their host organs.
Critically, although none of the mice developed cutaneous melanoma when NRASG12D was expressed in the melanocytes of embryonic or mature mice, when NRASG12D was expressed in the melanocytes of the embryos, the mice developed leptomeningeal melanoma that presented as neuronal symptoms at a median of 4 months for the homozygous animals and 12.5 months for the heterozygous animals. The tumors generally affected the frontoparietal region of the brain and presented as darkly pigmented lesions that followed the sulci and fissures and invaded the normal CNS parenchyma. The tumors were aggressive and had a high proliferative index, showed evidence of RAS pathway activation, and expression of melanocytic, but not neuronal cell markers.
Cerebral metastases of cutaneous melanomas are usually multifocal, homogenous and well-circumscribed nodules, whereas the tumors in our mice arose from areas connected to leptomeningeal melanocytic hyperproliferation. They were firmly adhered to the surface of the dura and diploe, and invaded the CNS in a highly destructive infiltrative pattern. There were no macroscopic or histological changes in the skin of our mice, and at time of sacrifice, the nevi in the skin retained their paucicellularity, benign architecture and cytomorphology. We also ruled out primary tumors in the other organs colonized by melanocytes. Thus, the brain melanomas arose in the absence of any other primary tumors, and we diagnosed primary melanoma of the CNS.
We were intrigued that although we observed melanoma of the CNS when we expressed NRASG12D off the endogenous Nras gene, when NRASQ61K was expressed using the tyrosinase promoter it did not induce leptomeningeal melanoma (26). However, as discussed below, the disease in our mice, which was driven by NRASG12D, mimicked the cardinal features of the disease in the children, which was driven by NRASQ61K/R. We therefore posit that the differences between our mouse and the mice that were previously reported lies in differences in the pattern, levels or timing of oncogenic NRAS expression, rather than differences in the biology of NRASG12D or NRASQ61K.
This is the first melanoma model driven by oncogenic RAS expressed using the endogenous gene that did not require additional genetic engineering of the mice, or exposure to carcinogens or tumor promoters. In most previously described models of RAS-driven melanoma, tumor induction was inefficient unless the mice carried a second genetic lesion or were exposed to an environmental stress. For example, HRASG12V-induced melanoma was inefficient unless the mice were exposed to ultraviolet (UV) light or the tumor promoter TPA (23, 35, 36). Similarly, deletion of p16INK4A cooperated with HRASG12V and NRASQ61K to induce melanoma (26, 28), and KRAS driven melanomagenesis was inefficient unless BRAFD594A was also expressed (30), or KRASG12V was strongly over-expressed using the Actb promoter (25). Note however that we do not interpret this to mean that NRASG12D alone was sufficient to induce leptomeningeal melanomagenesis and we are currently working to identify the cooperating events using insertional mutagenesis and genomics.
Importantly, our mice presented the cardinal clinical features of the disease presented by two children. As in the mice, the children presented with neurological symptoms, rapid health deterioration and death, and the cells in their tumors were highly proliferative, they invaded the CNS parenchyma and they stained positive for melanoma markers. We did not observe primary melanoma of the skin in either child and therefore diagnosed primary melanoma of the CNS. Sequencing revealed the presence of a NRAS mutation. Furthermore, one of the children presented with a giant congenital melanocytic nevus that shared the same NRAS mutation as his CNS melanoma. Since this child did not present cutaneous melanoma arising over CMN, it appears that his leptomeningeal melanocytes were more susceptible than his cutaneous melanocytes to transformation by oncogenic NRAS. Mouse leptomeningeal melanocytes also appear to be more susceptible than cutaneous melanocytes to transformation by oncogenic NRAS. Thus, despite the evidence that NRASG12D induced proliferation of both cutaneous and leptomeningeal melanocytes none of the animals developed cutaneous melanoma, whereas 75% of the heterozygous mice and all of the homozygous mice developed melanoma of the CNS.
It is unclear why developing leptomeningeal melanocytes more susceptible than cutaneous melanocytes to transformation by oncogenic NRAS, but it seems unlikely that this is due to differences in the numbers of melanocyte in these two sites. More plausibly, it seems likely that although oncogenic NRAS induced prenatal hyperproliferation of the melanocytes in both the skin and brains, intrinsic (cell autonomous) or extrinsic (microenvironment) differences between the melanocytes in these two sites mean that those in the leptomeninges were more susceptible to transformation. This is supported by the observation that one of the children’s tumors shared the same NRAS mutation as his giant congenital melanocytic nevus, suggesting a common ancestry, but nevertheless a more susceptible population in the leptomeninges. We anticipate that the identification of the events that cooperate with NRAS to transform melanocytes will go some way explaining the underlying biology.
Although we have reported two cases of childhood melanoma of the CNS that carry NRAS mutations, we note that in adults, melanoma of the CNS is associated with mutations in GNAQ and GNA11, whereas NRAS mutations are rare (17-19). Previous studies have shown that truly congenital cutaneous melanocytic nevi harbor NRAS mutations and patients with these lesions may also present congenital deposits of leptomeningeal melanocytes (20, 21). Furthermore, in humans, and in children in particular, CNS melanoma often arises in patients with giant cutaneous congenital melanocytic nevi (12-16), approximately 80% of which carry somatic mutations in NRAS but not in BRAF (20, 21). Our data show that leptomeningeal melanocytes are more susceptible to transformation by oncogenic NRAS than cutaneous melanocytes. We posit that there is a link between prenatal proliferation of melanocytes and early onset melanoma of the CNS and that acquisition of somatic oncogenic mutations in NRAS in the melanocytes of the CNS is a predisposing risk factor to melanoma of the CNS. We present a mouse model that can be used to study this rare and devastating disease and note that the tumor cells from our mice are susceptible to MEK inhibitors, suggesting a potential therapeutic approach for these patients.
METHODS
Animal procedures
All procedures involving animals were approved by the Animal Ethics Committees of the Institute of Cancer Research and the CR-UK London Research Institute in accordance with National Home Office regulations under the Animals (Scientific Procedures) Act 1986 and according to the guidelines of the Committee of the National Cancer Research Institute (37). Tamoxifen (Sigma-Aldrich T5648) was freshly prepared in 100% ethanol. For genotyping, genomic DNA was prepared from tail biopsies and PCR was performed using the primers previously described (32, 38). For allograft experiments, 0.5 × 106 Nras mutant cells in 0.1 ml PBS were inoculated intradermally into the flanks of female C57Bl/6 mice (Charles River; UK). Mice were treated daily by oral gavage with vehicle (n=9 mice) or PD184352 (25 mg/kg) (n=6 mice). Tumor volumes were determined using volume = length × width × depth (mm) × 0.5236.
RNA-extraction and sequence analysis of mouse tissue
RNA was extracted from frozen tissues using RNAeasy kit (QIAGEN) and first-strand cDNA synthesized and DNAse treated as previously described (24). Subsequentially, cDNA was amplified by PCR and the products were sequenced using dye-terminator chemistry using NRAS primers: 5′-ATGACTGAGTACAAACTGGTGGTGG-3′ and 5′-CCATCAATCACCACTTGCTTTCGGTAAG-3′. Sequences were visualized using Sequencher software.
Tumor DNA-extraction and sequence analysis of human cases
Three manually dissected sections of 10 μm formalin-fixed and paraffin-embedded tissue with an estimated tumor cell percentage of at least 80% were used for DNA extraction. DNA extraction and sequence analysis of BRAF, NRAS, HRAS, GNAQ, CDKN2A and TP53 was performed as previously described (17). The GNAQ gene sequence amplified was gene-specific as GNAQ-pseudogene specific nucleotides were not detected. Sequence analysis for GNA11 was performed using M13-tailed GNA11 specific primers 5′-TGTAAAACGACGGCCAGTGGTGGGAGCCGTCCTGGGATTGC-3′ and 5′-CAGGAAACAGCTATGACCCCCACCTCGTTGTCCGAC- 3′. All PCR reactions were performed in duplicate using two independent PCR products for sequence analysis.
Histology and immunohistochemistry (IHC)
Mouse tumors were formalin-fixed and analyzed as previously described (24), and subsequently stained with hematoxylin and eosin, Ki-67 (Dako M7249) and HMB45/MelanA (Abcam ab732).
All procedures concerning the use of human tissue were in accordance with valid standards for this type of investigation in The Netherlands (39). For tumor staining the following antibodies were used: S100 (Dako Z0311), HMB45 (Novocastra NCL-HMB45) and MelanA (Mart clone M2-7C10, Neomarkers MS-716). Sample preparation for S100 staining was performed without retrieval while sample preparation for HMB45 and MelanA staining was performed with retrieval (NAcitrate pH6.0 and pH9.0 respectively).
Immunofluoresence (IF) labeling of FFPE samples
Three mm sections of formalin-fixed paraffin embedded (FFPE) material were used. Slides were dewaxed and antigen retrieval was performed using citrate buffer pH6.0 followed by blocking in PBS-Tween 0.1% + 1% BSA for 15 minutes and over night incubation with primary antibody (1:100 in PBS+1%BSA). The following primary antibodies were used: a-phospho-ERK1/2 (Cell Signalling), Glial Fibrillary Acidic Protein, GFAP, (Dako), HMB45/MelanA and Cyclin D1 (Abcam), and S100 (Menapath). Antibody detection was performed using AlexaFluor-conjugated secondary antibodies (Invitrogen). Slides were counterstained with DAPI. Samples were analysed and pictures were taken using a Leica SP2 confocal scanning microscope (Leica Microsystems, Milton Keynes, Bucks, UK).
Cell culture and Western Blotting
The Nras mutant tumor cell line was established by collecting murine brain melanoma in sterile PBS on ice and mechanically dissociating tumors in Dulbecco’s modified Eagle’s Medium (DMEM). The cells were continuously cultured in DMEM supplemented with 10% fetal bovine serum and 10units/mL penicillin and 100mg/mL streptomycin. Cells were exposed to the MEK inhibitor PD184352 and cell lysates were prepared as previously described (40). The following primary antibodies were used: Anti-phospho-p42/p44 MAPK, and total ERK1/2 (Cell Signalling).
Supplementary Material
SIGNIFICANCE.
We show that the acquisition of NRAS mutations in melanocytes during embryogenesis is a risk factor for early onset melanoma of the CNS. We have developed a powerful mouse model to study this rare but devastating childhood disease, and to develop therapeutic approaches for its treatment.
ACKNOWLEDGEMENTS
This work was supported by the Wenner-Gren Foundations, Stockholm, Teggerstiftelsen, Cancer Research UK (ref: C107/A10433 and C5759/A12738), The Institute of Cancer Research and the EORTC Melanoma Group.
Footnotes
Conflict of interest: The authors do not have any conflicts of interest related to this work.
REFERENCES
- 1.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 2.Broekaert SM, Roy R, Okamoto I, van den Oord J, Bauer J, Garbe C, et al. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 2010;23:763–70. doi: 10.1111/j.1755-148X.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427–34. doi: 10.1001/archdermatol.2008.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Seddon JM, Gragoudas ES, Glynn RJ, Egan KM, Albert DM, Blitzer PH. Host factors, UV radiation, and risk of uveal melanoma. A case-control study. Arch Ophthalmol. 1990;108:1274–80. doi: 10.1001/archopht.1990.01070110090031. [DOI] [PubMed] [Google Scholar]
- 6.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 7.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–6. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 8.Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–9. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999;23:745–54. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Allcutt D, Michowiz S, Weitzman S, Becker L, Blaser S, Hoffman HJ, et al. Primary leptomeningeal melanoma: an unusually aggressive tumor in childhood. Neurosurgery. 1993;32:721–9. doi: 10.1227/00006123-199305000-00004. discussion 9. [DOI] [PubMed] [Google Scholar]
- 13.Makin GW, Eden OB, Lashford LS, Moppett J, Gerrard MP, Davies HA, et al. Leptomeningeal melanoma in childhood. Cancer. 1999;86:878–86. [PubMed] [Google Scholar]
- 14.Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24:747–55. doi: 10.1016/0190-9622(91)70115-i. [DOI] [PubMed] [Google Scholar]
- 15.Shah KN. The risk of melanoma and neurocutaneous melanosis associated with congenital melanocytic nevi. Semin Cutan Med Surg. 29:159–64. doi: 10.1016/j.sder.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Pavlidou E, Hagel C, Papavasilliou A, Giouroukos S, Panteliadis C. Neurocutaneous melanosis: report of three cases and up-to-date review. J Child Neurol. 2008;23:1382–91. doi: 10.1177/0883073808319069. [DOI] [PubMed] [Google Scholar]
- 17.Kusters-Vandevelde HV, Klaasen A, Kusters B, Groenen PJ, van Engen-van Grunsven IA, van Dijk MR, et al. Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2009;119:317–23. doi: 10.1007/s00401-009-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murali R, Wiesner T, Rosenblum MK, Bastian BC. GNAQ and GNA11 mutations in melanocytomas of the central nervous system. Acta Neuropathol. 2012;123:457–9. doi: 10.1007/s00401-012-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessi M, Hammes J, Lauriola L, Dorner E, Kirfel J, Kristiansen G, et al. GNA11 and N-RAS mutations: alternatives for MAPK pathway activating GNAQ mutations in primary melanocytic tumours of the central nervous system. Neuropath and Applied Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01288.x. [DOI] [PubMed] [Google Scholar]
- 20.Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. Br J Dermatol. 1994;131:72–7. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 21.Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127:179–82. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- 22.Kovalyshyn I, Braun R, Marghoob A. Congenital melanocytic naevi. The Australasian J Dermatol. 2009;50:231–40. doi: 10.1111/j.1440-0960.2009.00553_1.x. quiz 41-2. [DOI] [PubMed] [Google Scholar]
- 23.Powell MB, Hyman P, Bell OD, Balmain A, Brown K, Alberts D, et al. npigmentation and melanocytic hyperplasia in transgenic mice expressing the human T24 Ha-ras gene regulated by a mouse tyrosinase promoter. Mol Carcinogen. 1995;12:82–90. doi: 10.1002/mc.2940120205. [DOI] [PubMed] [Google Scholar]
- 24.Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010;70:5549–57. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–11. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 27.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes & Development. 1997;11:2822–34. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larue L, Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res. 2007;20:485–97. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 30.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhomen N, Da Rocha Dias S, Hayward R, Ogilvie L, Hedley D, Delmas V, et al. Inducible expression of (V600E) Braf using tyrosinase-driven Cre recombinase results in embryonic lethality. Pigment Cell Melanoma Res. 2010;23:112–20. doi: 10.1111/j.1755-148X.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 32.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–8. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yajima I, Belloir E, Bourgeois Y, Kumasaka M, Delmas V, Larue L. Spatiotemporal gene control by the Cre-ERT2 system in melanocytes. Genesis. 2006;44:34–43. doi: 10.1002/gene.20182. [DOI] [PubMed] [Google Scholar]
- 34.Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nature cell biology. 2012;14:882–90. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- 35.Kannan K, Sharpless NE, Xu J, O’Hagan RC, Bosenberg M, Chin L. Components of the Rb pathway are critical targets of UV mutagenesis in a murine melanoma model. Proc Natl Acad Sci U S A. 2003;100:1221–5. doi: 10.1073/pnas.0336397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hacker E, Irwin N, Muller HK, Powell MB, Kay G, Hayward N, et al. Neonatal ultraviolet radiation exposure is critical for malignant melanoma induction in pigmented Tpras transgenic mice. J Invest Dermatol. 2005;125:1074–7. doi: 10.1111/j.0022-202X.2005.23917.x. [DOI] [PubMed] [Google Scholar]
- 37.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. British Journal of Cancer. 2010;102:1555–77. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delmas V, Martinozzi S, Bourgeois Y, Holzenberger M, Larue L. Cre-mediated recombination in the skin melanocyte lineage. Genesis. 2003;36:73–80. doi: 10.1002/gene.10197. [DOI] [PubMed] [Google Scholar]
- 39.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packer LM, Rana S, Hayward R, O’Hare T, Eide CA, Rebocho A, et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell. 2011;20:715–27. doi: 10.1016/j.ccr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.