Abstract
ATP is critical for oocyte maturation, fertilization, and subsequent embryo development. Both mitochondrial membrane potential and copy number expand during oocyte maturation. In order to differentiate the roles of mitochondrial metabolic activity and mtDNA copy number during oocyte maturation, we used two inhibitors, FCCP (carbonyl cyanide p-(tri-fluromethoxy)phenyl-hydrazone) and ddC (2’3-dideoxycytidine), to deplete the mitochondrial membrane potential (Δφm) and mitochondrial copy number, respectively. FCCP (2000 nM) reduced ATP production by affecting mitochondrial Δφm, decreased the mRNA expression of Bmp15 (bone morphogenetic protein 15), and shortened the poly(A) tails of Bmp15, Gdf9 (growth differentiation factor 9), and Cyclin B1 transcripts. FCCP (200 and 2000 nM) also affected p34cdc2 kinase activity. By contrast, ddC did not alter ATP production. Instead, ddC significantly decreased mtDNA copy number (P < 0.05). FCCP (200 and 2000 nM) also decreased extrusion of the first polar body, whereas ddC at all concentrations did not affect the ability of immature oocytes to reach metaphase II. Both FCCP (200 and 2000 nM) and ddC (200 and 2000 µM) reduced parthenogenetic blastocyst formation compared with untreated oocytes. However, these inhibitors did not affect total cell number and apoptosis. These findings suggest that mitochondrial metabolic activity is critical for oocyte maturation and that both mitochondrial metabolic activity and replication contribute to the developmental competence of porcine oocytes.
Keywords: Mitochondrial metabolic activity, Mitochondrial replication, Oocyte maturation, Porcine
Mitochondria are major powerhouses in all eukaryotic cells, producing ATP through oxidative phosphorylation and the citric acid cycle. An increase in ATP production is required during oocyte maturation, fertilization, and early embryo development in mammals [1, 2]. Previous studies have also reported an association between mitochondrial DNA (mtDNA) copy number and oocyte quality during maturation [1, 3]. For example, mtDNA copy number is an indicator of fertilization potential and oocyte maturation, and oocytes with a low mtDNA copy number have a significantly lower developmental potency [4, 5]. mtDNA copy number also increases during the in vitro maturation of porcine oocytes and after the treatment of oocytes with follicular fluids or EGF (epidermal growth factor), which likely affects the developmental potential of oocytes [6].
Mitochondrial membrane potential (Δφm) is also critical for the production of ATP. During oocyte maturation, there is a significant increase in mitochondrial Δφm [7], and in the absence of an increase, the developmental potential of oocytes decreases [8, 9]. In addition, a high mitochondrial Δφm in mouse and human oocytes and early preimplantation stage embryos is associated with ionic and metabolic regulation [10].
To date, few maternal genes in mammalian oocytes have been characterized. Among these maternal transcripts, C-mos, Cyclin B1, Cdc2 (cell division cycle 2), Gdf9 (growth differentiation factor 9), and Bmp15 (bone morphogenetic protein 15) are well-studied genes considered to be markers of female germ cells. One of the essential regulators of meiosis resumption is formed by Cyclin B1 and Cdc2 kinase [11]. It has been reported that the dynamic change in levels of cyclin B1 is mainly controlled by cytoplasmic polyadenylation during mouse [12] and bovine [13] oocyte maturation. GDF9 and BMP15 belong to the transforming growth factor-β (TGF-β) superfamily, which contains many members with important roles in regulating fertility [14]. GDF9 and BMP15 were recently identified as oocyte-secreted factors involved in folliculogenesis and oocyte maturation, as well as in cooperative regulation of granulosa cells [15].
Recently Ge et al. [16] reported a connection between mouse oocyte quality and both mitochondrial metabolic activity and DNA copy number, specifically with spindle formation, chromosomal alignment, and embryo development. However, the underlying molecular mechanism has not been addressed. In vitro maturation of pig oocytes is useful in the study of the molecular mechanisms that underlie meiosis and fertilization as well as in the production of cloned and transgenic embryos and pigs [17,18,19]. While in vitro culture conditions and/or micromanipulations such as enucleation and injection of DNA or sperm can affect mitochondrial activity in oocytes from several species, this information is not available for porcine oocytes.
To determine the effects of mitochondrial metabolic activity and mtDNA copy number on oocyte maturation and developmental competence, we treated immature porcine oocytes with FCCP, which inhibited mitochondrial oxidative phosphorylation, and ddC (2’3-dideoxycytidine), which depleted mtDNA. The effects of these two inhibitors on oocyte dynamics were assessed by determining the mitochondrial Δφm, mtDNA copy number, ATP concentration, target mRNA expression and poly(A) tail length of maternal genes. P34cdc2 kinase activity and mitogen-activated protein kinase (MAPK) phosphorylation were also investigated following FCCP and ddC treatment. To the best of our knowledge, this is the first report to address the relationship among mitochondrial Δφm and copy number, the in vitro maturation of porcine oocytes, and the developmental competence of embryos.
Materials and Methods
Oocyte collection and in vitro maturation
Prepubertal porcine ovaries were obtained from a local slaughterhouse. Cumulus-oocyte complexes (COCs) were isolated and cultured in tissue culture medium 199 (TCM 199, Gibco, Grand Island, NY, USA) supplemented with 0.57 mM cysteine, 10 ng/ml EGF, 10 IU/ml PMSG, and 10 IU/ml hCG under light mineral oil at 38.5 C in 5% CO2 (v/v). Oocytes were treated with 20, 200, and 2000 nM FCCP, or 20, 200, 500, 1000 and 2000 µM ddC. Control oocytes were untreated. Oocytes with a first polar body were collected after 44–48 h of in vitro maturation.
Parthenogenic activation and culture of embryos
Upon maturation, cumulus cells were removed by repeated pipetting in the presence of 1 mg/ml hyaluronidase for 2–3 min. Oocytes were parthenogenetically activated with calcium ionophore A23187 (50 µM) for 5 min, followed by incubation in PZM-5 medium [20, 21] containing 7.5 µg/ml cytochalasin B (CB, Sigma-Aldrich, St. Louis, MO, USA) for 3 h. Embryos were cultured in PZM-5 medium supplemented with 0.4% bovine serum albumin (BSA, w/v) under light mineral oil for 7 days at 38.5 C in 5% CO2 (v/v) and then harvested.
Mitochondrial copy number analysis
Total DNA was isolated from 10 oocytes according to the manufacturer’s instructions provided in the Puregene DNA Isolation Kit (Invitrogen, Carlsbad, CA, USA). Oocyte DNA samples were then used for real-time polymerase chain reaction (PCR) experiments. Twenty-microliter PCR reactions were set up with final concentrations of 1 × buffer containing 4 mM/l MgCl2, 0.2 mM/l dNTPs, 0.5 mM/l of each primer, SYBR Green I dye and 0.25 U Taq DNA polymerase (Biotech International, Western Australia). The reactions were performed as follows: initial denaturation at 95 C for 2 min and then 40 cycles of denaturation at 95 C for 10 sec, annealing at 55 C for 20 sec, and elongation at 72 C for 20 sec. SYBR Green fluorescence was quantified at the end of each elongation step. The relative quantification of mitochondrial copy number was performed with the 2-ΔΔCt method. Mitochondrial copy number in the control group was arbitrarily set at 1. Three separate experiments were performed, with each experiment containing three replicates.
Membrane potential assay
To measure mitochondrial Δφm , denuded MII oocytes were washed three times with PBS and incubated in culture medium containing 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) (Invitrogen) at a concentration of 1 mM/l at 37C in 5% CO2 for 30 min. Membrane potential was calculated as a ratio of the red florescence, which corresponded to activated mitochondria (J-aggregates), to the green fluorescence, which corresponded to less-activated mitochondria (J-monomers)[16] . Fluorescence was visualized with a Zeiss inverted confocal microscope equipped with a 40 × oil immersion objective (Zeiss, Jena, Germany). Images were processed with ZEN software (Zen Software, Manchester, UK). The fluorescence intensity in the control group was arbitrarily set at 1, and the fluorescence intensity in the treatment groups was then measured. Three separate experiments were performed, with each experiment containing from 10 to 15 oocytes.
ATP content assay
The ATP content of 20 completely denuded mature oocytes was measured using a commercial assay (Invitrogen). Briefly, samples were washed three times with PBS and then transferred individually into 1 ml tubes on ice. Media were removed, and oocytes were then frozen and thawed to lyse them. Approximately 100 µl of ice-cold somatic cell reagent (FL-SAR) was added to each tube, and samples were incubated in an ice-water bath for 5 min. Thereafter, 100 µl of ice-cold assay buffer (diluted 1:25 with ATP assay buffer, FL-AAB) was added, and the tubes were maintained at room temperature for 5 min under limited light conditions. The ATP concentration was measured using a luminometer (Berthold, Wildbad, Germany) with a sensitivity of 0.01 pmol. The ATP concentration in the control group was arbitrarily set at 1. Three separate experiments were performed, with each experiment containing three replicates.
Real-time RT-PCR
Extraction of mRNA and cDNA synthesis were performed as previously described [22]. Briefly, mRNA was extracted from 20 oocytes with a Dynabeads mRNA Direct Kit (Dynal Biotech ASA, Oslo, Norway), followed by routine cDNA synthesis by reverse transcription of RNA using an oligo (dT)12–18 primer and SuperScript Reverse Transcriptase (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions.
Real-time RT-PCR or RT-PCR was performed using the four primer pairs listed in Table 1. Real-time RT-PCR was performed in a Bio-Rad PCR machine (Bio-Rad, Hercules, CA, USA). Relative gene expression was analyzed using the 2-ΔΔCt method [23]. Gapdh mRNA was used as an internal control. Three independent experiments were performed, with each experiment containing triplicate samples.
Table 1. List of porcine primers used for real-time RT-PCR.
| Gene | GenBank accession number | Primer sequence (5’–3’) | Length (bp) |
| Gdf9 | AY626786 | F: GAGCTCAGGACACTGTAAGCT R: CTTCTCGTGGATGATGTTCTG |
272 |
| Bmp15 | NM_001005155 | F: CCCTCGGGTACTACACTATG R: GGCTGGGCAATCATATCCT |
192 |
| Cyclin B1 | L48205 | F: GCTCCAGTGCTCTGCTTCTC R: ACAAACTTTATTAAAAGTAAATAAGTG |
177 |
| C-mos | NM_001113219 | F: TGGGAAGAAACTGGAGGACA R: TTCGGGTCAGCCCAGTTCA |
121 |
| Gapdh | AF017079 | F: GGGCATGAACCATGAGAAGTR: AAGCAGGGATGATGTTCTGG | 230 |
| Cdc2 | AB495208 | F: TGGGCACTCCCAATAATGAA R: TCCAAGCCATTTTCATCCAA |
133 |
PAT Assay: analysis of poly(A) tail lengths by PCR
To determine the poly(A) tail length of maternal transcripts, a PAT assay was performed as described previously [24]. In principle, the PAT assay is similar to the 3’-Rapid Amplification of cDNA Ends (RACE) method. Briefly, poly(A) RNAs from pools containing 10 denuded in vitro matured porcine oocytes were isolated and reverse transcribed with an oligo(dT)12 primer (5’–GCGAGCTCCGCGGCCGCGT12–3’) [25]. Subsequent PCR was performed using oligo(dT) and gene-specific upstream primers (GSP) corresponding to maternal transcripts (Table 1). The reactions were performed as follows: 8 min at 94 C, followed by 33 cycles of 30 sec at 93 C, 1 min at 59 C, and 50 sec at 72 C. A final extension of 5 min at 72 C was included at the end of the PCR run. PCR products were electrophoresed on a 3% agarose gel and stained with ethidium bromide. Differences in poly(A) tail length were observed as smears of different lengths. Since a gene may express many mRNA isoforms that vary in 3’-untranslated region (UTR) length, the PAT product may appear as several main bands and smears. Bands represented lengths that were in between that of the GSP and the poly(A) tail.
p34cdc2 kinase activity assay
p34cdc2 kinase activity was quantified with a Mesacup cdc2 Kinase Assay Kit (MBL, Nagoya, Japan) according to Shoujo et al. [26]. With this method, the correlation coefficient between p34cdc2 kinase activity (as determined by the cdc2 kinase assay) and histone H1 kinase activity (as measured by radioactive detection) can be as high as 0.9961.
Briefly, 5 µl of oocyte extract (containing 20 oocytes) was mixed with 45 µl of kinase assay buffer [25 mM HEPES pH 7.5 at 25 C containing 10 mM MgCl2, 10% MV peptide solution (SLYSSPGGAYC; MBL, w/v), and 0.1 mM ATP]. The mixture was incubated for 30 min at 30 C. The reaction was terminated with 200 µl PBS containing 50 mM EGTA. Phosphorylation of the MV peptide (Dynal Biotech ASA, Oslo, Norway) was detected by ELISA. The optical density (O.D.) of p34cdc2 kinase activity in the control group was arbitrarily set at 1, and the p34cdc2 kinase activity was then quantified in the FCCP or ddC treatment groups. This experiment was repeated three times.
Western blot analysis
Western blot analysis was performed as described previously [24]. Briefly, 200 pig oocytes or embryos were thawed at room temperature and added to 20 µl 1 SDS Sample Buffer [62.5 mM Tris–HCl pH 6.8 at 25 C containing 2% SDS (w/v), 10% glycerol (v/v), 50 mM DTT, and 0.01% bromophenol blue or phenol red (w/v)]. Samples were then heated at 95 C for 5 min. Total proteins were subsequently separated by electrophoresis in a Criterion Precast Gel (Bio-Rad) for 2 h at 100 V, followed by transfer to a PVDF membrane (iBlot Gel Transfer Stacks, Invitrogen). After blocking with 5% skim milk (w/v) in PBS for 1 h, the membrane was incubated with a primary anti-phospho-p44/42 MAPK antibody (Cell Signaling Technology, Danvers, MA, USA) diluted 1:1,000 in blocking solution [1 × TBS pH 7.4 at 25 C containing 0.1% Tween-20 (v/v) and 5% nonfat dry milk (w/v)] for 1.5 h at room temperature. After washing three times in Tris-buffered saline with Tween-20 [TBST, 20 mM Tris–HCl pH 7.5 at 25 C containing 250 mM NaCl, and 0.1% Tween-20 (v/v)], the membrane was incubated for 1 h with a 1:2,000-diluted HRP-linked anti-rabbit IgG secondary antibody (Cell Signaling Technology) in blocking solution at room temperature. Immunoreactive bands were visualized with chemiluminescence luminol reagent (Invitrogen) after three 10 min washes with TBST. Protein bands were analyzed, and the integrated O.D. of each band was determined with the Image-Pro Plus software (version 6.0, Media Cybernetics, Springfield, IL, USA).
TUNEL assay
Parthenogenetically activated embryos were fixed and permeabilized with 0.3% Triton X-100 (v/v) at room temperature for 1 h. After washing twice with PBS/polyvinyl alcohol (PVA), embryos were incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase enzyme (In Situ Cell Death Detection Kit, Roche, Mannheim, Germany) at 37 C for 1 h under light protected conditions. After counterstaining with Hoechst 33342 (40 µg/ml) and RNase A (4 µg/ml) at 37 C for 1 h to label nuclei, embryos were washed with PBS/PVA and mounted. Fluorescence was visualized by confocal microscopy. Excitation and emission wavelengths for Hoechst 33342 were 543 nm and 585 nm, respectively; for TUNEL, the wavelengths were 488 and 515 nm, respectively. This experiment was performed at least three times. Three separate experiments were performed, with each experiment containing from 10 to 15 blastocysts.
To determine total cell number, blastocysts were fixed in 4% paraformaldehyde (w/v), stained with Hoechst 33342 (4 µg/ml) for 5 min, and imaged by fluorescence microscopy (Eclipse Ti-U, Nikon, Tokyo, Japan).
Statistical analysis
Data are representative of at least three independent experiments. The general linear models (GLM) procedure in the SAS program (SAS Institute, Cary, NC, USA) was used for data analysis. Significant differences were determined by Tukey’s multiple range test, and P < 0.05 was considered significant.
Results
Effects of FCCP and ddC on mitochondrial membrane potential
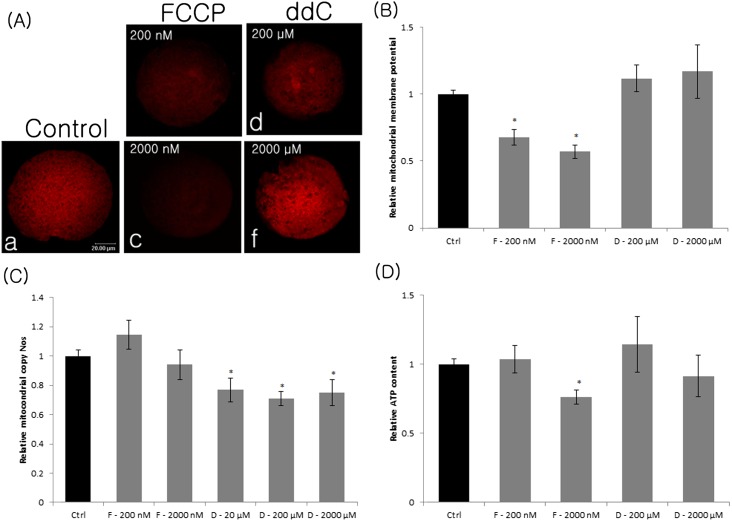
Mitochondrial Δφm was determined in FCCP-treated (200 and 2000 nM) and ddC-treated (200 and 2000 µM) oocytes by JC-1 staining. Both 200 and 2000 nM FCCP significantly reduced (P < 0.05) the mitochondrial Δφm compared with the control. However, ddC did not affect the mitochondrial Δφm (Fig. 1A, B).
Fig. 1.
Mitochondrial membrane potential (A, B), relative mtDNA copy number (C), and relative ATP content (D) in FCCP- or ddC-treated MII oocytes. Membrane potential was calculated as a ratio of the red florescence, which corresponded to activated mitochondria (J-aggregates) (A), to the green fluorescence, which corresponded to less-activated mitochondria (J-monomers, data not shown). Data corresponding to the control was arbitrarily set at 1. Ctrl, control; F, FCCP; D, ddC. Values represent means ± SEM from at least three separate experiments. *, P < 0.05, compared with the control.
Effects of FCCP and ddC on mitochondrial copy number
To determine if FCCP and ddC affect mitochondrial copy number, we treated immature porcine oocytes with different concentrations of these inhibitors. There was no significant difference in mtDNA copy number after treatment with FCCP (200 and 2000 nM) compared with untreated oocytes (Fig. 1C), illustrating that FCCP does not affect mtDNA transcription. However, the mtDNA copy number decreased in MII oocytes treated with ddC (20, 200 and 2000 µM, P < 0.05) compared with the control group (Fig. 1C).
Effects of FCCP and ddC on the concentration of ATP
To determine whether mitochondrial content and Δφm affect mitochondrial ATP synthesis, we quantified the concentration of ATP in oocytes after inhibition of these two parameters by ddC and FCCP. There was no difference in ATP content in oocytes treated with ddC (200 and 2000 µM) (Fig. 1D) or FCCP (200 nM) compared with untreated oocytes. By contrast, the concentration of ATP decreased significantly in oocytes treated with FCCP (2000 μM, P < 0.05) compared with the control group. These results illustrate that a high concentration of FCCP inhibited mitochondrial Δφm, resulting in the reduction of ATP synthesis.
Effects of FCCP and ddC on oocyte maturation
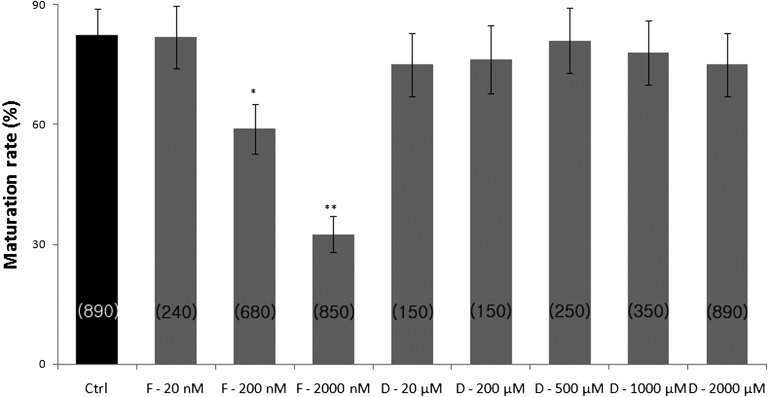
To investigate the effects of FCCP and ddC on the resumption of oocyte meiosis, COCs were cultured in IVM medium supplemented with increasing concentrations of FCCP (20, 200, or 2000 nM) or ddC (20, 200, 500, 1000, or 2000 µM). The percentage of oocytes that reached the MII stage was significantly lower (P < 0.05) in the presence of 200 and 2000 nM FCCP compared with the control group (58.9 ± 6.2% and 32.5 ± 4.5% vs. 82.5 ± 6.5% for 200 and 2000 nM FCCP vs. control, respectively, Fig. 2). ddC at all concentrations did not affect extrusion of the first polar body.
Fig. 2.
Percentage of maturation in FCCP- or ddC-treated MII oocytes. The number of oocytes examined in each treatment group is shown in parentheses. Ctrl, control; F, FCCP; D, ddC. Values represent means ± SEM from at least five separate experiments. *P < 0.05 compared with the control, **P < 0.01 compared with the control.
Effects of FCCP and ddC on maternal mRNA expression and poly(A) tail length
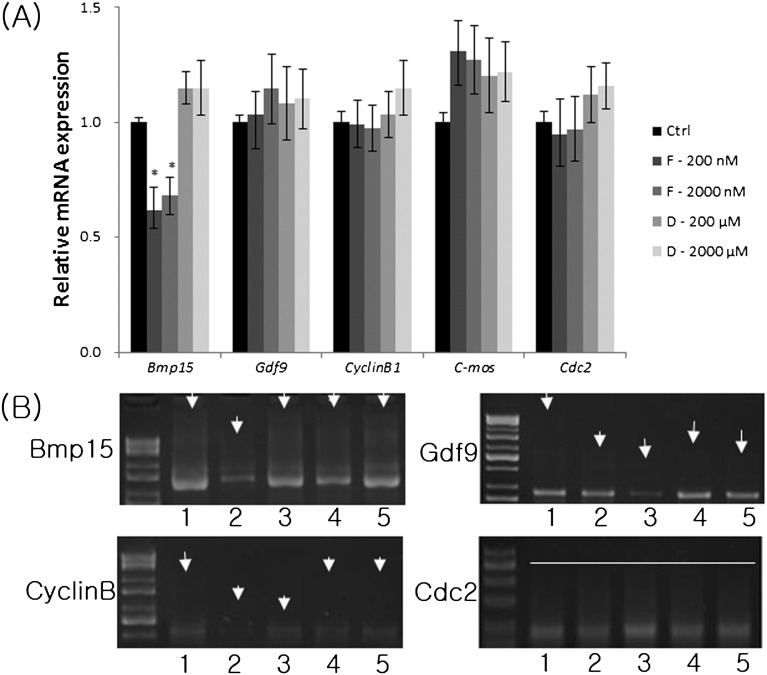
We examined the mRNA expression of several putative maternally expressed genes and analyzed the poly(A) tail length after FCCP and ddC treatment. While the mRNA expression of Bmp15 was decreased significantly by FCCP, it did not affect the mRNA expression of Gdf9, Cyclin B1, C-mos and Cdc2 in MII oocytes (Fig. 3A). However, there were no changes in the mRNA expression of Bmp15, Gdf9, Cyclin B1, and C-mos in MII oocytes treated with ddC. In addition, FCCP also shortened the poly(A) tails of Bmp15, Cyclin B, and Gdf9 compared with the control group (Fig. 3B). ddC also shortened the poly(A) tail of Gdf9. The length of the poly(A) tail of Cdc2 was unaffected by both FCCP and ddC.
Fig. 3.
Relative mRNA expression of maternal genes (Bmp15, Gdf9, cyclin B1, and c-mos) (A) and poly(A) tail length (B) in FCCP- or ddC-treated MII oocytes. Porcine Gapdh mRNA expression was used as an internal control, and the control group was set at 1. Ctrl, control; F, FCCP; D, ddC. Lane 1, control; lanes 2 and 3, oocytes treated with 200 or 2000 nM FCCP, respectively; lanes 4 and 5, oocytes treated with 200 or 2000 µM ddC, respectively. Arrows point to the initial length of the poly(A) tail. Values represent means ± SEM from three separate experiments. *P < 0.05, compared with the control.
Effects of FCCP and ddC on p34cdc2 kinase activity
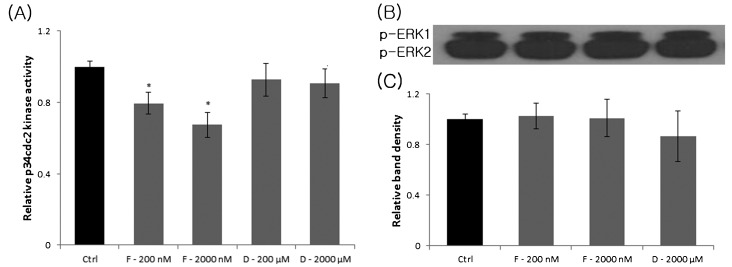
We also quantified p34cdc2 kinase activity in FCCP- and ddC-treated MII oocytes. There was no change in p34cdc2 kinase activity in MII oocytes treated with ddC compared with the control group. However, FCCP (200 and 2000 nM) significantly reduced p34cdc2 kinase activity in MII oocytes compared with untreated oocytes (Fig. 4A).
Fig. 4.
Relative p34cdc2 kinase (A), p-ERK1/2 activity (B) and relative density of p-ERK1/2 activity (C) in FCCP- or ddC-treated MII oocytes. The upper panel in (B) is an immunoblot showing the levels of p-ERK1 and p-ERK2. The lower panel in (B) is a histogram summarizing immunoblotting results. Ctrl, control; F, FCCP; D, ddC, p, phospho. Values represent means ± SEM from three separate experiments. *P < 0.05, compared with the control.
Effects of FCCP and ddC on MAPK phosphorylation
Oocytes were incubated in the presence of different concentrations of FCCP and ddC for 44 h, and MAPK phosphorylation in MII oocytes was assessed by Western blot analysis. FCCP and ddC did not affect the relative levels of p-ERK44 and p-ERK42 in MII oocytes (Fig. 4B).
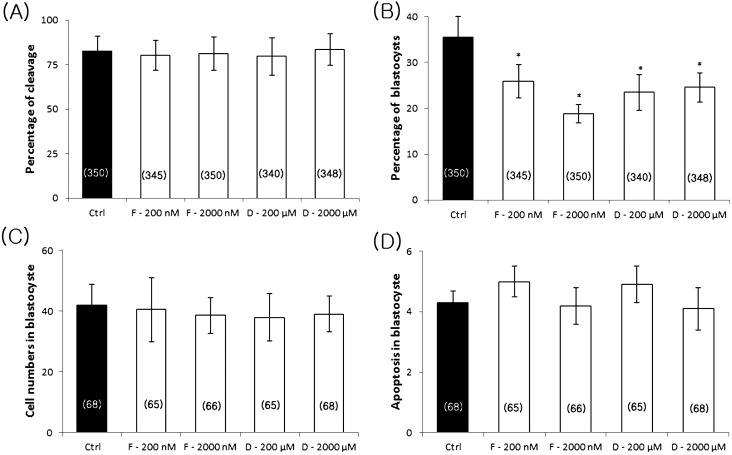
Effects of FCCP and ddC on parthenotes development, cell number, and apoptosis
The effects of FCCP and ddC on the developmental competence of oocytes was investigated. Following the in vitro maturation of oocytes in the presence of FCCP (200 or 2000 nM) or ddC (200 or 2000 µM), all MII oocytes were artificially activated and subsequently cultured for 7 days. The percentage of blastocysts and the total number of cells in blastocysts were determined on day 7. While FCCP and ddC did not affect parthenote cleavage (Fig. 5A), both inhibitors reduced the blastocyst rate compared with the control group (25.9 ± 3.6% for 200 nM FCCP, 18.9 ± 2.1% for 2000 nM FCCP, 23.5 ± 3.9% for 200 µM ddC, and 24.6 ± 3.2% for 2000 µM ddC vs. 36.6 ± 4.4% for the control, Fig. 5B). There was no significant difference in total cell number and apoptosis in blastocysts from all groups (Fig. 5C, D).
Fig. 5.
Effects of FCCP and ddC on the developmental competence of oocytes. Percentage of cleavage (A), blastocyst formation (B), total cell number (C), and apoptosis (D) in blastocysts. Ctrl, control; F, FCCP; D, ddC. The number of oocytes examined in each treatment group is shown in parentheses. Values represent means ± SEM from at least four separate experiments. *P < 0.05, compared with the control.
Discussion
In the present study, two inhibitors, namely FCCP (an uncoupler of OXPHOS) and ddC (a nucleoside analogue), were used to inhibit mitochondrial activity and replication, respectively. While FCCP (2000 nM) significantly reduced membrane potential and first polar body extrusion, ddC reduced mitochondrial copy number but failed to affect first polar body extrusion. These results are consistent with a previous study that reported FCCP (10 and 100 nM) reduces the rate of oocyte maturation in the mouse and that ddC (10 and 100 µM) has no effects on first polar body extrusion in vitro [16]. Other studies suggest that certain developmental events in oocytes and preimplantation stage embryos may be spatially regulated by the magnitude of mitochondrial Δφm [10, 27]. Moreover, a further disruption of mitochondrial function with FCCP delays microtubule formation due to ATP deficiency [28]. Collectively, meiotic maturation processes such as polar body extrusion and maintenance of metaphase stages in mammals need proper ATP supply by mitochondrial Δφm, but little related to mitochondria copy numbers.
Oocyte maturation is mainly controlled by the activity of MAPK and maturation promoting factor (MPF). To understand the involvement of mitochondria on pig oocyte maturation, MAPK and MPF activity was quantified in mature oocytes in the presence of FCCP and ddC. While FCCP and ddC did not affect MAPK activity in mature oocytes, FCCP decreased MPF activity. It is unclear that why declined ATP only affect the activity of MPF, but not activity of MAPK. MPF is a complex comprised of a catalytic unit, Cdc2/CDK1, and a regulatory unit, Cyclin B. The activity of MPF is regulated by the phosphorylation of Cyclin B. ATP is also required for the activation of MPF because ATP is needed for phosphorylation of Cyclin B and Cyclin-dependent kinase 1 (CDK1) [29]. During oocyte maturation, MAPK is activated from germinal vesicle breakdown until MII [30]. Since MAPK activity is regulated by external signals such as FSH and estrogen, it might be less sensitive to cytoplasmic ATP.
In the present study, we evaluated whether mitochondrial replication and activity affect maternal gene expression and poly(A) tail length. Cyclin B1, C-mos, Cdc2, Bmp15, and Gdf9 were the maternal genes selected, and their polyadenylation statuses were investigated. FCCP and ddC did not change the mRNA expression of Cyclin B1 and C-mos, but the poly(A) tail of Cyclin B1 was shortened by FCCP treatment. These results suggest that mitochondrial production of ATP may involve maternal mRNA stability via the maintenance of poly(A) tail length. Spikings et al. [31] also reported that ATP was essential for both cleavage and polyadenylation for mRNA. C8-modified ATP analogues coupled with the decline in cellular ATP potential inhibite the polyadenylation. [32]. Bmp15 and Gdf9 are important for oocyte maturation and fertility. In the present study, FCCP significantly reduced the mRNA expression of Bmp15 in MII oocytes. Oocyte maturation in vitro is achieved by the distribution of active mitochondria and by cumulus cell expansion. It is widely reported that mammalian cumulus cell expansion requires Bmp15 and Gdf9. In the present study, FCCP significantly reduced the mRNA expression of Bmp15 in MII oocytes. mRNA stability was related to the length of the poly(A) tail. Cytoplasmic polyadenylation and nuclear polyadenylation are two important roles in protecting mRNAs from degradation and in stimulating the translation of mRNAs [33]. The results of the poly(A) tail assay confirmed that FCCP treatment shortened the poly(A) tail length of Bmp15, which may cause mRNA instability and induce degradation and low expression.
While ATP activity is required for normal maternal gene expression, additional research is needed to determine which ATP-dependent signaling pathways are involved.
Reduction of the mitochondrial Δφm, ATP concentration or mtDNA copy did not affect the ability of oocytes to undergo the first cleavage step. However, a lower rate of blastocyst formation was observed in oocytes treated with FCCP or ddC. Spikings et al. [34] demonstrated that mtDNA degradation may accompany the early stages of embryonic development, because a marked decline in mtDNA copy number occurred during cleavage (a 96% decrease between the 2- and 8-cell stages). This is consistent with previous findings in which mitochondrial dysfunction in mouse [35] and porcine [36] oocytes had a negative effect on blastocyst formation.
Mitochondrial Δφm is a critical factor for the establishment of oocyte and embryo competence [10, 37, 38]. Sequential analysis of individual oocytes and embryos indicates that mitochondria with a high Δφm remain spatially stable during oocyte maturation, fertilization, and initial cleavage [39]. It is likely that FCCP slows the rate of early embryo development. Similarly, ddC treatment decreased mtDNA copy number, resulting in a reduction in blastocyst formation. These results are in agreement with studies by several research groups [16, 34, 40] and Ge et al., who showed low mtDNA copy numbers affect mitochondrial activity and disrupt the balance between nuclear and mitochondrial genomes.
Taken together, we investigated the association of mitochondrial potential and copy number with pig oocyte maturation and developmental potential in vitro. Our findings suggested that mitochondrial membrane potential, an important regulator of ATP production, is critical for pig oocyte maturation, quality, and subsequent parthenogenetically activated embryo development. Furthermore, the negative effects of lower mitochondrial copy number are mainly restricted to parthenotes developmental potential in pigs. The present study highlights the need to further elucidate the function of mitochondria in early embryo development.
Acknowledgments
This work was sponsored by a grant from the Next-Generation BioGreen 21 Program (PJ00956302, PJ00909801 and PJ009594), Rural Development Administration, Republic of Korea, and a research grant from Chungbuk National University in 2012, Republic of Korea.
References
- 1.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Gonçalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod 2001; 64: 904–909 [DOI] [PubMed] [Google Scholar]
- 2.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14: 53–61 [DOI] [PubMed] [Google Scholar]
- 3.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril 2006; 85: 584–591 [DOI] [PubMed] [Google Scholar]
- 4.Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod 2001; 7: 425–429 [DOI] [PubMed] [Google Scholar]
- 5.Pikó L, Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol 1976; 49: 1–10 [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Whitworth KM, Spate LD, Walters EM, Zhao J, Prather RS. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology 2012; 78: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod 2007; 13: 759–770 [DOI] [PubMed] [Google Scholar]
- 8.Quinn P, Warnes GM, Kerin JF, Kirby C. Culture factors in relation to the success of human in vitro fertilization and embryo transfer. Fertil Steril 1984; 41: 202–209 [DOI] [PubMed] [Google Scholar]
- 9.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod 2004; 10: 23–32 [DOI] [PubMed] [Google Scholar]
- 10.Van Blerkom J, Davis P, Alexander S. Inner mitochondrial membrane potential (DeltaPsim), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod 2003; 18: 2429–2440 [DOI] [PubMed] [Google Scholar]
- 11.Newman B, Dai Y. Transcription of c-mos protooncogene in the pig involves both tissue-specific promoters and alternative polyadenylation sites. Mol Reprod Dev 1996; 44: 275–288 [DOI] [PubMed] [Google Scholar]
- 12.Tay J, Hodgman R, Richter JD. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev Biol 2000; 221: 1–9 [DOI] [PubMed] [Google Scholar]
- 13.Tremblay K, Vigneault C, McGraw S, Sirard MA. Expression of cyclin B1 messenger RNA isoforms and initiation of cytoplasmic polyadenylation in the bovine oocyte. Biol Reprod 2005; 72: 1037–1044 [DOI] [PubMed] [Google Scholar]
- 14.Juengel JL, Bodensteiner KJ, Heath DA, Hudson NL, Moeller CL, Smith P, Galloway SM, Davis GH, Sawyer HR, McNatty KP. Physiology of GDF9 and BMP15 signalling molecules. Anim Reprod Sci 2004; 82–83: 447–460 [DOI] [PubMed] [Google Scholar]
- 15.McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 2005; 129: 481–487 [DOI] [PubMed] [Google Scholar]
- 16.Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev 2012; 79: 392–401 [DOI] [PubMed] [Google Scholar]
- 17.Cabot RA, Kühholzer B, Chan AW, Lai L, Park KW, Chong KY, Schatten G, Murphy CN, Abeydeera LR, Day BN, Prather RS. Transgenic pigs produced using in vitro matured oocytes infected with a retroviral vector. Anim Biotechnol 2001; 12: 205–214 [DOI] [PubMed] [Google Scholar]
- 18.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002; 295: 1089–1092 [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, Cowell-Lucero JL, Wells KD, Colman A, Polejaeva IA, Ayares DL. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol 2002; 20: 251–255 [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Sui L, Li Y, Ji S, Zhang X, Zhang Y. Effects of chemically defined medium on early development of porcine embryos derived from parthenogenetic activation and cloning. Zygote 2012; 20: 229–236 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki C, Iwamura S, Yoshioka K. Birth of piglets through the non-surgical transfer of blastocysts produced in vitro. J Reprod Dev 2004; 50: 487–491 [DOI] [PubMed] [Google Scholar]
- 22.Cui XS, Li XY, Jeong YJ, Jun JH, Kim NH. Gene expression of cox5a, 5b, or 6b1 and their roles in preimplantation mouse embryos. Biol Reprod 2006; 74: 601–610 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 24.Zhang DX, Cui XS, Kim NH. Involvement of polyadenylation status on maternal gene expression during in vitro maturation of porcine oocytes. Mol Reprod Dev 2009; 76: 881–889 [DOI] [PubMed] [Google Scholar]
- 25.Sallés FJ, Strickland S. Analysis of poly(A) tail lengths by PCR: the PAT assay. Methods Mol Biol 1999; 118: 441–448 [DOI] [PubMed] [Google Scholar]
- 26.Shoujo ATH, Terada T. Effect of aging on parthenogenetic activation with cycloheximide and alteration of the activity of maturation promoting factor during aging of bovine oocytes. J Mamm Ova Res Microbiol 2000; 17: 35–41 [Google Scholar]
- 27.Van Blerkom J, Davis P. High-polarized (Delta Psi m(HIGH)) mitochondria are spatially polarized in human oocytes and early embryos in stable subplasmalemmal domains: developmental significance and the concept of vanguard mitochondria. Reprod Biomed Online 2006; 13: 246–254 [DOI] [PubMed] [Google Scholar]
- 28.Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL, Liang XY. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod 2007; 22: 1681–1686 [DOI] [PubMed] [Google Scholar]
- 29.Timofeev O, Cizmecioglu O, Settele F, Kempf T, Hoffmann I. Cdc25 phosphatases are required for timely assembly of CDK1-cyclin B at the G2/M transition. J Biol Chem 2010; 285: 16978–16990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goudet G, Belin F, Bézard J, Gérard N. Maturation-promoting factor (MPF) and mitogen activated protein kinase (MAPK) expression in relation to oocyte competence for in-vitro maturation in the mare. Mol Hum Reprod 1998; 4: 563–570 [DOI] [PubMed] [Google Scholar]
- 31.Shankarling GS, Coates PW, Dass B, Macdonald CC. A family of splice variants of CstF-64 expressed in vertebrate nervous systems. BMC Mol Biol 2009; 10: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LS, Du-Cuny L, Vethantham V, Hawke DH, Manley JL, Zhang S, Gandhi V. Chain termination and inhibition of mammalian poly(A) polymerase by modified ATP analogues. Biochem Pharmacol 2010; 79: 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weill L, Belloc E, Bava FA, Méndez R. Translational control by changes in poly(A) tail length: recycling mRNAs. Nat Struct Mol Biol 2012; 19: 577–585 [DOI] [PubMed] [Google Scholar]
- 34.Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod 2007; 76: 327–335 [DOI] [PubMed] [Google Scholar]
- 35.Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod 2004; 71: 1936–1942 [DOI] [PubMed] [Google Scholar]
- 36.Xu YN, Shen XH, Lee SE, Kwon JS, Kim DJ, Heo YT, Cui XS, Kim NH. Autophagy influences maternal mRNA degradation and apoptosis in porcine parthenotes developing in vitro. J Reprod Dev 2012; 58: 576–584 [DOI] [PubMed] [Google Scholar]
- 37.Wilding M, Carotenuto R, Infante V, Dale B, Marino M, Di Matteo L, Campanella C. Confocal microscopy analysis of the activity of mitochondria contained within the ‘mitochondrial cloud’ during oogenesis in Xenopus laevis. Zygote 2001; 9: 347–352 [DOI] [PubMed] [Google Scholar]
- 38.Jones A, Van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod 2004; 19: 1861–1866 [DOI] [PubMed] [Google Scholar]
- 39.Van Blerkom J, Davis P, Thalhammer V. Regulation of mitochondrial polarity in mouse and human oocytes: the influence of cumulus derived nitric oxide. Mol Hum Reprod 2008; 14: 431–444 [DOI] [PubMed] [Google Scholar]
- 40.Cummins JM. Fertilization and elimination of the paternal mitochondrial genome. Hum Reprod 2000; 15(Suppl 2): 92–101 [DOI] [PubMed] [Google Scholar]