Abstract
The literature on positron emission tomography and computed tomography using 18fluoro-deoxyglusose (FDG-PET/CT) in the diagnosis of pediatric inflammatory bowel disease (IBD) is presented. Only five papers representing independent studies were identified and included in this review. Of these, two studies dealt with both stand-alone FDG-PET and FDG-PET/CT, while three were about stand-alone FDG-PET only. No studies could be found that focused on FDG-PET/CT only. The five studies comprised analysis of a total 181 pediatric patients (0-18 years of age). They unanimously indicated that FDG-PET/CT is a versatile method with a diagnostic high sensitivity ranging from 70% to 97%. In conclusion, the pediatric literature on FGD-PET/CT’s role in the diagnosis of IBD is very limited. Prospective studies of well characterized populations are needed in order to validate this novel imaging modality in pediatric IBD.
Keywords: Inflammatory bowel disease, ulcerative colitis, Crohn’s disease, positron-emission tomography and computed tomography
Introduction
Inflammatory bowel disease (IBD) is a disease entity primarily comprising Crohn’s disease (CD) and ulcerative colitis (UC). CD and UC are conditions with both acute and chronic inflammation in the gastrointestinal tract. In UC, inflammation is restricted to the colon, whereas CD can be found from the oral cavity to the anus. IBD as a whole constitutes a diagnostic and therapeutic challenge especially in children and adolescents, not only at initial disease presentation, but also during suspected disease flares. Compared to IBD in adults, children often display more extensive disease at initial presentation and in the majority of children UC presents with a pancolitis [1,2]. At present, invasive endoscopic procedures are required to ascertain the specific IBD subtype and to evaluate disease extension, and in the pediatric and adolescent population this frequently requires general anesthesia. Thus, non-invasive alternatives are in high demand. Positron emission tomography using the radioactive glucose analogue 18fluoro-deoxyglusose (FDG-PET) has been available for decades and studies have found it to be useful in the diagnostic workup in pediatric patients with suspected IBD [3-5]. However, the invention of combined positron emission tomography and computed tomography (FDG-PET/CT) has allowed a more precise evaluation of disease extent and the involvement of the gut wall, which could be important in diagnosing the specific IBD subtype [6]. There has been an increasing awareness of the potential benefit of PET/CT in the diagnostic workup and follow up in adult patients with IBD [7-12]. Despite the promising results in adults, only few original pediatric papers have been published. The purpose of this mini-review was to present the results of the existing limited literature on this subject.
FDG-PET/CT in inflammatory bowel disease
PET/CT is a non-invasive imaging modality combining metabolic assessment of pathophysiologic processes with morphologic correlation. The most common tracer is FDG, a glucose analogue taken up by cells proportional to their metabolic activity. It is widely used in cancer imaging because of the inherently high glycolytic activity of most malignant cells providing the basis for a sensitive whole-body scan for staging, assessment of treatment response, and detection of recurrences [13]. The rationale for employing this modality in IBD is an increasing recognition that inflammatory cells display similar hypermetabolic features due to an up-regulation of glucose transporters to meet the increased metabolic demands in the inflamed state. This knowledge has prompted an increasing interest in recent years to employ FDG-PET/CT in inflammatory diseases because it offers a sensitive whole-body survey especially suited for mapping the frequent systemic manifestations seen in this category of diseases. This is also true for IBD, as FDG-PET/CT is the only modality available allowing both functional and morphological visualization of the whole gastrointestinal tract as well as detection of extra-intestinal areas of inflammation [14].
Pediatric IBD
The use of FDG-PET/CT in patients with IBD has mainly been described in adult patients, in whom it has been shown to be a reliable and non-invasive way to visualize the gastrointestinal tract, but its role in the diagnosis of IBD is yet to be established. Figure 1 presents an example of an FDG-PET/CT scan done in a pediatric patient with CD.
Figure 1.
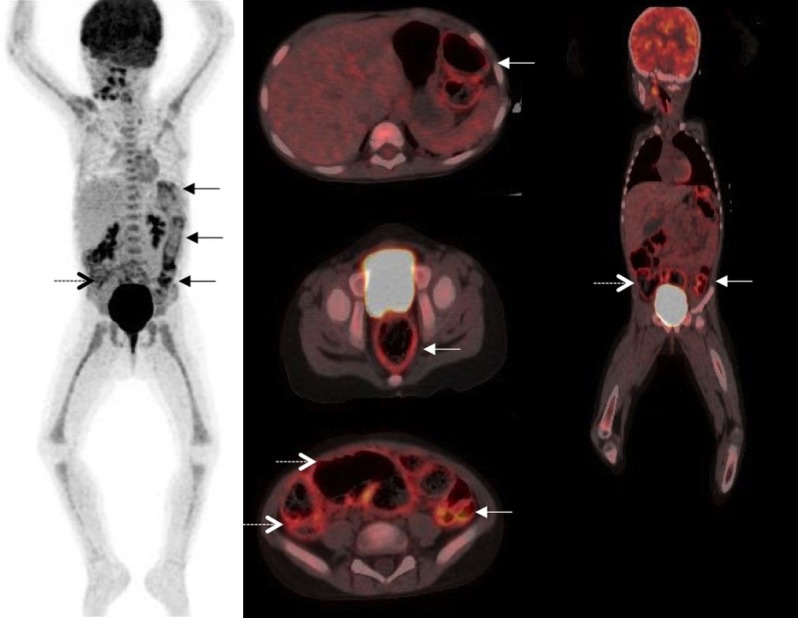
An example of FDG-PET/CT in pediatric IBD. This figure shows an FDG-PET/CT scan done at our clinic of a 3 year old girl with Crohn’s disease to assess the possibility of surgical intervention. The displayed images are maximum intensity projection (left column), fused transaxial images (middle column), and fused coronal images (right column). Images show intensely increased FDG uptake in the recto-sigmoid and the descending colon (solid arrows), and in the cecum and ascending colon (dotted arrow), but no pathologic uptake in the small bowel, stomach or esophagus. Note the intense, diffuse FDG uptake in the bone marrow consistent with the reactive response of the bone marrow to systemic inflammation. The patient was subsequently considered candidate for therapeutic colectomy.
In a recent review, the pooled per segment diagnostic sensitivity/specificity in adults was 85%/87% [6]. In the pediatric setting, literature on FDG-PET and FDG-PET/CT is sparse. We have found three studies on stand alone FDG-PET in IBD and two studies describing the utility of both FDG-PET and FDG-PET/CT in the diagnosis of IBD. These will be presented chronologically below and are summarized in Table 1.
Table 1.
Overview of included articles
| Author | Year | n | Study design | Modality studied | Reference examination | Sensitivity/specificity (%) |
|---|---|---|---|---|---|---|
| Skehan et al. | 1999 | 25 | Retro-spective | FDG-PET | Colonoscopy and/or SBFT | 81/851 |
| Lemberg et al. | 2005 | 65 | Pro-spective | FDG-PET | Colonoscopy and SBFT | PET vs colonoscopy: 86/50 |
| PET vs. SBFT: 59/1001 | ||||||
| Löffler et al. | 2006 | 23 | Retro-spective | FDG-PET | Colonoscopy and/or ultrasound | 98/682 |
| Dabritz et al. | 2011 | 45 | Retro-spective | FDG-PET (±CT) | Colonoscopy, ultrasound and gastroscopy | 97/1001 |
| 82/972 | ||||||
| Berthold et al. | 2013 | 23 | Retro-spective | FDG-PET (±CT) | Colonoscopy, MRI and gastroscopy | Stomach & duodenum: 25/1002 |
| Remaining bowel: 73/892 |
Per-patient sensitivity and specificity.
Per-segment sensitivity and specificity.
The first paper appeared in 1999 and was a retrospective study by Skehan et al. including 25 patients between 7 and 18 years of age with suspected IBD [3]. Within 14 days of their reference examination (small bowel follow through (SBFT) or colonoscopy with biopsies), an FDG-PET scan was performed. A dosage of 1.85 MBq/kg was given 1 h prior to FDG-PET scan. The FDG uptake in the gut was compared to the uptake of the spine. FDG uptake greater than that of the spine was considered pathological. Compared to the reference standard, PET showed a per-patient sensitivity and specificity of 81% and 85%, respectively. In 16 patients who in addition had a colonoscopy, the ileum was not reached in 10 and in eight of these, FDG-PET scans showed signs of inflammation in the colon proximal of the endoscopically visualized area. The authors concluded that FDG-PET was a useful technique for the diagnosis of IBD and could be used as an adjunct diagnostic tool.
In 2005, Lemberg et al. [5] published the only, prospective study to date addressing this subject. They included 55 pediatric patients with known IBD and 10 patients with recurrent abdominal pain. Colonoscopy with biopsies and/or a SBFT served as reference. The mean time interval from the reference examination to the FDG-PET scan was 30 days (range 1-62). The FDG dose used was 3.7 MBq/kg given 45 min prior to the PET scan. The FDG uptake in the gut was compared to the uptake of the spine. FDG uptake greater than that of the spine was considered pathological. FDG-PET exhibited a per-patient sensitivity with regards to the presence of UC, CD, and recurrent abdominal pain, of 76%, 82%, and 100%, respectively. The specificity of FDG-PET versus SBFT was 100%, versus colonoscopy it was 50% for CD and 81% and for UC. However, this study had several limitations, which may have reduced the diagnostic probabilities. Firstly, a gap of up to 62 days between the reference examination and the FDG-PET scan meant that the two examinations could easily depict different situations. In addition, most patients had been started on anti-inflammatory therapy in the meantime, a circumstance known to decrease or eliminate FDG avidity of inflammatory lesions [12]. Furthermore, three of the CD patients had fibrotic non-inflammatory strictures, which do not show on an FDG-PET scan, as FDG is not taken up by fibrotic, non-inflammatory tissue. As these investigations were considered false negative they contributed to the relatively poor sensitivity of FDG-PET seen in CD. However, as pointed out by Jacene et al. in adult patients [15], this finding should instead be considered as true negative. Accordingly, these authors advocated the use of FDG-PET to distinguish between inflammatory and fibrostenotic lesions with consequent implications for therapy, i.e. anti-inflammatory drugs vs. surgery. Lastly, Lemberg et al. also noted, that in patients who did not have a full colonoscopy (only 20 of 40 patients) PET was positive in the colon not visualized by the endoscopy. However, these were regarded as false positives, and thus contributed – perhaps erroneously – to the relatively poor specificity.
Löffler et al. [4] published a retrospective study in 2006, including 23 children with a median age of 12 years (range 2-16). An FDG-PET scan was performed within 10 days before or after the reference examination, i.e. colonoscopy with biopsies and/or ultrasonographic evaluation of the abdomen. Prior to the PET scan a dose of 3-5 MBq/kg FDG was injected. The time from injection to the PET scan was not stated in the article. FDG uptake was calculated semiquantitatively using the maximal standardized uptake values (SUVmax) and compared with the FDG uptake in the liver. If SUVmax/SUVliver was greater than 1.2 it was considered as a sign of pathological inflammation. Compared to histology, FDG-PET showed per-segment sensitivity and specificity of 98% and 68%, respectively. Interestingly, the sensitivity was higher than the sensitivity of endoscopy when compared to histology. The authors concluded that FDG-PET could be used in the diagnostic workup in pediatric IBD and in the follow up of patients with IBD. However, inclusion criteria were not stated, and it seems as if some patients were included more than once, as 26 FDG-PET scans in 23 patients were evaluated. It is also not clear why only 18 patients had a colonoscopy.
In 2011, the same group published the first paper addressing combined FDG-PET/CT for the diagnosis of pediatric IBD [16]. This was a retrospective study including 45 patients between four and seventeen years of age (median 13.2). All patients were known with either CD or UC, and most patients were actively treated with anti-inflammatory drugs at inclusion. Patients were examined with either an FDG-PET or an FDG-PET/CT between 27 days before and 2 days after the reference examination, i.e. endoscopies (gastroscopy and colonoscopy) with biopsies and ultrasound of the small bowel. With both PET/CT and PET a dose of 3 MBq was injected 1 h prior to scanning. With PET/CT the total radiation exposure was estimated to 5-7 mSv whereas it was estimated to 4-5 mSv with stand alone PET. The FDG uptake in the gut was compared to the uptake of the liver. FDG uptake greater than that of the liver was considered as pathological inflammation. Thirty-five of the 45 patients included had a colonoscopy, but in only 25 was the terminal ileum intubated. Gastroscopy was performed in 30 patients. The study showed per-patient sensitivity and specificity of 97% and 100%, respectively, and per-segment sensitivity and specificity of 82% and 97%, respectively. No difference was found in the sensitivity or specificity between FDG-PET and FDG-PET/CT. The authors concluded that FDG-PET/CT seems to be a reliable tool for detecting inflamed gut segments in IBD with high sensitivity and high specificity. However, it is unclear why and how patients were included, and the FDG-PET or FDG-PET/CT were performed up to 27 days before the reference examination again opening the possibility that the two examinations did not show the same clinical situations. The lack of difference in diagnostic ability described may be the case when focusing only on inflammation in different segments of the bowel. However, as noted by Lemberg et al. [5], FDG avidity is limited in non-inflammatory changes in CD, like strictures. This, and the ability to better localize extra intestinal inflammation, would make the FDG-PET/CT superior to FDG-PET alone.
The most recent paper by Berthold et al. [17], included retrospectively 23 patients between 8 and 17 years of age (median 15), who had undergone endoscopies (gastroscopy and colonoscopy) with biopsies, magnetic resonance imaging (MRI), and FDG-PET (±CT) as part of the diagnostic workup. In all patients, reference examinations (endoscopies and MRI) were performed during initial admission, but the time interval until FDG-PET (±CT) was not systematically reported and in three patients the time span was up to three months. The FDG dose given in this study was not stated, neither in the PET nor the PET/CT scans. Positive PET signal was defined as a diffuse and coherent tracer uptake in the bowel that was visually increased compared to normal intestinal uptake. If endoscopies were insufficient, the segments not accessible were analyzed by MRI. In this study, sensitivity and specificity of FDG-PET (±CT) was 25% and 100%, respectively, in the stomach and duodenum, and 73% and 89%, respectively in the remaining bowel. The authors concluded that FDG-PET (±CT) has to be further evaluated as a tool for determination of the extent and degree of inflammation, especially in the small bowel, where endoscopy is limited. The study contained some uncertainties regarding the inclusion process and the indication for FDG-PET (±CT) was not clearly stated. As FDG-PET (±CT) was not stated as a reference examination, it is not likely that FDG-PET (±CT) was part of the routine workup. Therefore, if FDG-PET (±CT) was done in diagnostically challenging patients only, this would mean a selection bias reducing sensitivity and specificity. Again, FDG-PET and FDG-PET/CT were pooled as one modality, although the sensitivity and specificity of FDG-PET with and without CT are not comparable.
Conclusion and future perspectives
The pediatric literature on the value of FDG-PET and FDG-PET/CT in IBD is limited, heterogeneous, and mostly restricted to retrospective studies. Also, knowledge of the optimal FDG dosage for visualizing pathology in gastrointestinal tract is virtually nonexistent in the pediatric setting. Currently used radiation doses are less for FDG-PET/CT with low-dose, non-enhanced CT compared to other modalities such as SBFT and contrast-enhanced CT. Nonetheless, the literature does reflect a potential for diagnosing inflammatory changes in the gastrointestinal tract with a high sensitivity and a reasonable specificity. New developments may add to the versatility of PET/CT, as Saboury et al. [18] recently published a paper where they employed a quantitative volume-based technique to FDG-PET/CT in adult CD. They showed, that by applying this technique they could calculate the global disease activity, which correlated to both clinical and endoscopic findings. Studies, applying this technique in pediatric IBD, would be of great interest, as it could be a noninvasive way to monitor the effect of treatment.
Developments in instrumentation have made stand-alone FDG-PET obsolete, and early reports based on this modality can hardly be compared to studies using FDG-PET/CT as it has been shown in for instance cancer that hybrid PET/CT has greatly improved sensitivity and specificity due to a more accurate CT-based attenuation correction and a much better anatomical mapping [19-22]. Thus, it is reasonable to hypothesize that future studies with FDG-PET/CT in pediatric IBD will show further improved overall results, despite the results by one study (Löffler et al.) showing no apparent advantages of PET/CT over PET. Moreover, in the adult literature, the ability of the PET/CT scan to visualize the extra-intestinal manifestations of IBD has proven valuable in the diagnostic work up [10].
In the reported studies, almost no patients under the age of 7 years were included, and thus, the current knowledge on the value of FDG-PET/CT for IBD in the youngest children is very limited. Finally, it is noteworthy that in articles reporting on the incidence of incomplete colonoscopies, 51 out of 91 colonoscopies were incomplete. If this number is applicable to the clinical pediatric routine, 44% of our “gold standard” examinations in children suspected of having IBD are incomplete. FDG-PET/CT seems to have great potential as a non-invasive whole-body examination for assessment of several important aspects of pediatric IBD adding to the accuracy of the existing techniques, i.e. in the preliminary assessment of disease extent, for follow up of indeterminate cases, for suspected flares or recurrence, for therapy planning in patients with stenosis, and for assessing the response to treatment. Of particular importance for pediatric use, the general availability, the easy administration of FDG and fast procedure time for PET/CT procedures make this technique almost universally feasible.
This review calls for prospective studies of well characterized patients to shed light upon the undoubtedly positive impact that FDG-PET/CT will have on the management of patients with known or suspected IBD.
Disclosure of conflict of interest
None.
References
- 1.Pigneur B, Seksik P, Viola S, Viala J, Beaugerie L, Girardet JP, Ruemmele FM, Cosnes J. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis. 2010;16:953–961. doi: 10.1002/ibd.21152. [DOI] [PubMed] [Google Scholar]
- 2.Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT, Bisset WM, Mahdi G, Arnott ID, Satsangi J, Wilson DC. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 3.Skehan SJ, Issenman R, Mernagh J, Nahmias C, Jacobson K. 18F-fluorodeoxyglucose positron tomography in diagnosis of paediatric inflammatory bowel disease. Lancet. 1999;354:836–837. doi: 10.1016/S0140-6736(99)80021-X. [DOI] [PubMed] [Google Scholar]
- 4.Loffler M, Weckesser M, Franzius C, Schober O, Zimmer KP. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379–385. doi: 10.1196/annals.1326.014. [DOI] [PubMed] [Google Scholar]
- 5.Lemberg DA, Issenman RM, Cawdron R, Green T, Mernagh J, Skehan SJ, Nahmias C, Jacobson K. Positron emission tomography in the investigation of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:733–738. doi: 10.1097/01.mib.0000172810.49619.cb. [DOI] [PubMed] [Google Scholar]
- 6.Treglia G, Quartuccio N, Sadeghi R, Farchione A, Caldarella C, Bertagna F, Fania P, Cistaro A. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography in patients with chronic inflammatory bowel disease: a systematic review and a meta-analysis. J Crohns Colitis. 2013;7:345–354. doi: 10.1016/j.crohns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi A, Li Q, Muller K, Collins D, Valentine JF, Drane W, Polyak S. Diagnostic value of noninvasive combined fluorine-18 labeled fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography enterography in active Crohn’s disease. Inflamm Bowel Dis. 2010;16:974–981. doi: 10.1002/ibd.21153. [DOI] [PubMed] [Google Scholar]
- 8.Shyn PB, Mortele KJ, Britz-Cunningham SH, Friedman S, Odze RD, Burakoff R, Goldberg JE, Erturk M, Silverman SG. Low-dose 18F-FDG PET/CT enterography: improving on CT enterography assessment of patients with Crohn disease. J Nucl Med. 2010;51:1841–1848. doi: 10.2967/jnumed.110.080796. [DOI] [PubMed] [Google Scholar]
- 9.Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053–1059. doi: 10.2967/jnumed.107.040436. [DOI] [PubMed] [Google Scholar]
- 10.Das CJ, Makharia GK, Kumar R, Kumar R, Tiwari RP, Sharma R, Malhotra A. PET/CT colonography: a novel non-invasive technique for assessment of extent and activity of ulcerative colitis. Eur J Nucl Med Mol Imaging. 2010;37:714–721. doi: 10.1007/s00259-009-1335-2. [DOI] [PubMed] [Google Scholar]
- 11.Groshar D, Bernstine H, Stern D, Sosna J, Eligalashvili M, Gurbuz EG, Niv Y, Fraser G. PET/CT enterography in Crohn disease: correlation of disease activity on CT enterography with 18F-FDG uptake. J Nucl Med. 2010;51:1009–1014. doi: 10.2967/jnumed.109.073130. [DOI] [PubMed] [Google Scholar]
- 12.Spier BJ, Perlman SB, Jaskowiak CJ, Reichelderfer M. PET/CT in the evaluation of inflammatory bowel disease: studies in patients before and after treatment. Mol Imaging Biol. 2010;12:85–88. doi: 10.1007/s11307-009-0232-1. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49:17N–21N. 37N. [PubMed] [Google Scholar]
- 14.Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124–145. doi: 10.1053/j.semnuclmed.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Jacene HA, Ginsburg P, Kwon J, Nguyen GC, Montgomery EA, Bayless TM, Wahl RL. Prediction of the need for surgical intervention in obstructive Crohn’s disease by 18F-FDG PET/CT. J Nucl Med. 2009;50:1751–1759. doi: 10.2967/jnumed.109.065466. [DOI] [PubMed] [Google Scholar]
- 16.Dabritz J, Jasper N, Loeffler M, Weckesser M, Foell D. Noninvasive assessment of pediatric inflammatory bowel disease with 18-F-fluorodeoxyglucose-positron emission tomography and computed tomography. Eur J Gastroenterol Hepatol. 2011;23:81–9. doi: 10.1097/MEG.0b013e3283410222. [DOI] [PubMed] [Google Scholar]
- 17.Berthold LD, Steiner D, Scholz D, Alzen G, Zimmer KP. Imaging of Chronic Inflammatory Bowel Disease with 18F-FDG PET in Children and Adolescents. Klin Padiatr. 2013;225:212–217. doi: 10.1055/s-0033-1334878. [DOI] [PubMed] [Google Scholar]
- 18.Saboury B, Salavati A, Brothers A, Basu S, Kwee TC, Lam MG, Hustinx R, Louis E, Torigian DA, Alavi A. FDG PET/CT in Crohn’s disease: correlation of quantitative FDG PET/CT parameters with clinical and endoscopic surrogate markers of disease activity. Eur J Nucl Med Mol Imaging. 2014;41:605–14. doi: 10.1007/s00259-013-2625-2. [DOI] [PubMed] [Google Scholar]
- 19.Bockisch A, Freudenberg LS, Schmidt D, Kuwert T. Hybrid imaging by SPECT/CT and PET/CT: proven outcomes in cancer imaging. Semin Nucl Med. 2009;39:276–289. doi: 10.1053/j.semnuclmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48(Suppl 1):78S–88S. [PubMed] [Google Scholar]
- 21.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007;11:iii–iv. xi–267. doi: 10.3310/hta11440. [DOI] [PubMed] [Google Scholar]
- 22.Histed SN, Lindenberg ML, Mena E, Turkbey B, Choyke PL, Kurdziel KA. Review of functional/ anatomical imaging in oncology. Nucl Med Commun. 2012;33:349–361. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]


